Abstract
Background: The activity of V in myeloma was first described in the 1970’s. Although Phase II data suggest that V demonstrates single agent activity, subsequent reports have questioned its role. Due to these conflicting results, we conducted a subanalysis investigating the effect of V dose in the phase II DVd-T regimen that we have previously reported (Agrawal et al ASH 2003). We evaluated the effects of V dose on progression free survival (PFS) and overall survival (OS) in newly diagnosed and relapsed/refractory patients treated with DVd-T.
Patients and Methods: As previously reported, this Phase II study enrolled 102 patients with newly diagnosed or relapsed/refractory multiple myeloma with evidence of end organ damage. DVd-T was administered as previously reported. After best response, patients were maintained on prednisone 50mg every other day and the maximum tolerated dose of thalidomide until disease progression. For patients experiencing grade 1 neuropathy, V was reduced by 25%, and for grade 2, by 50%. Patients developing grade 3/4 neuropathy had V discontinued and thalidomide suspended until toxicity decreased by at least one grade. Univariate analyses were conducted to assess the effect of V dose reduction or elimination on PFS and OS. Multivariate analyses were performed to adjust for the impact of age, platelet count, stage, quality of response (CR or near CR versus SD or PR), and thalidomide dose.
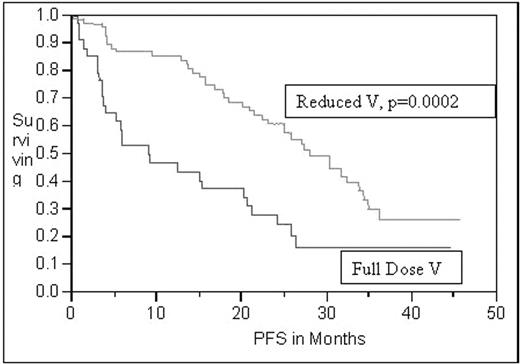
Results: Trial included 53 newly diagnosed and 49 relapsed/refractory patients. Median age was 62.9 years. 59% had stage 3 or 4 disease. 37% had abnormal cytogenetics. Median beta-2 microglobulin was 4.1. Overall response rate of 87% was seen in newly diagnosed patients (36% achieved CR; 13% near CR; 38% PR; 8% SD; 6% PD). In the relapsed/refractory patients, overall response rate of 87% was achieved (21% achieved CR; 26% near CR; 40% PR; 13% SD). Median follow up was 28.1 months. Median PFS for the newly diagnosed group was 28.2 months and 15.5 for the relapsed/refractory group. Median OS was 39.9 months for the relapsed/refractory group. After 50 months of follow-up for the newly diagnosed group, median OS has not been reached. In total, 464 cycles were administered, of which 225 were given with full dose V and 242 with reduced dose or eliminated V. Grade 3/4 neuropathy occurred in 22 patients. Univariate analysis revealed that reducing or eliminating V had a significant positive effect on PFS and OS (p = 0.0002 and 0.02 respectively). Multivariate analysis adjusting for age at start of study, platelet count, stage, quality of response [CR or near CR versus SD or PR], and thalidomide dose, similarly found that reducing or eliminating the dose of V had a significant positive effect (p = 0.0121) on PFS. However, multivariate analysis did not reveal the same effect on OS (p = 0.11).
Conclusions: This subanalysis suggests that the use of full dose V in the DVd-T regimen may have a negative effect on PFS. The exact mechanism by which V affects PFS is not clear. Studies are now on-going investigating this regimen without V.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal