Abstract
Background: Peripheral T-cell lymphoma, unspecified (PTCL-US) represents the largest subtype of PTCLs among Western populations. The prognosis of this heterogeneous group is poor with a 5-year overall survival (OS) of approximately 30%. The biological diversity of this subtype has prompted various attempts at refining clinical risk groups. For instance, the International Prognostic Index (IPI), first validated in DLBCL, has also been validated in PTCL-US in several studies. Recently, however, a new Prognostic Index for PTCL-US (PIT) has been proposed (
Methods: The BCCA Lymphoid Cancer Database was screened to identify all patients over 18 y diagnosed with PTCL-US by the World Health Organization classification system between January 1981 and June 2004. Patients were excluded if the diagnosis occurred outside of British Columbia or if the pertinent prognostic information was incomplete. Five year OS estimates were calculated for each variable in both the IPI (age >60 y, LDH> normal, PS ≥2, Stage III/IV and >1 extranodal sites) and PIT models (age, LDH> normal, PS ≥2 and bone marrow involvement). Five year OS estimates were then calculated for IPI groups 1, 2 and 3 (0/1, 2/3 and 4/5 factors, respectively) as well as PIT groups 1 through 4 (0, 1, 2 and 3/4 factors, respectively).
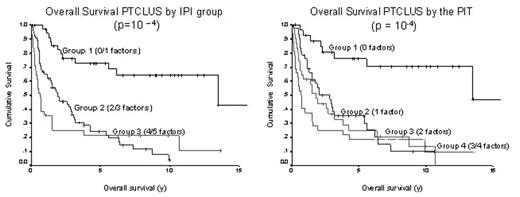
Results: Of the 134 patients identified, the median age was 61 y and the male to female ratio was 1.6. The predominant sites of extranodal involvement were bone marrow (10%), bone (6%), liver (4%), skin (4%), soft tissue (4%), lung (3%) and GI (3%). As demonstrated in figure 1, 5 year OS estimates were 73%, 24% and 22% for IPI groups 1, 2 and 3 (p=10−4), respectively and 76%, 35%, 25% and 19% for PIT groups 1 through 4, respectively (p=10−4 ). There was no difference between the prognostic models in the subset of 107 (80%) patients treated with CHOP-based chemotherapy.
Conclusions: Although the PIT was as effective as the IPI in defining clinical risk groups among PTCL-US patients, it did not provide any additional information in this study. Since the IPI is a well-established, familiar tool with similar prognostic capacity, it is reasonable to continue to apply this model in PTCL-US. With ongoing advances in gene expression profiling, it is likely that new biological models will emerge which may be used in combination with the IPI to better prognosticate this heterogeneous group.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal