Abstract
Normal human hematopoietic progenitors as well as leukemia/lymphoma cells express kinase-defective EphB6 receptors. The only unique high affinity ligand for EphB6 among eight known mammalian ephrins, ephrins-B2 is expressed not only on hematopoietic malignancies, but also on mesenchymal stem cells. However, the biological functions of the receptor and its ligand in hematopoietic cells are largely unknown.
In the present study, we showed that the interaction between EphB6 and ephrinB2 could initiate forward as well as reverse signaling in vitro. Both pre-clustered and unclustered ligands could trigger the signal transduction, but pre-clustered ones more rapidly down-regulated the signaling. We also examined the EphB6/ephrinB2 function in cell adhesion and migration.
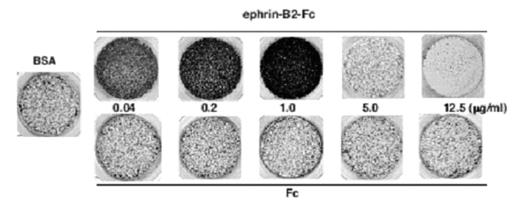
HEK-EphB6 cells placed in the upper chamber of a Transwell apparatus, in which the lower side of filter was coated with different concentrations of ephrin-B2-Fc or Fc, were allowed to migrate to the lower side at 37°C overnight. Vector-transfected cells were used as controls. The cells that had migrated to the lower side of filter were stained, photographed. A BSA-coated filter is shown as a control. EphB6 exerted biphasic effects in response to different concentrations of the ephrin-B2. EphB6 promoted cell adhesion and migration when stimulated with low concentrations of ephrin-B2, whereas it induced repulsion and inhibited migration upon stimulation with high concentrations of ephrin-B2. A truncated EphB6 receptor lacking the cytoplasmic domain showed monophasic positive effects on cell adhesion and migration, indicating that the cytoplasmic domain is essential for the negative effects. We further explored the signal transduction of the biphasic effects.
The Src family kinase, Fyn was co-immunoprecipitated with anti-EphB6 antibody in the absence or presence of ephrin-B2 stimulation. High concentrations of ephrin-B2 induced tyrosine phosphorylation of EphB6 through a Src family kinase activity. These results indicate that EphB6 can both positively and negatively regulate cell adhesion and migration, and suggest that tyrosine phosphorylation of the kinase-defective EphB6 receptor by a Src family kinase acts as the molecular switch for the functional transition.
Thus, EphB6 expressed on hematopoietic cells may play an important role in the regulation of cell homing to hematopoietic tissues as well as leukemia cell infiltration.
Author notes
Corresponding author


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal