Abstract
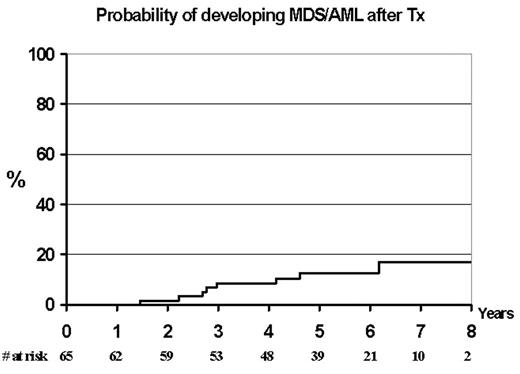
In the MRC CLL Pilot Study of autografting conducted between 1996 and 2001 115 newly diagnosed patients were treated with a median of 6 cycles of fludarabine (range 3–8). In addition 26 patients received further treatment, mainly the addition of cyclophosphamide to fludarabine, CHOP or alemtuzumab, in order to achieve either CR, nPR or PR. Progenitor cells were mobilised with cyclophosphamide 2g/m2 and G-CSF. Sixty-five patients were autografted with cyclophosphamide and TBI conditioning or BEAM (20%). The median CD34 cell dose for transplanted patients was 2.6x106/Kg (range 0.75–3.9). Ten patients have subsequently developed AML/MDS at a median 3.5 years after starting treatment (range 2.5–7 years). Eight patients had undergone an autograft and 2 had received fludarabine alone and not proceeded to transplant. The actuarial risk of developing MDS/AML post autograft was 12.4% at 5 years (CI 0.7–24%). At the time of diagnosis of MDS/AML all the patients had either complex cytogenetics or/and deletion of chromosomes 5 or 7. These abnormalities were not detectable by FISH on the pre-transplant harvested material. Analysis of potential risk factors (age, stage of disease, previous treatment, cell dose, conditioning type) demonstrated only age as a possible factor (p<0.05). This frequency of post transplant MDS has been reported in low grade lymphomas but not previously in autografts for CLL. We hypothesise that this patient population may be particularly at risk of secondary MDS/AML and that potential causative factors are fludarabine, a low cell dose and transplant conditioning.
Probability of developing MDS/AML after Tx
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal