Abstract
1,25-dihydroxyvitamin D3 (1,25(OH)2D3) is a secosteroid hormone that renders dendritic cells (DCs) tolerogenic, favoring the induction of regulatory T cells. Induction of DCs with tolerogenic properties by 1,25(OH)2D3 is associated with increased selective expression of immunoglobulin-like transcript 3 (ILT3), suggesting its involvement in the immunoregulatory properties of this hormone. Here we show an in vivo correlate of the increased ILT3 expression on DCs in healing psoriatic lesions following topical treatment with the 1,25(OH)2D3 analog calcipotriol. Analysis of DC subsets reveals a differential regulation of ILT3 expression by 1,25(OH)2D3, with a marked up-regulation in myeloid DCs but no effect on its expression by plasmacytoid DCs. A regulatory role for ILT3 expressed on DCs is indicated by the increased interferon-γ (IFN-γ) secretion promoted by anti-ILT3 addition to cultures of DCs and T cells, but this effect is blunted in 1,25(OH)2D3-treated DCs, suggesting ILT3-independent mechanisms able to regulate T-cell activation. Although ILT3 expression by DCs is required for induction of regulatory T cells, DC pretreatment with 1,25(OH)2D3 leads to induction of CD4+Foxp3+ cells with suppressive activity irrespective of the presence of neutralizing anti-ILT3 monoclonal antibody (mAb), indicating that ILT3 expression is dispensable for the capacity of 1,25(OH)2D3-treated DCs to induce regulatory T cells.
Introduction
The activated form of vitamin D, 1,25-dihydroxyvitamin D3 (1,25(OH)2D3), is a secosteroid hormone that has, in addition to its central function in calcium and bone metabolism, important effects on the growth and differentiation of many cell types, and pronounced immunoregulatory properties.1-5 The biologic effects of 1,25(OH)2D3 are mediated by the vitamin D receptor (VDR), a member of the superfamily of nuclear hormone receptors functioning as a ligand-activated transcription factor that binds to specific DNA sequence elements in vitamin D–responsive genes and ultimately influences their rate of RNA polymerase II–mediated transcription.6
Antigen-presenting cells (APCs), and notably dendritic cells (DCs), express the VDR and are key targets of VDR agonists. A number of studies have clearly demonstrated that 1,25(OH)2D3 and its analogs inhibit the differentiation and maturation of DCs.7-11 These studies have consistently shown that in vitro treatment of DCs with 1,25(OH)2D3 and its analogs leads to down-regulated expression of the costimulatory molecules CD40, CD80, and CD86, and to decreased interleukin-12 (IL-12) and enhanced IL-10 production, resulting in decreased T-cell activation. The block of maturation, coupled with abrogation of IL-12 and strongly enhanced production of IL-10, can explain the capacity of VDR agonists to induce DCs with tolerogenic properties that favor regulatory T-cell enhancement. These effects are not limited to in vitro activity: 1,25(OH)2D3 and its analogs can also induce DCs with tolerogenic properties in vivo, as demonstrated in models of allograft rejection by oral administration directly to the recipient,12 or by adoptive transfer of in vitro–treated DCs.13
To further characterize mechanisms accounting for the induction of DCs with tolerogenic properties by VDR agonists, we have examined the expression of immunoglobulin-like transcripts (ILTs) by 1,25(OH)2D3-treated DCs. ILTs are receptors structurally and functionally related to killer-cell inhibitory receptors (KIRs),14 and they have been shown to be involved in immunoregulation.15 ILT family members can be subdivided into 2 main types: one, comprising ILT1, ILT7, ILT8, and leukocyte immunoglobulin (Ig)–like receptor 6, is characterized by a short cytoplasmic tail delivering an activating signal through the immunoreceptor tyrosine-based activatory motif (ITAM) of the associated common γ chain of the Fc receptor; members of the second type, including ILT2, ILT3, ILT4, and ILT5, contain a cytoplasmic immunoreceptor tyrosine-based inhibitory motif (ITIM) transducing a negative signal.16 When inhibitory ILTs are activated, their ITIM domains become phosphorylated, and recruit p56lck and src homology 2 (SH2)-containing protein-tyrosine-phosphatase 1 (SHP-1), leading to downstream events and gene modulation.17 The high homology between ILTs and KIRs suggests that ILTs can also interact with class I major histocompatibility complex (MHC) molecules, but this has been so far confirmed only for ILT2 and ILT4.18,19
Most cell types involved in innate or acquired immune responses, including myeloid, lymphoid and dendritic cells, express at least 1 member of the ILT family, which may play an important role in immunoregulation.17 For example, the inhibitory receptor ILT3 has been shown to negatively regulate APC activation.20 In addition, human leukocyte antigen–G (HLA-G), an inhibitory molecule involved in immune tolerance, has been shown to up-regulate ILT2, ILT3, and ILT4 in APCs, natural killer (NK), and T cells.21 A connection between ILTs and tolerance induction has been established by the observation that CD8+CD28– suppressor T cells up-regulate ILT3 and ILT4 expression on DCs, rendering them tolerogenic.22 Such tolerogenic DCs have been reported to anergize alloreactive CD4+CD45RO+CD25+ T cells, converting them into regulatory T cells which, in turn, continue the cascade of suppression by tolerizing other DCs.23 Alloantigen-specific CD8+CD28–Foxp3+ T suppressor cells have also been shown to induce ILT3+ ILT4+ tolerogenic endothelial cells, inhibiting alloreactivity.24
Treatment of DCs with 1,25(OH)2D3 leads to a marked up-regulation of ILT3 but not ILT4,25,26 suggesting its direct involvement in regulatory T-cell induction. Results in the present study show that ILT3 expressed by DCs is involved in induction of CD4+Foxp3+ regulatory T cells, further supporting an important role of this inhibitory receptor in the tolerogenic function of DCs. Although the marked up-regulation of ILT3 induced by 1,25(OH)2D3 in DCs could represent a mechanism contributing to the tolerogenic properties of VDR agonists, it is dispensable, and ILT3-independent mechanisms induced by 1,25(OH)2D3 are sufficient to promote DCs with the capacity to induce regulatory T cells.
Materials and methods
Cell-culture reagents
Cells were cultured in RPMI 1640 culture medium supplemented with 10% fetal calf serum (FCS; Hyclone Laboratories, Logan, UT), 2 mM l-glutamine, 50 μg/mL gentamicin, 1 mM sodium pyruvate, and 1% nonessential amino acids (complete medium). DCs were stimulated with 1 μg/mL lipopolysaccharide (LPS; Escherichia coli 0111:B4; Sigma Chemical, Saint Louis, MO); CD154-transfected J558L cells at a ratio of 4:1; and 5 μM cytosine phosphate guanosine (CpG) oligonucleotide 2216 type A or CpG oligonucleotide 2006 type B (InvivoGen, San Diego, CA). CD4+ T cells were stimulated with anti–human CD3 mAb (clone TR66). Crystalline 1,25(OH)2D3 was a gift of Milan Uskokovic (BioXell, Nutley, NJ), dexamethasone was purchased from Sigma Chemical, and recombinant human (rh) IL-10 from PharMingen (San Diego, CA).
Cell purification
Peripheral-blood mononuclear cells (PBMCs) were isolated from buffy coats by Ficoll gradient (Pharmacia Biotec AB, Uppsala, Sweden). Peripheral-blood myeloid DCs (M-DCs) and plasmacytoid DCs (P-DCs) were magnetically sorted with blood dendritic-cell antigen-1 (BDCA-1) and BDCA-4 cell-isolation kits (Miltenyi Biotec, Bergish Gladblach, Germany), respectively, as described,27 to a purity of 95% to 98% in both cases. DC subsets were analyzed either immediately or after culture in complete medium in the presence of 10 ng/mL recombinant human granulocyte-macrophage colony-stimulating factor (rhGM-CSF) or 20 ng/mL IL-3 (Pharmingen), respectively. To generate immature monocyte-derived DCs, monocytes, obtained from PBMCs by positive selection with CD14 beads (Miltenyi Biotec), were grown for 6 to 7 days in complete medium plus 10 ng/mL rhGM-CSF and 10 ng/mL IL-4 (PharMingen). CD4+ T cells were purified from PBMCs by negative selection with CD4 T-cell-isolation kit (Miltenyi Biotec), and CD4+CD25+ T cells were subsequently positively selected with CD25 beads (Miltenyi Biotec).
Flow cytometric analysis
Flow cytometric analysis was performed as previously described,7 in the presence of 100 μg/mL mouse IgG, using the mAbs anti-CD1c (BDCA-1) fluoroscein isothiocyanate (FITC) or phycoerythrin (PE), anti–BDCA-2 FITC or PE (Miltenyi Biotec), anti-CD1a FITC/PE, and anti-CD83 FITC/PE, all from Pharmingen, or with mAbs specific for ILT1 (clone 135.1), ILT2 (clone GHI75), ILT3 (clone ZM3.1), ILT4 (clone 42D.1), and ILT5 (clone 7H5). Cells were analyzed with an LSR flow cytometer (Becton Dickinson, Mountain View, CA) using CellQuest software (Becton Dickinson).
T-cell activation
Peripheral-blood DC subsets were cultured for 5 days in complete medium and for an additional 7 days in the presence of CD4+ T cells with or without 20 μg/mL anti-ILT3 antibody. Intracellular cytokine production by CD4 T-cell lines was analyzed as described.28 CD4+ T cells were cultured in 96-well flat-bottom plates with graded amounts of immature monocyte-derived DCs, which had been cultured for the last 48 hours with or without 10 nM 1,25(OH)2D3. The coculture of DCs and T cells was carried out in the presence or absence of anti-ILT3 mAb. After 5 days of culture, interferon-γ (IFN-γ) production was measured by 2-site enzyme-linked immunosorbent assay (ELISA). Alternatively, CD4+ T cells were cultured in the presence of immature monocyte-derived DCs (ratio, 1:10) with or without anti-ILT3 and 7 to 10 days after primary stimulation, were restimulated under the same conditions. After 2 rounds of stimulation, CD4+ T cells were cultured in 96-well round-bottom plates precoated by overnight incubation with anti–human CD3 mAb. After 72 hours, cytokine production was quantified by 2-site ELISA. Paired mAbs specific for IFN-γ, IL-4, and IL-10 (Pharmingen), were used as described.7 The detection limit for all cytokines was 15 pg/mL.
Suppression assay
The read-out system was composed of CD4+CD25– cells from donor A PBMCs labeled with Vybrant CFDA (carboxyfluorescein diacetate) succinimidyl ester (SE) cell tracer kit (Molecular Probes, Leiden, the Netherlands) and cultured in the presence of 1:10 LPS-matured monocyte-derived DCs from donor B. To evaluate the induction of regulatory T cells, graded amounts of CD4+ T cells from donor C were generated by 3 rounds of restimulation with allogeneic immature monocyte-derived DCs from donor D, which were cultured for the last 48 hours with or without 1,25(OH)2D3. The coculture was carried out with or without anti-ILT3 mAb (20 μg/mL). The suppression assay was performed in the presence of 1 μg/mL anti–human CD3 mAb. After 72 to 96 hours of culture, cells were recovered and analyzed with an LSR flow cytometer (Becton Dickinson) using CellQuest software.
Real-time quantitative RT-PCR
RNA was extracted using Trizol (Invitrogen, Carlsbad, CA) according to the manufacturer's instruction, followed by a cleanup with the RNeasy Kit (Qiagen, Hilden, Germany). Reverse transcription (RT) was performed, and real-time quantitative RT–polymerase chain reaction (PCR) of total cDNA using specific primers was carried out using an ABI PRISM 7000 Sequence Detection System (Applied Biosystems, Foster City, CA) and Taqman chemistry. The primers used are commercially available from Applied Biosystems as assays on demand. Relative quantification of target cDNA was determined by arbitrarily setting the control value to 1 and changes in cDNA content of a sample were expressed as a multiple thereof. Differences in cDNA input were corrected by normalizing to β actin or GAPDH (glyceraldehyde-3-phosphate dehydrogenase) signals. To exclude amplification of genomic DNA, RNA samples were treated with DNAse (Sigma Chemical).
Immunohistology
Skin biopsies were obtained from untreated psoriatic plaques or from psoriatic lesions of 5 patients treated topically for 30 days with 0.005% calcipotriol cream (Psorcutan; Schering, Milan, Italy) twice daily, or for 20 days with 0.1% mometasone furoate cream (Elocon; Schering-Plough, Milan, Italy) twice daily, and snap-frozen in liquid nitrogen. Immunohistology was performed on 5-μm-thick cryostat sections fixed in acetone and dried overnight, as described.29 Before incubation with specific mAbs or control isotype mouse Ig's, sections were hydrated for 10 minutes with 0.05 M Tris (tris(hydroxymethyl)aminomethane)–aminomethane saline buffer (TBS), pH 7.6, and incubated for 10 minutes with a mixture of normal human AB serum and normal rabbit serum (20% in TBS), to minimize nonspecific staining. Then, after washing in TBS, the sections were incubated for 30 minutes with specific mAbs, followed by polyclonal rabbit antimouse serum diluted 1/30 in TBS (Dako-Patt, Copenhagen, Denmark) and alkaline phosphatase anti–alkaline phosphatase complex diluted 1/50 in TBS (Dako-Patt). Finally, stainings were revealed using new fucsin (75 μL of a new fucsin–sodium-nitrite solution) in 10 mL of 0.05 M TBS, pH 8.7; 5 mg naphthol AS-BI (7-bromo-3-hydroxy-2-naphtho-o-anisidine) sodium salt; and 1 mM levamisole (Sigma Chemical). Sections were counterstained with Mayer haematoxylin, washed in water, and dried at room temperature before mounting. Slides were observed with a Leica light microscope (DMR; Leica Microsystems, Milan, Italy) equipped with an HC Plans 10×/2.2 ocular and an N plan 20×/0.40 objective lens.
Immunofluorescent stainings were performed on 5-μm-thick frozen sections from skin biopsies of calcipotriol-treated psoriatic plaques. After fixing for 15 minutes in 4% paraformaldehyde and blocking with 0.1 M glycine for 10 minutes at room temperature, slides were incubated overnight at 4°C in blocking solution with affinity-purified rabbit anti–mouse CD11c (Jackson Immunoresearch, West Grove, PA), washed 5 times with wash buffer (0.45 M NaCl, 0.24 M Na2HPO4, 0.24 M NaH2PO4, and 0.3% Triton X-100), and incubated for 60 minutes with polyclonal antirabbit FITC (Sigma Chemical). Alternatively, slides were incubated overnight at 4°C with biotinylated rat anti–mouse CD123 (Jackson Immunoresearch) followed by Rhodamine Red-X-streptavidin (Jackson Immunoresearch). Negative controls were performed by incubation with appropriate isotype-matched primary antibodies. The slides were then washed again and mounted with 90% glycerol/phosphate-buffered saline (PBS). Slides were analyzed with an MRC-1024 laser-scanning confocal microscope (Bio-Rad Laboratories, Hercules, CA). Images were acquired and processed with Laser Sharp 3.2 software (Bio-Rad).
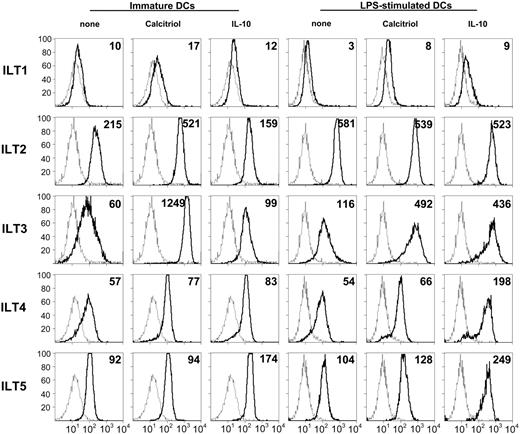
1,25(OH)2D3 enhances ILT3 expression on DCs. Monocyte-derived human DCs were incubated for 48 hours with medium alone or containing 10 nM 1,25(OH)2D3 or 10 ng/mL IL-10, either when immature or during LPS-induced (100 ng/mL) maturation. Surface expression of the indicated ILT molecules was determined by cytofluorimetry. Histograms represented by broken lines show staining with an isotype control; those with thick lines show staining with the indicated anti-ILT mAbs. Geometric mean fluorescence intensity (MFI) values are shown in the top right corner. A representative experiment out of 6 performed is shown.
1,25(OH)2D3 enhances ILT3 expression on DCs. Monocyte-derived human DCs were incubated for 48 hours with medium alone or containing 10 nM 1,25(OH)2D3 or 10 ng/mL IL-10, either when immature or during LPS-induced (100 ng/mL) maturation. Surface expression of the indicated ILT molecules was determined by cytofluorimetry. Histograms represented by broken lines show staining with an isotype control; those with thick lines show staining with the indicated anti-ILT mAbs. Geometric mean fluorescence intensity (MFI) values are shown in the top right corner. A representative experiment out of 6 performed is shown.
Results
1,25(OH)2D3 up-regulates ILT3 expression on dendritic cells
We and others have previously shown that VDR agonists can induce DCs to acquire tolerogenic properties, characterized by arrest of maturation, decreased expression of costimulatory molecules, abrogation of IL-12, and marked increase of IL-10 production,7-9 leading to hyporesponsiveness of effector T cells and to enhancement of CD4+CD25+ regulatory T cells.12,30 Since preliminary experiments have indicated increased expression of ILT3 by 1,25(OH)2D3-treated DCs,25,26 we have examined the expression of ILT1 to ILT5 in immature and mature DCs. Immature monocyte-derived DCs express all the ILT molecules tested, with a relatively higher expression of ILT2 (Figure 1). Incubation of immature monocyte-derived DCs with 1,25(OH)2D3 induced up to a 20-fold up-regulation of ILT3 expression, whereas only a slight up-regulation was induced by IL-10. Addition of 1,25(OH)2D3 during LPS-induced maturation led to a 4-fold enhancement of ILT3 expression, similar to IL-10. In 6 independent experiments, incubation with 1,25(OH)2D3 was found to enhance ILT3 expression, as detected by mean fluorescence intensity (MFI), by 9-fold (range, 3- to 21-fold) in immature DCs and by 4-fold (range, 2- to 7-fold) in LPS-treated DCs. No modulation of the other ILT receptors examined was induced by 1,25(OH)2D3, except for a modest increase of ILT2 expression in immature DCs. Conversely, IL-10 induced a 4-fold up-regulation of ILT4 and a 2-fold increase of ILT5 expression (Figure 1).
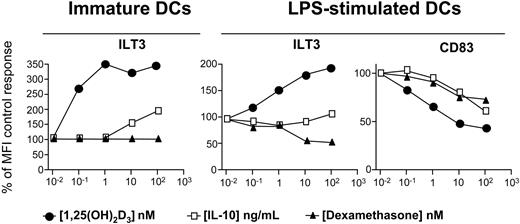
Dexamethasone fails to enhance ILT3 expression on DCs. Monocyte-derived human DCs were incubated for 48 hours with medium alone or medium containing 10 nM 1,25(OH)2D3, 10 ng/mL IL-10, or 100 nM dexamethasone, either when immature or during LPS-induced (100 ng/mL) maturation. Surface expression of ILT3 and CD83 molecules was determined by cytofluorimetry. Results are expressed as percent of the control geometric MFI. A representative experiment out of 2 performed is shown.
Dexamethasone fails to enhance ILT3 expression on DCs. Monocyte-derived human DCs were incubated for 48 hours with medium alone or medium containing 10 nM 1,25(OH)2D3, 10 ng/mL IL-10, or 100 nM dexamethasone, either when immature or during LPS-induced (100 ng/mL) maturation. Surface expression of ILT3 and CD83 molecules was determined by cytofluorimetry. Results are expressed as percent of the control geometric MFI. A representative experiment out of 2 performed is shown.
As tolerogenic DCs induced by different pharmacologic agents share several properties,26,31 we analyzed modulation of ILT3 expression by 1,25(OH)2D3, dexamethasone, and IL-10. 1,25(OH)2D3 and IL-10 induced a dose-dependent increase of ILT3 expression in immature monocyte-derived DCs, whereas no effect was observed in the presence of dexamethasone (Figure 2). However, the up-regulation of ILT3 expression was markedly higher in DCs incubated with 1,25(OH)2D3 compared with the increase induced by IL-10. Addition of 1,25(OH)2D3 during LPS-induced maturation enhanced ILT3 expression, and a marginal increase was also induced by IL-10. DC maturation was confirmed by the 3-fold enhanced expression of CD83. Expression of this DC maturation marker was inhibited by 1,25(OH)2D3, and to a lower extent by IL-10 or dexamethasone, showing efficacy of all in vitro treatments and confirming the capacity of these agents to maintain DCs in an immature stage.7,32
VDR agonists induce in vivo up-regulation of ILT3 on dendritic cells in psoriatic lesions
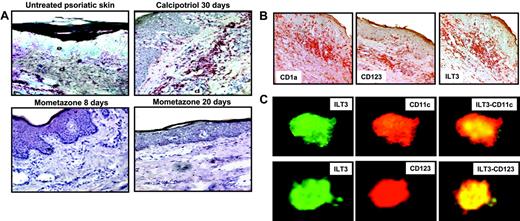
To assess the capacity of VDR agonists to up-regulate in vivo ILT3 expression on DCs, immunohistochemical analysis was performed on skin plaques from 5 patients treated topically with the 1,25(OH)2D3 analog calcipotriol or with the glucocorticoid mometasone furoate. In untreated psoriatic lesions (Figure 3A, top left panel), a low expression of ILT3 was observed, but this was strongly enhanced following a 30-day treatment with calcipotriol, concomitant with a reduction of histologic lesions such as acanthosis, hyperkeratosis, papillomatosis, and lymphoid infiltrate (Figure 3A, top right panel). Conversely, no up-regulation of ILT3 expression was observed in biopsies from psoriatic plaques treated topically with mometasone furoate for 8 or 20 days, although also in this case an amelioration of histologic lesions was observed (Figure 3A, bottom panels). As shown in serial sections stained for CD1a, CD123, and ILT3 expression, ILT3 is expressed by both myeloid and plasmacytoid DCs (Figure 3B). In contrast, endothelial cells lining capillary and postcapillary venules appear to be negative (Figure 3B). Expression of ILT3 by both myeloid and plasmacytoid DCs following topical treatment with calcipotriol (Figure 3C) was confirmed by the colocalization of ILT3 and CD11c or CD123, respectively, in the same skin biopsies shown in the top right panel of Figure 3A.
VDR agonists induce in vivo up-regulation of ILT3 on M-DCs and P-DCs in psoriatic lesions. (A) Skin biopsies from untreated psoriatic lesions, psoriatic plaques treated for 30 days with calcipotriol, and psoriatic plaques treated for 8 or 20 days with mometazone furoate were stained with anti-ILT3 mAb and counterstained with hematoxylin, as described in “Materials and methods.” Original magnification × 200, except top left panel (× 50); e indicates epidermis; d, dermis. (B) Noncounterstained serial sections from calcipotriol-treated psoriatic lesions shown in panel A stained with anti-CD1a, anti-CD123, and anti-ILT3. Original magnification × 100. (C) Expression of ILT3 in M-DCs and P-DCs from the calcipotriol-treated psoriatic lesions shown in panel A. Sections were stained with anti-ILT3 and anti-CD11c or anti-CD123, respectively, and analyzed by confocal microscopy. Merged stainings are shown in the right panels. The results refer to analysis of 1 representative patient out of 5 examined.
VDR agonists induce in vivo up-regulation of ILT3 on M-DCs and P-DCs in psoriatic lesions. (A) Skin biopsies from untreated psoriatic lesions, psoriatic plaques treated for 30 days with calcipotriol, and psoriatic plaques treated for 8 or 20 days with mometazone furoate were stained with anti-ILT3 mAb and counterstained with hematoxylin, as described in “Materials and methods.” Original magnification × 200, except top left panel (× 50); e indicates epidermis; d, dermis. (B) Noncounterstained serial sections from calcipotriol-treated psoriatic lesions shown in panel A stained with anti-CD1a, anti-CD123, and anti-ILT3. Original magnification × 100. (C) Expression of ILT3 in M-DCs and P-DCs from the calcipotriol-treated psoriatic lesions shown in panel A. Sections were stained with anti-ILT3 and anti-CD11c or anti-CD123, respectively, and analyzed by confocal microscopy. Merged stainings are shown in the right panels. The results refer to analysis of 1 representative patient out of 5 examined.
Modulation of ILT3 expression by different maturation-inducing stimuli in myeloid and plasmacytoid DC subsets: selective up-regulation by 1,25(OH)2D3 in myeloid DCs
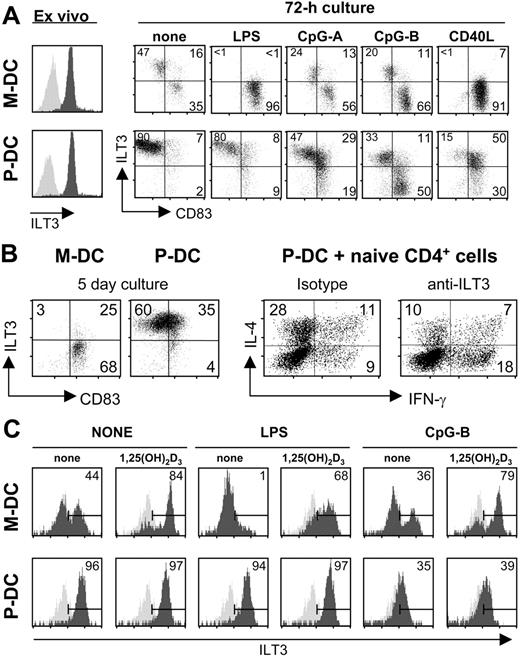
Both M-DCs and P-DCs from human peripheral blood express ILT3 ex vivo (Figure 4A) as previously shown.33,34 About 50% of blood M-DCs, following a 3-day culture without stimulation, down-regulate ILT3 and mature, as indicated by the increased expression of CD83 (Figure 4A). LPS and CD40 ligation induce complete down-regulation of ILT3 expression and maturation of the entire M-DC population, whereas CpGs have no effect compared with unstimulated M-DCs, as predicted by the absence of Toll-like receptor 9 (TLR9) in this cell population.35,36 P-DCs are not induced to mature or modulate ILT3 expression by culture in the absence of stimuli, but stimulation with CpGs induces a population of P-DCs, more sizeable with CpG-B, to mature and down-regulate ILT3 expression. No obvious effects are induced by LPS, consistent with the absence of TLR4 in P-DCs.35,36 Interestingly, CD40 ligation induces maturation in the majority of P-DCs, which, however, continue to maintain ILT3 expression (Figure 4A).
Modulation of ILT3 expression by different maturation-inducing stimuli in M-DC and P-DC subsets: selective up-regulation by 1,25(OH)2D3 in M-DCs. (A) Maturation stimuli modulate ILT3 expression in myeloid and plasmacytoid DC subsets. DC subsets were incubated for 72 hours with 1 μg/mL LPS, 5 μg/mL CpG-A or CpG-B sequence, or CD154-transfected J558L cells at a ratio of 1:4. Surface expression of ILT3 and CD83 was determined by cytofluorimetry. The left panels show expression of ILT3 (dark gray) by ex-vivo purified M-DCs and P-DCs. Light gray histograms represent staining with isotype controls. (B) Blocking ILT3 on P-DCs enhances Th1-cell development. DC subsets were cultured for 5 days in the absence of any exogenous stimulus before analysis of ILT3 and CD83 expression by cytofluorimetry. P-DCs were subsequently cocultured with naive CD4+ cells for an additional 7 days in the presence of anti-ILT3 antibody or isotype control. The T-helper–cell phenotype was evaluated by cytofluorimetry following intracellular staining for IFN-γ and IL-4, and the percentages of positive cells are shown. A representative experiment out of 3 performed is shown. (C) Selective up-regulation of ILT3 in M-DCs treated with 1,25(OH)2D3. DC subsets were cultured for 48 hours without stimulation or with 1 μg/mL LPS or 5 μg/mL CpG-B sequence, in medium alone or medium containing 10 nM 1,25(OH)2D3. Surface expression of ILT3 was determined by cytofluorimetry. A representative experiment out of 3 performed is shown.
Modulation of ILT3 expression by different maturation-inducing stimuli in M-DC and P-DC subsets: selective up-regulation by 1,25(OH)2D3 in M-DCs. (A) Maturation stimuli modulate ILT3 expression in myeloid and plasmacytoid DC subsets. DC subsets were incubated for 72 hours with 1 μg/mL LPS, 5 μg/mL CpG-A or CpG-B sequence, or CD154-transfected J558L cells at a ratio of 1:4. Surface expression of ILT3 and CD83 was determined by cytofluorimetry. The left panels show expression of ILT3 (dark gray) by ex-vivo purified M-DCs and P-DCs. Light gray histograms represent staining with isotype controls. (B) Blocking ILT3 on P-DCs enhances Th1-cell development. DC subsets were cultured for 5 days in the absence of any exogenous stimulus before analysis of ILT3 and CD83 expression by cytofluorimetry. P-DCs were subsequently cocultured with naive CD4+ cells for an additional 7 days in the presence of anti-ILT3 antibody or isotype control. The T-helper–cell phenotype was evaluated by cytofluorimetry following intracellular staining for IFN-γ and IL-4, and the percentages of positive cells are shown. A representative experiment out of 3 performed is shown. (C) Selective up-regulation of ILT3 in M-DCs treated with 1,25(OH)2D3. DC subsets were cultured for 48 hours without stimulation or with 1 μg/mL LPS or 5 μg/mL CpG-B sequence, in medium alone or medium containing 10 nM 1,25(OH)2D3. Surface expression of ILT3 was determined by cytofluorimetry. A representative experiment out of 3 performed is shown.
We next tested the functional implications of ILT3 expression by P-DCs. Addition of anti-ILT3 mAb to cultures of P-DCs, which maintain expression of ILT3, and naive CD4+ cells could switch, compared with addition of an isotype control, T-cell development from the T-helper 2 (Th2) to the Th1 phenotype, as shown by the increased percentage of IFN-γ–producing cells concomitant with a reduction of IL-4–producing cells (Figure 4B). No effect of anti-ILT3 was observed in cocultures of M-DCs that had completely down-regulated ILT3 expression following a 5-day culture and naive CD4+ cells (data not shown).
Interestingly, 1,25(OH)2D3 has a differential effect on ILT3 expression by unstimulated DC subsets, inducing its up-regulation only in M-DCs (Figure 4C). LPS stimulation induces down-regulation of ILT3 expression in M-DCs, and this effect is inhibited by 1,25(OH)2D3 (Figure 4C). As expected, no effect, compared with unstimulated M-DCs, is induced by CpG-B. Similarly, no effect is observed in LPS-stimulated P-DCs, but stimulation with CpG-B markedly down-regulates ILT3 expression, which, in contrast to LPS-stimulated M-DCs, is not affected by 1,25(OH)2D3.
1,25(OH)2D3 treatment of DCs inhibits the enhanced alloreactive T-cell response induced by ILT3 blockade
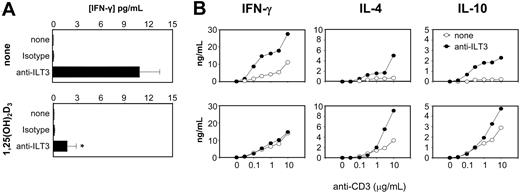
To determine if ILT3 could modulate T-cell responsiveness, we first blocked ILT3 in mixed leukocyte cultures of immature monocyte-derived DCs and CD4+ cells, using the same antibody previously shown to have ILT3-neutralizing activity.22 Addition of anti-ILT3 mAb increased IFN-γ secretion by 2 orders of magnitude (Figure 5A, top panel), confirming the inhibitory role played by ILT3.22 Similar results were obtained by addition of anti-ILT3 mAb to cultures of immature DCs and allogeneic CD8+ cells (data not shown). Surprisingly, the stimulatory effect of ILT3 blockade was significantly decreased (P = .002) by pretreatment of immature DCs for 48 hours with 10 nM 1,25(OH)2D3 (Figure 5A, bottom panel), indicating that this hormone can affect ILT3-independent pathways leading to inhibition of T-cell responsiveness.
To analyze functional properties of alloreactive CD4+ T cells obtained after 2 rounds of restimulation with allogeneic immature DCs, cytokine production was quantified following stimulation with plate-bound anti-CD3 and soluble anti-CD28 mAbs. Results in Figure 5B show that T cells generated in the presence of anti-ILT3 mAb display enhanced cytokine production. Both Th1-type cytokines, like IFN-γ, and Th2-types like IL-4 and IL-10, were enhanced, indicating that ILT3 expressed by immature monocyte-derived DCs plays an overall inhibitory role on T-cell activation. 1,25(OH)2D3 pretreatment of DCs inhibited the anti-ILT3–induced enhancement of IFN-γ secretion, but increased IL-4 and IL-10 secretion by T cells regardless of ILT3 blockade, indicating the existence of ILT3-independent mechanisms induced by 1,25(OH)2D3 in DCs able to modulate T-cell responsiveness.
ILT3 is dispensable for induction of CD4+Foxp3+ regulatory T cells by 1,25(OH)2D3-treated DCs
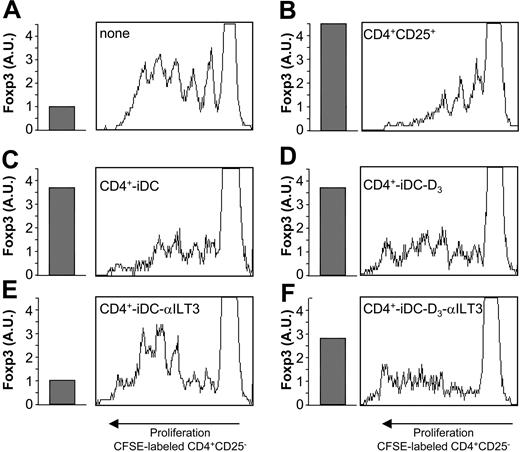
To evaluate the role of ILT3 in the induction of CD4+Foxp3+ regulatory T cells, immature monocyte-derived DCs were cultured with allogenic CD4+ cells for 3 rounds of restimulation. Results in Figure 6 demonstrate that the marked proliferation of CFSE (carboxyfluorescein diacetate succinimidyl ester)–labeled CD4+CD25– cells (Figure 6A) is profoundly inhibited by addition of blood CD4+CD25+ cells (Figure 6B). ILT3 blockade prevents the induction of CD4+ T cells with suppressive activity, as shown by the strong proliferation of CFSE-labeled CD4+CD25– cells (Figure 6E) compared with the inhibition observed in control cultures (Figure 6C). Prevention of T-cell induction is also indicated by the 4-fold reduction of Foxp3 expression in CD4+ regulatory T cells generated in the presence of anti-ILT3 mAb. Pretreatment of immature monocyte-derived DCs with 1,25(OH)2D3 before coculture with allogeneic CD4+ cells leads to induction of CD4+ Ts cells (Figure 6D) regardless of the presence of anti-ILT3 mAb (Figure 6F), indicating the existence of ILT3-independent mechanisms promoted by 1,25(OH)2D3 that are able to induce the generation of regulatory T cells. This is in agreement with the comparable expression of Foxp3 transcripts in CD4+ cells generated in the presence of 1,25(OH)2D3 with (Figure 6F) or without (Figure 6D) ILT3 blockade.
1,25(OH)2D3 affects ILT3-independent T-cell responses. (A) Monocyte-derived human DCs, incubated for 48 hours with or without 10 nM 1,25(OH)2D3, were cocultured with allogeneic CD4+ cells for 5 days in the presence or absence of anti-ILT3 mAb or isotype control. Results in panel A refer to mean ± standard error from five separate experiments. (B) CD4+ cells were restimulated with anti-CD3 after 2 rounds of restimulation under the same conditions as in panel A. After 72 hours of culture, the indicated cytokines were measured by 2-site ELISA. A representative experiment out of 2 performed is shown in panel B. *P = .002 versus anti-ILT3 without 1,25(OH)2D3 by Mann-Whitney U test.
1,25(OH)2D3 affects ILT3-independent T-cell responses. (A) Monocyte-derived human DCs, incubated for 48 hours with or without 10 nM 1,25(OH)2D3, were cocultured with allogeneic CD4+ cells for 5 days in the presence or absence of anti-ILT3 mAb or isotype control. Results in panel A refer to mean ± standard error from five separate experiments. (B) CD4+ cells were restimulated with anti-CD3 after 2 rounds of restimulation under the same conditions as in panel A. After 72 hours of culture, the indicated cytokines were measured by 2-site ELISA. A representative experiment out of 2 performed is shown in panel B. *P = .002 versus anti-ILT3 without 1,25(OH)2D3 by Mann-Whitney U test.
ILT3 is dispensable for induction of CD4+Foxp3+ regulatory T cells by 1,25(OH)2D3-treated DCs. The read-out system to test for suppressive activity was composed of CFSE-labeled CD4+CD25-cells from donor A PBMCs cultured with 1:10 LPS-matured monocyte-derived DCs from donor B in the presence of 1 μg/mL anti–human CD3 mAb. To evaluate the induction of Ts, CD4+ T cells from donor C were generated by 3 rounds of restimulation with allogeneic immature monocyte-derived DCs from donor D, which were cultured for the last 48 hours with or without 10 nM 1,25(OH)2D3. The coculture was carried out with or without anti-ILT3 mAb (20 μg/mL). After 96 hours of culture, cells were analyzed by flow cytometry. Expression of Foxp3 transcripts, evaluated by real time RT-PCR, is shown as arbitrary units (AU) normalized to GAPDH signals. (A) Proliferation of CFSE-labeled CD4+CD25– cells (5 × 104/well). Basal levels of Foxp3 transcripts are expressed as AU. (B) Addition of blood CD4+CD25+ cells (6.5 × 103/well) inhibits CD4+CD25– cell proliferation. Foxp3 expression of blood CD4+CD25+ cells is indicated. (C) CD4+ cells (6.5 × 103/well) stimulated for 3 rounds with allogeneic immature monocyte-derived DCs (CD4-iDC) show enhanced Foxp3 expression and inhibit proliferation of CFSE-labeled CD4+CD25– cells. (D) CD4+ cells stimulated for 3 rounds with allogeneic immature monocyte-derived DCs in the presence of 10 nM 1,25(OH)2D3 (CD4-iDC-D3) show enhanced Foxp3 expression and inhibit proliferation of CFSE-labeled CD4+CD25– cells. (E) Addition of anti-ILT3 mAb to CD4+ cells stimulated for 3 rounds with allogeneic immature monocyte-derived DCs (CD4-iDC-αILT3) show basal levels of Foxp3 expression and fail to inhibit proliferation of CFSE-labeled CD4+CD25– cells. (F) Addition of anti-ILT3 mAb to CD4+ cells stimulated for 3 rounds with allogeneic immature monocyte-derived DCs in the presence of 10 nM 1,25(OH)2D3 (CD4-iDC-D3-αILT3) show enhanced Foxp3 expression and inhibit proliferation of CFSE-labeled CD4+CD25– cells. A representative experiment out of 2 performed is shown.
ILT3 is dispensable for induction of CD4+Foxp3+ regulatory T cells by 1,25(OH)2D3-treated DCs. The read-out system to test for suppressive activity was composed of CFSE-labeled CD4+CD25-cells from donor A PBMCs cultured with 1:10 LPS-matured monocyte-derived DCs from donor B in the presence of 1 μg/mL anti–human CD3 mAb. To evaluate the induction of Ts, CD4+ T cells from donor C were generated by 3 rounds of restimulation with allogeneic immature monocyte-derived DCs from donor D, which were cultured for the last 48 hours with or without 10 nM 1,25(OH)2D3. The coculture was carried out with or without anti-ILT3 mAb (20 μg/mL). After 96 hours of culture, cells were analyzed by flow cytometry. Expression of Foxp3 transcripts, evaluated by real time RT-PCR, is shown as arbitrary units (AU) normalized to GAPDH signals. (A) Proliferation of CFSE-labeled CD4+CD25– cells (5 × 104/well). Basal levels of Foxp3 transcripts are expressed as AU. (B) Addition of blood CD4+CD25+ cells (6.5 × 103/well) inhibits CD4+CD25– cell proliferation. Foxp3 expression of blood CD4+CD25+ cells is indicated. (C) CD4+ cells (6.5 × 103/well) stimulated for 3 rounds with allogeneic immature monocyte-derived DCs (CD4-iDC) show enhanced Foxp3 expression and inhibit proliferation of CFSE-labeled CD4+CD25– cells. (D) CD4+ cells stimulated for 3 rounds with allogeneic immature monocyte-derived DCs in the presence of 10 nM 1,25(OH)2D3 (CD4-iDC-D3) show enhanced Foxp3 expression and inhibit proliferation of CFSE-labeled CD4+CD25– cells. (E) Addition of anti-ILT3 mAb to CD4+ cells stimulated for 3 rounds with allogeneic immature monocyte-derived DCs (CD4-iDC-αILT3) show basal levels of Foxp3 expression and fail to inhibit proliferation of CFSE-labeled CD4+CD25– cells. (F) Addition of anti-ILT3 mAb to CD4+ cells stimulated for 3 rounds with allogeneic immature monocyte-derived DCs in the presence of 10 nM 1,25(OH)2D3 (CD4-iDC-D3-αILT3) show enhanced Foxp3 expression and inhibit proliferation of CFSE-labeled CD4+CD25– cells. A representative experiment out of 2 performed is shown.
Discussion
Results in this study show that the VDR agonist 1,25(OH)2D3 is able, in vitro and in vivo, to markedly up-regulate expression of the inhibitory receptor ILT3 on DCs. ILT3 expression by DCs is required to induce CD4+Foxp3+ regulatory T cells, but is dispensable for their induction by 1,25(OH)2D3-treated DCs, although this hormone strongly up-regulates ILT3 expression in immature and mature DCs. Thus, ILT3-independent mechanisms promote regulatory T-cell induction by 1,25(OH)2D3-treated DCs.
Several immunosuppressive and anti-inflammatory drugs share the capacity to target DCs, rendering them tolerogenic and, in some cases, fostering the induction of regulatory T cells.26,31 Among immunosuppressive and anti-inflammatory drugs, VDR agonists are immunomodulatory agents able to directly target both DCs and T cells, leading to the inhibition of pathogenic effector T cells and enhancing the frequency of T cells with suppressive properties, effects at least in part mediated via induction of tolerogenic DCs.37 Multiple mechanisms probably contribute to induction of DC tolerogenicity by VDR agonists, and a potentially critical one could be based on their capacity to up-regulate expression of inhibitory receptors, like ILT3, on DCs.
As tolerogenic DCs induced by different pharmacologic agents share several properties,26,31 we compared up-regulation of ILT3 expression in immature and mature DCs by selected immunomodulatory agents. Our in vitro results demonstrate that while 1,25(OH)2D3 markedly up-regulates ILT3 expression on both immature and mature DCs, IL-10 has a much less pronounced effect, and the glucocorticoid dexamethasone has no ILT3-increasing activity. In the same experiments, all the 3 agents inhibited DC maturation, as shown by decreased CD83 expression. An in vivo correlate could be established by the marked up-regulation of ILT3 expression in DCs present in psoriatic lesions treated with the VDR agonist calcipotriol, whereas no ILT3 expression was induced by topical treatment of psoriatic plaques with the glucocorticoid mometazone. Psoriasis is a Th1-mediated autoimmune disease of the skin that can be topically treated with VDR agonists and glucocorticoids,38 first-line drugs, while novel biologic agents are being developed.39 Our in vivo data, demonstrating up-regulation of ILT3 expression on DCs by treatment with calcipotriol but not with mometasone furoate, parallel the in vitro observations on DC cultures, indicating specific VDR-mediated effects in DCs that are not shared by glucocorticoids.40
These results indicate that ILT3 up-regulation is not a general feature of DC-targeting anti-inflammatory or immunosuppressive drugs, as proposed,25 and are consistent with the view that VDR agonists and glucocorticoids modulate DCs using distinctive pathways.41 Conversely, IL-10, already noted as sharing some immunomodulatory activities with 1,25(OH)2D3,7 up-regulates ILT3 expression, further extending these similarities. In agreement with the IL-10–mediated increase of ILT4 expression observed in monocytes from HIV-positive individuals,42 we could also document an IL-10–induced up-regulation of ILT4 expression in LPS-treated DCs. However, 1,25(OH)2D3 did not up-regulate DC expression of ILT4, an inhibitory receptor induced, together with ILT3, in tolerogenic DCs by CD8+CD28– suppressor T cells.22
Analysis of blood myeloid and plasmacytoid DCs reveals interesting differences in regulation of ILT3 expression by maturation-inducing stimuli, and a differential modulation by 1,25(OH)2D3. As predicted by differential TLR expression in M-DCs and P-DCs,35,36 LPS and CpG oligodeoxynucleotides induced maturation, associated with down-regulation of ILT3, in M-DCs and P-DCs, respectively. The latter result is consistent with the decreased ILT3 expression observed in P-DCs stimulated with CpG sequences.43 It is conceivable that down-regulation of inhibitory receptors like ILT3 on mature DCs could contribute to induction of T-cell responses. In addition, our data showing that CD40 ligation, a signal mimicking interaction with T cells, reduces ILT3 expression on M-DCs suggest that ILT3 down-regulation may be involved also in maintaining T-cell responses. Interestingly, ILT3 expression is not decreased by CD40 triggering in P-DCs, although this is sufficient to induce their maturation.
Maintaining ILT3 expression on P-DCs matured via CD40 ligation could have immunoregulatory potential, because this cell population has been shown to induce CD8+ as well as CD4+CD25+ regulatory T cells,44,45 and to skew the response to the Th2 pathway.46 In addition, our data show that expression of ILT3 by P-DCs is associated with induction of Th2 cells, and its blockade by anti-ILT3 mAb skews T-cell differentiation to the Th1 pathway. Interestingly, while incubation with 1,25(OH)2D3 did not affect ILT3 expression by P-DCs, it markedly increased its expression on M-DCs, indicating a differential VDR agonist-induced effect in the 2 DC subsets.
The present data show that ILT3 expression by DCs is required for induction of CD4+Foxp3+ regulatory T cells, suggesting that interaction with the ILT3 ligand, not yet identified, likely expressed by CD4+ cells could selectively promote induction of regulatory T cells. The up-regulation of ILT3 induced by 1,25(OH)2D3 in M-DCs could thus suggest an additional mechanism contributing to the induction of tolerogenic DCs by this hormone, besides the well-established inhibition of costimulatory molecule expression, abrogation of IL-12, and up-regulation of IL-10 production.7-11 Nevertheless, our data show that ILT3 is dispensable for the generation of CD4+Foxp3+ regulatory T cells by 1,25(OH)2D3-treated DCs, revealing the capacity of 1,25(OH)2D3 to promote dominant ILT3-independent mechanisms enabling DCs to induce regulatory T cells. This does not exclude a contribution of ILT3 to other immunomodulatory properties of 1,25(OH)2D3-treated DCs.
ILT3 overexpression inhibits T-helper cell–induced nuclear factor–κB (NF-κB) activation in DCs, possibly because ILT3 acts via SHP phosphatases to downmodulate IκB phosphorylation and degradation, thus preventing NF-κB nuclear translocation.22 A similar mechanism is induced by 1,25(OH)2D3 in DCs, leading to inhibition of NF-κB activation,47 and up-regulation of IκBα transcripts with arrest of NF-κB translocation has been observed in islet cells treated with 1,25(OH)2D3.48 It is possible that NF-κB activation can be independently inhibited in DCs by both ILT3 expression and VDR ligation, thus explaining the ILT3-independent effect of 1,25(OH)2D3 on CD4+Foxp3+ regulatory T-cell induction. Alternatively, the multiple genes modulated by VDR agonists in DCs,40 in particular those encoding costimulatory molecules and cytokines,49 may have a dominant effect in promoting DCs able to induce regulatory T cells, irrespective of ILT3 expression.
Prepublished online as Blood First Edition Paper, July 19, 2005; DOI 10.1182/blood-2005-05-2044.
Supported in part by a European Community grant (QLRT-2000-02103 to L.A.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal