Abstract
Introduction: Two approaches to improve progression-free survival (PFS) in MCL are intensifying induction, as with hyperCVAD/M-A regimen, or intensifying consolidation with high dose chemoradiotherapy (HDT) and ASCT as in the prospective European MCL Network Trial. At MSKCC the strategy is to incorporate both approaches by administering an anthracycline-containing regimen in a dose dense fashion (CHOP- or R-CHOP-14) followed by consolidation with ICE (ifosfamide, carboplatin, etoposide) and HDT/ASCT.
Patients: Forty six patients with newly diagnosed MCL underwent HDT/ASCT between 11/96 and 2/05. The median age was 55 years; 74% were male; 72% had bone marrow involvement, 39% had GI involvement, 7% were in leukemic phase, and 91% presented with stage IV disease. Splenomegaly was seen in 35%, B symptoms in 9%, KPS>70 in 93%, elevated LDH in 23%, and blastoid histology in 9%.
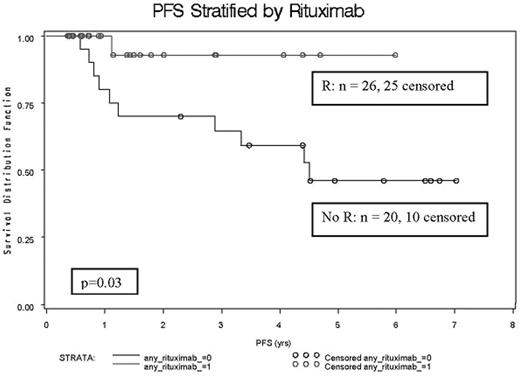
Results: Induction was 4 to 6 cycles of CHOP-14 (43%), R-CHOP-14 (37%), or other doxorubicin-containing regimen (20%). Consolidation was performed with 2–3 cycles of ICE in 53% or R-ICE in 39%. Upfront treatment was well tolerated and permitted adequate stem cell collection and prompt transition to HDT/ASCT. Conditioning regimens were TBI/CY/VP-16 (59%) and BEAM (41%). Involved field radiation therapy was administered to 65%. Post-ASCT rituximab maintenance was given to 39%, with 57% of patients receiving rituximab as part of their treatment. Anthracycline-based induction led to CRu of 44% and ORR of 98%. Seventy two percent of patients were transplanted in CR, while the remaining 28% were in PR. At a median follow-up of 2.5 years (range 0.4–8.0 years) 17% of the patients have died and 24% have had progressive disease. The median OS and PFS have not been reached (lower 95% CI, 5.7 years and 4.4 years, respectively). The 5-year PFS and OS are 58% and 83%, respectively. The use of rituximab at any point during treatment prolonged PFS - only 1 of 26 patients receiving rituximab relapsed, as compared to 10 of 20 patients who were rituximab naive (p=0.03); thus far there is no significant difference in OS. There was no day 100 treatment related mortality. One patient developed bronchiolitis obliterans after ASCT and died of pulmonary fibrosis 6.5 years later; 3 patients have died of secondary cancers - one case each of MDS (1.6 years after ASCT), melanoma and lung cancer.
Conclusion: These data provide evidence that dose-dense induction with CHOP-14 or R-CHOP-14 and consolidation with ICE/HDT/ASCT appears to be safe and effective, with minimal acute toxicity. Although the median follow-up is short, the use of rituximab appears to improve PFS. This contrasts with the findings of German LGLSG and may be a consequence of in vivo rituximab purging. Future therapy could incorporate all the successful elements of prior treatment programs, including R-CHOP-14, R-ICE, and radioimmunotherapy with high dose chemotherapy conditioning regimen, followed by ASCT and rituximab-based maintenance.
PFS stratified by Rituximab
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal