Abstract
The 2nd. Nordic Lymphoma Group mantle cell lymphoma (MCL2) protocol has demonstrated the importance of Ara-C and Rituximab in the induction chemotherapy and stem-cell mobilisation before high-dose therapy and autologous stem-cell transplant (1). By July 2005, 128 patients (83% stage IV) had completed protocol treatment consisting of 3 series of R-CHOP and 3 series of R-Ara-C, stem-cell harvest and high-dose therapy with BEAM/BEAC with ASCT. The 5-year failure-free and overall survival is 50% and 83% respectively, significantly higher than the historic control group of the Nordic MCL1 protocol with the same treatment without HD-Ara-C and Rituximab (P<0.0001). Patients with a molecular marker (t(11;14) or clonal IgH rearrangement) identified at the time of diagnosis in bone marrow and blood, undergo regular molecular follow-up posttransplant,. Patients who turn PCR-positive or increase their qPCR signal, without clinical disease, are offered preemptive treatment with Rituximab 375 mg/m2 Wx4.
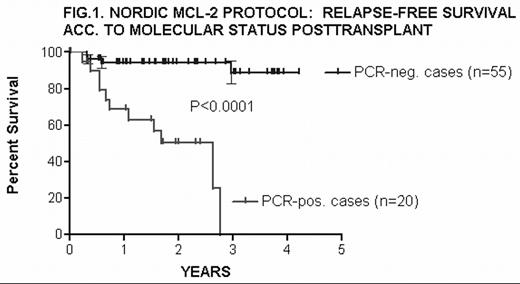
Of 75 patients with molecular markers who had completed treatment, 55 remain PCR-negative and 20 have become/remained PCR-pos. posttransplant. Clinical relapse ocurred significantly more often in the latter group (11 of 20) than in the PCR-neg. patients (4 of 55) (P<0.0001) (Fig.1).
Ten of the 20 PCR-positive patient did not receive preemptive rituximab: five due to immediate clinical relapse, 2 due to stable qPCR signals, one due to protocol error and two await treatment. Of 10 patients who did receive preemptive rituximab 8 again became PCR-negative and 2 remain PCR-positive. Six of the 10 Rituximab treated patients remain in clinical and molecular remission 200–600 days after the Rituximab treatment (Fig. 2).
Conclusions:
In MCL, molecular relapse is a harbinger of imminent clinical relapse, whereas continuous molecular remission is associated with prolonged disease-free survival (89% at 4 years)
Rituximab preemptive treatment can reinduce molecular remission and may delay clinical relapse. Following molecular relapse, only Rituximab treated patients (6 of 8 evaluable) remain disease-free.
NORDIC NCL-2 PROTOCOL: RELAPSE-FREE SURVIVAL ACC. TO MOLECULAR STATUS POSTTRANSPALNT
NORDIC NCL-2 PROTOCOL: RELAPSE-FREE SURVIVAL ACC. TO MOLECULAR STATUS POSTTRANSPALNT
NORDIC NCL-2 PROTOCOL: RELAPSE-FREE SURVIVAL FROM TIME OF MOLECULAR RELAPSE.
NORDIC NCL-2 PROTOCOL: RELAPSE-FREE SURVIVAL FROM TIME OF MOLECULAR RELAPSE.
Supported by the Nordic Cancer Union and the Danish Cancer Society
Author notes
Corresponding author


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal