Abstract
Mature erythrocytes are unable to synthesize AMP from IMP due to the developmental loss of adenylosuccinate synthetase, which also effectively prevents the synthesis of adenine nucleotides from circulating hypoxanthine, the major salvageable purine compound. Consequently, AMP deaminase (AMPD) activity must be tightly regulated in order to avoid irreversible depletion of the erythrocyte adenine nucleotide pool during periods of energy imbalance. However, increased intracellular calcium promotes activation of erythrocyte AMPD (AMPD3) through a protein-protein interaction with calmodulin (
Effect of Autoincubation on Erythrocyte AMP and IMP Pools
| GROUP . | HOURS . | AMPa . | IMP . | IMP/AMP . |
|---|---|---|---|---|
| a, metabolite concentrations reported as μmol/g Hb; †, p<.05 when compared to 0 time in a paired student’s t-test; ‡, p<.05 when compared to control in an unpaired student’s t-test | ||||
| Control (N=4) | 0 | .29±.08 | .15±.06 | .58±.28 |
| 6 | .29±.01 | .33±.11† | 1.15±.35† | |
| SCD (N=5) | 0 | .22±.04 | .08±.03 | .36±.10 |
| 6 | .14±.03† | .74±.24†‡ | 5.15±1.25†‡ | |
| GROUP . | HOURS . | AMPa . | IMP . | IMP/AMP . |
|---|---|---|---|---|
| a, metabolite concentrations reported as μmol/g Hb; †, p<.05 when compared to 0 time in a paired student’s t-test; ‡, p<.05 when compared to control in an unpaired student’s t-test | ||||
| Control (N=4) | 0 | .29±.08 | .15±.06 | .58±.28 |
| 6 | .29±.01 | .33±.11† | 1.15±.35† | |
| SCD (N=5) | 0 | .22±.04 | .08±.03 | .36±.10 |
| 6 | .14±.03† | .74±.24†‡ | 5.15±1.25†‡ | |
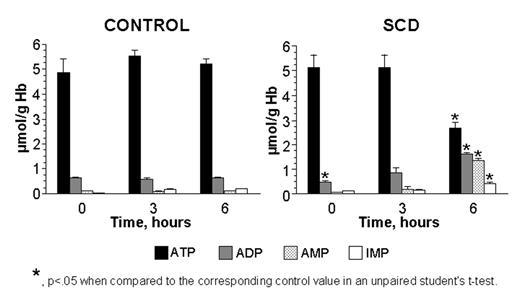
Data in the figure show an accelerated depletion of ATP and increased accumulation of IMP in glucose-starved SCD erythrocytes compared to control cells (*, p<.05 when compared to control in an unpaired student’s t-test).
These combined results demonstrate AMPD3 enzyme activation and accelerated adenine nucleotide pool depletion during periods of energy imbalance in SCD erythrocytes. This metabolic imbalance in SCD erythrocytes could contribute to 1) an increased rate of hemolysis, 2) cation channel dysregulation, 3) modulation of adhesive membrane components, and 4) diminished protein phosphorylation. Furthermore, strategies directed against this metabolic imbalance, e.g., diffusible AMPD inhibitors and adenine supplementation, may have a therapeutic benefit in SCD.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal