Abstract
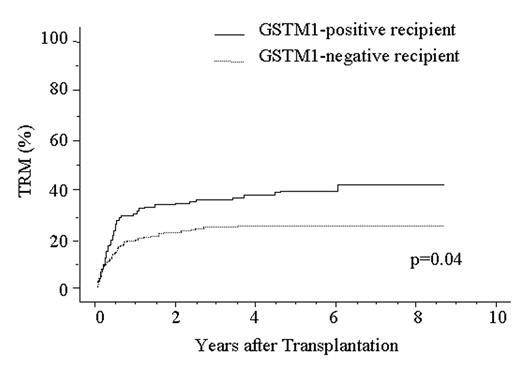
Treatment-related mortality (TRM) is a major obstacle to successful allogeneic hematopoietic stem cell transplantation (HSCT). A variety of drugs are used in allogeneic HSCT, such as chemotherapeutic agents, immunosuppressants, antibiotics, antifungal and antiviral drugs, thus, a genetic polymorphism in metabolic enzymes would affect the metabolism of drugs and subsequent TRM after HSCT. We recently reported a metabolic enzyme UDP glycosyltransferase 2 family, polypeptide B17 (UGT2B17) has a null phenotype, and the use of a UGT2B17-positive donor is an independent risk factor for higher TRM and lower survival after HSCT (ASH annual meeting 2004, abstract# 1837). Here, we focused attention on glutathione S-transferase (GST) M1 and GSTT1 enzymes. These enzymes mainly metabolize chemotherapeutic agents, chemical carcinogens and by-products of oxidative stress and are absent from more than 50% of some populations. To assess the significance of homozygous GSTM1 and GSTT1 gene deletion in allogeneic HSCT, we analyzed DNA from 373 patients with a hematological disease and their HLA-A, B, C, DRB1-identical unrelated bone marrow donors using PCR method. All patients received an intensive myeloablative pretransplant conditioning regimen, an unmanipulated marrow graft, and cyclosporine A or tacrolimus as GVHD prophylaxis. Homozygous GSTM1 and GSTT1 gene deletion were observed in 56% and 45% of patients with a hematological disease, respectively, and 57% and 46% of healthy donors, respectively. There was no difference in the frequencies between disease group and donor group. We next applied Cox proportional hazard model to multivariate analysis for TRM which was defined as any death that occurred while the patient was in remission, acute GVHD, chronic GVHD, relapse, disease-free survival (DFS) and overall survival (OS). The analysis showed no significant association between GSTT1 polymorphism and any outcome. However, GSTM1-positive recipient was significantly associated with higher TRM and lower survival: TRM; Odds ratio (OR), 1.49; 95% confidence interval (CI), 1.03–2.16; P=0.036: DFS; OR, 1.50; 95% CI, 1.08–1.97; P=0.013: OS; OR, 1.41; 95% CI, 1.04–1.92; P=0.030. TRM was analyzed in relation to GSTM1 genotype in the patient by the Kaplan-Meier method (Fig 1). TRM in the GSTM1-positive patients (42.9%, n=166) was significantly higher than that in the GSTM1-negative patients (29.3%, n=201) (P=0.04). These data suggest the presence of the GSTM1 null genotype in recipient provide better protection against TRM after allogeneic HSCT. Some reactive intermediates or toxic metabolites generated by GSTM1 may initiate or promote the development of TRM.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal