Abstract
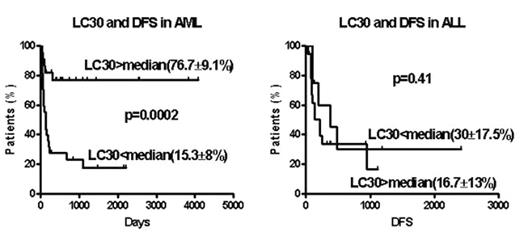
Between 05/1994 and 07/2005, 78 patients with acute leukemia (AML=49; ALL=29) received a T cell depleted stem cell transplant from an HLA-identical sibling, with 1–2 infusions of donor T cells on days 30–100. The conditioning regimen included 12–13.5 Gy total body irradiation (TBI) and cyclophosphamide (Cy) with or without fludarabine. Cyclosporine (CSA) was given as the sole GVHD prophylaxis after transplant. The stem cell source was peripheral blood in 75 and bone marrow in 3. The T cell dose ranged between 0.2 to 2 × 105CD3+ cells/kg. AML and ALL patients were distributed equally among transplant regimen and T cell dose variations. The median CD34 cell dose was 5.4 × 106 cells/kg, (5.4 for AML, and 5.5 for ALL; p=0.50). Actuarial disease free survival (DFS), transplant related mortality (TRM) and relapse rate was 42±8, 20±6 and 40±9 % for AML and 22±10; 35±19 and 64±11% for ALL patients. The median day 30 post-transplantation lymphocyte count (LC30) for AML patients was 418/μl (31–2105) and 778/μl (265–3295) for ALL patients; p=0.063). In multivariate analysis, LC30 emerged as an independent risk factor for transplant outcome in AML but not ALL patients (see figure). In AML patients LC30 above median was associated with superior DFS, lower TRM and lower relapse. CD34 dose and LC30 were highly correlated for AML and ALL analyzed together or separately. Lymphocyte subset analysis of 20 post-transplant samples on day 30 identified a large proportion of CD56+16+ natural killer (NK) cells (median 44%, range 15–86%). Since early post-transplant NK cells are immature and unregulated by KIR-expression they may exert anti-host reactivity. The more favorable outcome for AML but not ALL patients achieving higher lymphocyte counts early post-transplant recapitulates the myeloid leukemia-restricted benefit from superior NK cell recovery observed in AML but not ALL recipients of mismatched T cell depleted stem cell transplants reported by Ruggeri et al (
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal