Abstract
Blood-derived stem cell grafts contain large quantities of platelets. Platelets contain cytokines which could contribute to GVHD. However, the role of the number of infused platelets in GVHD has never been explored. Platelet dose data were available for 61 patients (27–66 y, median 52) undergoing submyeloablative HSCT from HLA-matched sib (n=35), 10/10 allele-matched unrelated donors (n=19), or 1-locus/allele mismatched donors (n=7) for hematologic malignancies from 9/01 to 5/04 (minimum follow-up >1 y). The conditioning comprised 100 mg/m2 melphalan with (n=42; no prior autograft) or without (n=19; prior autograft) 50 mg/kg cyclophosphamide. GVHD prophylaxis comprised cyclosporine-mycophenolate with HLA-matched sibs and tacrolimus-mycophenolate with the rest. G/GM-CSF were not administered post-transplant. Supportive care was uniform. The platelet dose was 21–236 (median 77) x 108/kg ideal body weight. The CD3+ cell dose was 0.9–14.9 (median 3) x 108/kg ideal body weight. The table shows the cumulative incidence and relative risk of acute and chronic GVHD with 95% CI by the number of platelets infused.
| Cumulative incidence (%) . | Platelet dose ≤100 (n=39) . | Platelet dose >100 (n=22) . | RR . | P . |
|---|---|---|---|---|
| Acute GVHD (any grade) | 46 (33–65) | 82 (67–100) | 2.45 (1.26–4.75) | 0.008 |
| Acute GVHD (grade III–IV) | 23 (12–42) | 70 (47–100) | 2.75 (1.04–7.24) | 0.041 |
| Chronic GVHD | 28 (17–47) | 23 (11–49) | 0.76 (0.26–2.21) | 0.62 |
| Cumulative incidence (%) . | Platelet dose ≤100 (n=39) . | Platelet dose >100 (n=22) . | RR . | P . |
|---|---|---|---|---|
| Acute GVHD (any grade) | 46 (33–65) | 82 (67–100) | 2.45 (1.26–4.75) | 0.008 |
| Acute GVHD (grade III–IV) | 23 (12–42) | 70 (47–100) | 2.75 (1.04–7.24) | 0.041 |
| Chronic GVHD | 28 (17–47) | 23 (11–49) | 0.76 (0.26–2.21) | 0.62 |
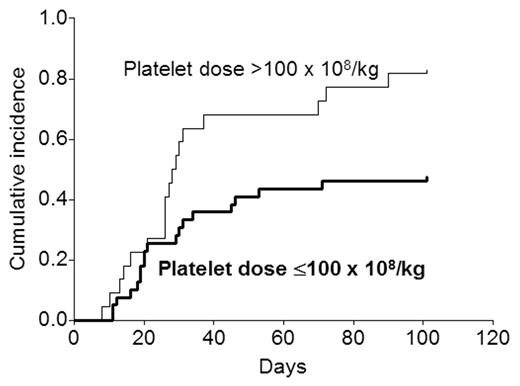
There was strong correlation between the platelet dose and the T cell dose (r=0.62; P<0.0001). Only 4 of 30 patients receiving <3 CD3+ cells received >100 platelets compared with 18 of 31 receiving ≥3 CD3+ cells (P=0.001). Since higher CD3+ cell numbers can be associated with more acute GVHD, Cox analysis was undertaken to determine the independent contribution of the platelet dose. When both platelet and CD3+ cell numbers were analyzed as categoric variables (in addition to several other factors potentially likely to affect GVHD), a platelet dose >100 was the only factor independently associated with any grade of acute GVHD (RR 2.5; 95% CI 1.3–4.8; P=0.008), and a CD3+ cell dose ≥3 the only factor associated with grade III–IV acute GVHD (RR 10.0; 95% CI 2.3–43.5; P=0.002). When both were analyzed as continuous variables, the relationship was similar with higher platelet doses being associated with a higher risk of any grade of acute GVHD (P=0.006) and higher CD3+ cell doses being associated with a higher risk of grade III–IV acute GVHD (P<0.0001). The figure shows the effect of the platelet dose on the cumulative incidence of any grade of acute GVHD.
The number of platelets infused had no effect on the incidence of chronic GVHD, transplant-related mortality, relapse rates, and disease-free or overall survival. This novel observation needs to be confirmed in a larger series of patients as well as the relative contribution of the T cell dose and the platelet dose to the occurrence of GVHD better defined. If confirmed, reducing the platelet content of blood stem cell grafts may represent a simple way to reduce the risk of acute GVHD partially.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal