Abstract
Background: Although initially responsive to chemotherapy, multiple myeloma ultimately relapses. Replacing Adriamycin with pegylated liposomal doxorubicin in the VAD regimen results in a regimen with better safety profile (less neutropenia, alopecia, fewer hospital and clinic visit). DVd resulted in normalizing the angiogenic process however this did not improve PFS or OS. Thalidomide because of its antiangiogenic, immunomodulatory effects and chemo-sensitizing activity through modulating integrins was added to the DVd regimen. Adding the thalidomide to the DVd regimen resulted in an overall response rate of 89% with a CR/NCR rate of 49 % as compared to 60% and 18 % respectively for the DVd. At this stage it is not clear if the quality of response affects outcome in multiple myeloma patients receiving conventional dose therapy. To better assess the prognostic implication of improved quality responses and to define the role of up front Thalidomide, we compared the DVd to the DVd-T regimen by retrospectively reviewing the two trial data, the follow up for both trials is mature. It is still not clear whether thalidomide is more appropriately used up front or in the management of relapse.
Methods: we recruited a total of 68 patients in our DVd trial versus 105 patient in the DVd-T trial. A total of 155 patients in both groups were evaluable for follow-up and response (58 received DVd and 97 received DVd-T). Patients were matched for age, disease prognosticators, disease stage, and bone marrow involvement. The newly diagnosed patients were comparable in both groups except for the b 2 microglobulin which was higher in the DVd-T group as compared to the DVd (3.2 vs. 5.0; respectively. p0.05). In the relapsed/refractory group of patients, all variables were comparable.
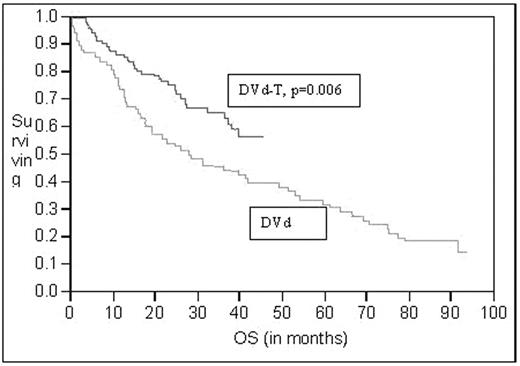
Results: The median follow up was 5 years for the DVd group and 2 years for the DVd-T group. Median age for the DVd was 62 years, which was similar to the DVd-T. For the DVd vs. the DVd-T group of patients achieving complete and near complete remission was 17% vs. 49.5% respectively with a p value of 0.001. Progression free survival was significantly longer for the DVd-T regimens vs. the DVd (28 vs. 13 months p= 0.0002) Figure 1., and for over all survival the median is not reached for the DVd-T vs. 28 months for he DVd (p=0.006).
Conclusion: we conclude that the addition of thalidomide significantly improved the quality of response and this appears to translate in to a PFS and OS benefit. While, recently published SWOG data suggests that the overall survival is determined by the duration of the PFS and not the quality of the response, this discrepancy could be related to the small number of patients in our study or the lack of use of biologic immune modulators among SWOG patients.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal