Abstract
Purpose: Primary nasal NK/T-cell lymphoma is a highly aggressive tumor associated with poor response to therapy, frequent relapse, and a short median survival of 7–12 months. Moreover, the optimal treatment has not yet been established as most published studies were retrospective in nature. We performed a prospective study to evaluate the efficacy of combined m-BACOD chemotherapy and involved field radiotherapy (RT) for patients with previously untreated or relapsed nasal NK/T-cell lymphoma.
Methods: Between January 1998 and December 2004, all previously untreated or relapsed patients (pts), aged > 18 years, with biopsy proven nasal NK/T-cell lymphoma, ECOG performance status < 2, and adequate marrow, cardiac, liver and renal functions were included in this study. Previously untreated pts were treated with m-BACOD (iv methotrexate 200mg/m2 D8, D15 with leucovorin rescue, iv bleomycin 4mg/m2 D1, iv doxorubicin 45mg/m2 D1, iv cyclophosphamide 600mg/m2 D1, and iv vincristine 1mg/m2, and dexamethasone 6mg/m2 D1-5 PO) every 3 weeks for 6 cycles, followed by involved field RT (54 Gy in 27 daily fractions over 5.4 weeks). Relapsed pts with prior anthacycline-based chemotherapy were treated with 3 cycles of m-BACOD, followed by RT. Stage I/II pts with static response (SR) after 3 cycles m-BACOD were treated with early RT. Young pts (<60 years) who achieved complete remission (CR) with m-BACOD were offered a course of high-dose therapy BEAM before RT. Endpoints were objective response (OR), treatment tolerability, progression-free-survival (PFS), and overall survival (OS).
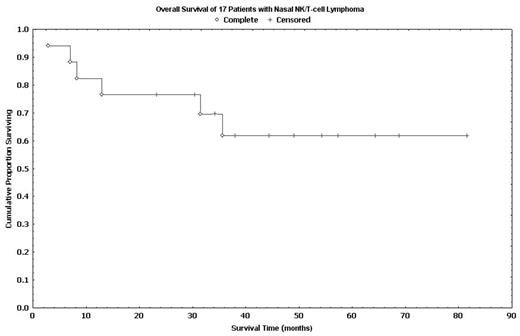
Results: Seventeen pts (14 untreated, 3 relapsed) were included. The median age was 49 years (range: 34–90) with a sex ratio of 1:1.1 (M:F). Fifteen pts had stage I disease, 1 had stage II and 1 had stage IV disease. A median of 5 cycles (range: 2–6) of m-BACOD were delivered to each pt. An OR was observed in 15 pts (88%) after m-BACOD induction with CR in 13 pts (76%), PR in 1 pt (6%) and SR in 1 pt (6%). Two pts (12%) had progressive disease during m-BACOD therapy. The two pts with PR and SR were converted to CR after RT, resulting in a final CR of 88%. Grade 3/4 adverse events were leukopenia (70%), thrombocytopenia (12%), infection, (24%), pneumonitis (12%). There was 1 (6%) therapy-related death. Only 5 pts opted for BEAM high-dose therapy. With a median follow-up of 49 months (range: 3–82), the median PFS and median OS were 31.5 months (range: 1.9–68.9) and 35.6 months (range: 2.9–81.6), respectively. The 5-year PFS and OS were 55% and 63%, respectively. These results appears superior than our historic control (5-yr OS: 33%, Clin Oncol 1999) and that reported by others (5-yr OS: 37.9%, Int J Radiat Oncol Biol Phys. 2002)
Conclusion: m-BACOD chemotherapy in combination with involved field RT is a highly active and well tolerated treatment for pts with aggressive nasal NK/T-cell lymphoma.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal