Abstract
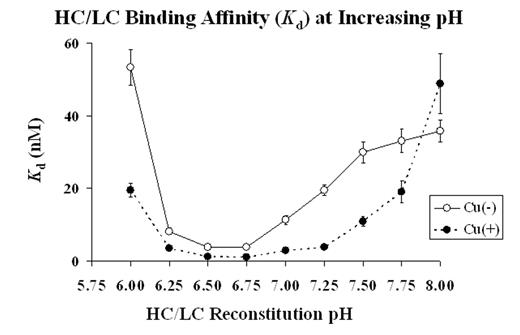
Factor VIII circulates as a heterodimer composed of a heavy chain (HC) and light chain (LC). The structural integrity of the heterodimer is crucial for factor VIII function. In this study we found that the reconstitution of factor VIII from isolated HC and LC was pH-dependent. In the presence of Ca2+, up to 80% of native factor VIII activity was recovered over a wide range of pH values. The affinity of HC and LC was maximal at pH 6.5-6.75 (Kd ~ 4 nM), whereas a Kd of ~20 nM was observed at physiological pH (7.25) (Figure). The effect of Cu2+ (0.5 μM total Cu2+) on maximal activity regenerated was negligible at pH 6.25–8.0. However, this level of Cu2+ increased the inter-chain affinity by ~5 fold at pH 7.25 (Figure). This effect resulted from an ~1.5-fold increased association rate constant (kon) and an ~3-fold reduced dissociation rate constant (koff). Addition of Cu2+ to a mixture of HC and LC at equilibrium resulted in an increase in cofactor activity that followed a single exponential, suggesting that association was defined by a simple reaction model. High affinity (Kd = 5.3 fM) of the factor VIII chains for Cu2+, as estimated by increases in cofactor activity, suggested a rapid association rate for the metal ion. Together, these results suggest that the protonation state of specific residues, His in particular, modulate inter-chain affinity. Furthermore, copper ion contributes to the maintenance of the heterodimer at physiologic pH by a mechanism consistent with bridging the two chains.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal