Abstract
X-linked agammaglobulinemia (XLA) is a human immunodeficiency caused by mutations in Bruton’s tyrosine kinase (Btk) and characterized by an arrest in early B-cell development, absence of serum immunoglobulin, and recurrent bacterial infections. Using Btk and Tec double deficient (Btk/Tec−/ −) mice as a model for XLA, we recently showed that onco-retroviral-mediated Btk gene transfer into hematopoietic stem cells (HSC) reconstituted in vivo Btk-dependent B-cell development and function (Yu et al. Blood 104(5):1281–90).
In order to increase the safety of this approach, we developed a SIN-lentiviral vector with a B cell specific enhancer/promoter element, Eμ B29. Using SIN-lentiviral vectors expressing GFP, we observed that Eμ B29 consistently promoted 3–5 fold higher GFP expression in human B lineage cells derived from transduced HSC in vitro and in vivo (ASGT 2002 abstract #1302). We also evaluated this vector, CSOM-Eμ B29-GFP-WPRE, in lentiviral transgenic mice where it exhibited the highest GFP expression in peripheral B cells compared with all other hematopoietic lineages. Specifically, in more than 8 independent founder strains the MFI for GFP expression in B cells was > 3 fold higher than that in T cells (p=0.0002).
Based upon these findings we developed Eμ B29-huBtk SIN-lentiviral vectors with or without the insulator element derived from the chicken β-globulin insulator (HS4). Using both vectors to transduce Btk −/ − DT40 B cells, followed by cloning by limiting dilution, we demonstrate Btk protein expression by intracellular staining and western blotting and full rescue of Btk-dependent, B cell receptor (BCR)-mediated Ca2+ signaling in all clones evaluated including those exhibiting a single viral integration. Next we tested the capacity of these vectors to reconstitute Btk-dependent B-cell development and function in a cohort of Btk/Tec−/ − mice. Marrow from 5-FU treated Btk/Tec −/ − mice was harvested, cultured on fibronectin coated plates with growth factors (mIL-3,mIL-6, mSCF, mTPO and mFLT3ligand) and concentrated lentivirus (2.3x107pg/106 cells measured by p24 level).
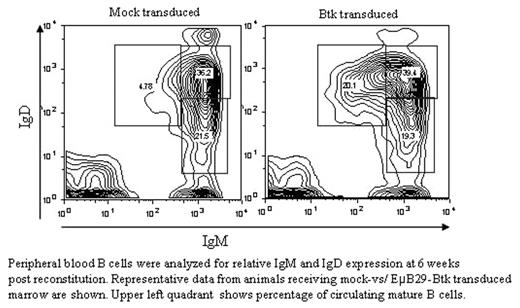
After 48h of in vitro culture, cells were transplanted into lethally irradiated animals and transplanted animals were serially evaluated for presence of B cells in the peripheral blood. B-cell numbers progressively increased with a significant difference as early as within 6 weeks in mice receiving transduced (16–18% B220+ cells) vs. control marrow (8–9%; mock transduced). Further, mature B cells (B220+IgMlowIgDhi) represented 14–20% of total B cells in treated compared to <5% in control mice. Finally, mice receiving transduced cells exhibited a rescue of total serum IgM and IgG3 levels and responses to TI-II dependent immunization. Results of two additional animal cohorts will be presented. In summary, our data demonstrate that Eμ B29-Btk SIN-lentiviral vector specifically promotes Btk expression in B lineage cells, and correction of the Btk-deficient phenotype in vitro and in vivo.
Peripheral blood B cells were analyzed for relative IgM and IgD expression at 6 weeks post reconstitution. Representative data from animals receiving mock-vs/ EμB29-Btk transduced marrow are shown. Upper left quadrant shows percentage of circulating mature B cells.
Peripheral blood B cells were analyzed for relative IgM and IgD expression at 6 weeks post reconstitution. Representative data from animals receiving mock-vs/ EμB29-Btk transduced marrow are shown. Upper left quadrant shows percentage of circulating mature B cells.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal