Abstract
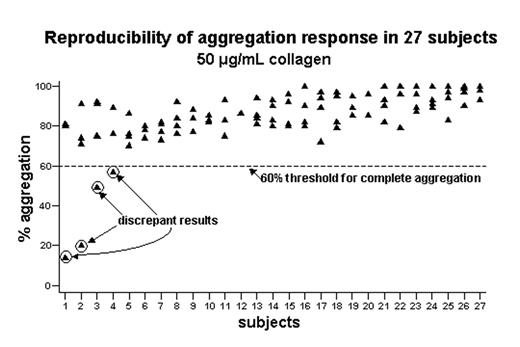
Platelet hypofunction occurs commonly in various settings and may contribute to clinically important bleeding; however, its laboratory evaluation remains a rather imprecise science. Although some platelet assays are well established in clinical practice, concerns about their reliability abound. Furthermore, few data exist on which to base laboratory criteria for identifying individuals with platelet hypofunction. To address these areas of need, we studied 449 healthy adults using assays commonly used in clinical laboratories to evaluate for impaired platelet function. These assays included optical aggregometry (using high concentrations of agonists in citrated platelet-rich plasma) and the PFA-100. In addition, we assessed the reproducibility of these assays by performing them on a subgroup of 27 individuals four times each on a weekly basis in a controlled setting. For each agonist and agonist concentration studied, we observed that a platelet aggregation response >60% clearly distinguished those subjects with complete aggregation from those who did not; we then identified minimal agonist concentrations at which ≥ 80% of healthy individuals demonstrated this degree of response (see table). Regarding reproducibility, a significant number of subjects showed widely discrepant results on repeated testing (see figure for example); the percentage of subjects who did so varied with the particular agonist employed (see table). Furthermore, our data obtained using the PFA-100 were less reproducible than has previously been reported and reproducibility varied with the cartridge employed (within-subject coefficient of variation=20.3% for epinephrine versus 8.5% for ADP). Aggregometry assays using different agonists correlated only partially with each other and with the PFA-100; the highest correlation coefficient was r=0.42 (p<.001, between ADP and epinephrine). In summary, our data provide a rational basis for specifying assay conditions and criteria (<60% aggregation in response to high concentrations of agonist) by which individuals with platelet hypofunction can be identified. However, despite studying only healthy subjects in a controlled research setting, we found the reproducibility of some commonly used platelet function tests to be rather poor; aggregometry using high concentrations of ADP or ristocetin and the PFA-100 using the ADP cartridge provided the most reliable results and are preferred assays in evaluating patients with suspected platelet hypofunction. Given the incomplete correlation between different assays, the other, less reliable assays may still provide useful information for an individual patient, but caution should be exercised in interpreting the results. Such assays may bear repeating, especially when the aggregation response to agonist is marginal (near 60%).
Aggregation assay characteristics
| Platelet agonist . | Required* concentration . | % of subjects with discrepant** results . |
|---|---|---|
| *to yield complete aggregation in ≥ 80% of subjects; **>30% difference in assay results on separate occasions | ||
| ADP | 4 μ M | 7 |
| Arachidonic acid | 0.5 mg/mL | 25 |
| Collagen-related peptide | 0.02 μg/mL | 19 |
| Collagen | 50 μ g/mL | 15 |
| Epinephrine | 10 μ M | 29 |
| Ristocetin | 1 mg/mL | 7 |
| Platelet agonist . | Required* concentration . | % of subjects with discrepant** results . |
|---|---|---|
| *to yield complete aggregation in ≥ 80% of subjects; **>30% difference in assay results on separate occasions | ||
| ADP | 4 μ M | 7 |
| Arachidonic acid | 0.5 mg/mL | 25 |
| Collagen-related peptide | 0.02 μg/mL | 19 |
| Collagen | 50 μ g/mL | 15 |
| Epinephrine | 10 μ M | 29 |
| Ristocetin | 1 mg/mL | 7 |
Reproducibility of aggregation response in 27 subjects 50 μg/mL collagen
Reproducibility of aggregation response in 27 subjects 50 μg/mL collagen
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal