Abstract
The thromboelastograph produces a continuous profile of the rheological changes that occur during the process of coagulation using whole blood. This information can be transformed into a dynamic velocity profile of the changes in blood elasticity occurring during clotting. We used the TEG® hemostasis analyzer in patients with hemophilia A or B with and without inhibitors and other coagulation factor deficiencies (OFD), to study the thromboelastographic profiles in these patients.
Materials and Methods: 62 children (6 months-19 years old) were enrolled according to IRB regulations. 29 children had severe hemophilia A (SHA), 4 moderate hemophilia A or B (Mod.H), 2 severe factor VII deficiency, 1 combined factor V and VIII deficiency, 1 VWD (type II B), 1 severe factor V deficiency, 1 Severe PAI deficiency, 19 normal controls (NC), and 4 SHA with inhibitors (SHA+I). All patients were studied 72 hours after the last dose of factor. Citrated whole blood was activated using recombinant human tissue factor (Innovin, Dade Behring Inc®) and recalcified using 0.2M CaCl2. In patients with central lines with heparin, a heparinase cup was used. The TEG® was run for ≥ 90 min. CBC with differential was obtained on all subjects.
Results: There was no significant difference in the CBC parameters among patients. Analysis of the TEG data revealed the following:
| TEG Parameters (mean values) . | SHA (n=29) . | Mod.H (n=4) . | SHA+I (n=4) . | OFD (n=6) . | Control(n=19) . |
|---|---|---|---|---|---|
| MTG:Max rate of thrombin generation; TMG: Time to MTG; R: Reaction Time; K: Time to reach an amplitude of 20mm; MA: Max. Amplitude | |||||
| MTG(mm*100/sec) | 8.7 | 9.6 | 1.3 | 9 | 17 |
| TMG(min) | 27.5 | 16.6 | 62.7 | 17.5 | 8.9 |
| R(min) | 22 | 14 | 56 | 15 | 7 |
| K(min) | 7 | 4 | 41 | 4 | 2 |
| Max.Amplitude, MA (mm) | 59 | 56 | 12 | 58 | 62 |
| TEG Parameters (mean values) . | SHA (n=29) . | Mod.H (n=4) . | SHA+I (n=4) . | OFD (n=6) . | Control(n=19) . |
|---|---|---|---|---|---|
| MTG:Max rate of thrombin generation; TMG: Time to MTG; R: Reaction Time; K: Time to reach an amplitude of 20mm; MA: Max. Amplitude | |||||
| MTG(mm*100/sec) | 8.7 | 9.6 | 1.3 | 9 | 17 |
| TMG(min) | 27.5 | 16.6 | 62.7 | 17.5 | 8.9 |
| R(min) | 22 | 14 | 56 | 15 | 7 |
| K(min) | 7 | 4 | 41 | 4 | 2 |
| Max.Amplitude, MA (mm) | 59 | 56 | 12 | 58 | 62 |
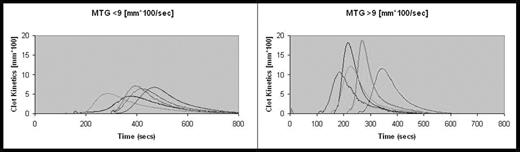
The rate of thrombin generation as visualized by plotting the 1st derivative of the TEG course, in patients with SHA without inhibitors, showed that they could be divided into 2 groups based on MTG (</>9). When analysed the 2 groups showed the following characteristics (5 representative curves from each group are shown):
| TEG Parameters (Mean values) . | MTG < 9 (n=16) . | MTG > 9 (n=13) . | p value . |
|---|---|---|---|
| TMA: Time to MA; | |||
| MTG(mm*100/sec) | 5.5 | 12.6 | <0.001 |
| TMG (min) | 33 | 20 | 0.009 |
| R(min) | 26 | 16 | 0.004 |
| K(min) | 9 | 3.4 | 0.03 |
| MA(mm) | 56.1 | 62.3 | 0.01 |
| TMA(min) | 60 | 38 | 0.006 |
| TEG Parameters (Mean values) . | MTG < 9 (n=16) . | MTG > 9 (n=13) . | p value . |
|---|---|---|---|
| TMA: Time to MA; | |||
| MTG(mm*100/sec) | 5.5 | 12.6 | <0.001 |
| TMG (min) | 33 | 20 | 0.009 |
| R(min) | 26 | 16 | 0.004 |
| K(min) | 9 | 3.4 | 0.03 |
| MA(mm) | 56.1 | 62.3 | 0.01 |
| TMA(min) | 60 | 38 | 0.006 |
13/29 children with SHA had target joints and 69%of patients with target joints had a MTG<9.
Conclusions: SHA patients have variable bleeding tendencies as seen by the variation in MTG. A lower MTG is associated with a higher incidence of target joints. This may provide a clue as to which patients may have the greatest benefit from primary prophylaxis. Patients with OFD have a TEG® profile similar to Mod.H patients. SHA+I have poor thrombin generation as seen by a significantly longer TMG and R time (p <0.05), compared to all subjects. The TEG may provide valuable clues to the severity of bleeding tendencies in patients with factor deficiencies. In additional observations (not shown), it appears that the TEG may be used to monitor the response to treatment with factor concentrates and tailor treatment with rFVIIa.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal