The t(4;14) translocation detected by fluorescence in situ hybridization (FISH) is an independent prognostic factor for an adverse outcome of multiple myeloma (MM). Because t(4;14) uniquely results in fibroblast growth factor receptor 3 (FGFR3) expression, decalcified, paraffin-embedded bone marrow biopsies were immunostained for FGFR3, and its expression was correlated with the t(4;14) status. FISH detected t(4;14) in 16 (19%) of 85 MM patient specimens, and immunocytochemistry detected aberrant FGFR3 expression in 13 (15%). Twelve (75%) t(4;14)-positive cases expressed FGFR3, and 12 (92%) FGFR3-positive cases harbored a t(4;14). FGFR3 expression and t(4;14) were strongly correlated (P < .001). FGFR3 expression by immunohistochemistry was associated with the immunoglobulin A (IgA) isotype (P < .001), a shorter progression-free survival (median, 11.5 versus 25.8 months; P < .001), and a shorter overall survival (median, 19.2 versus 46.3 months; P < .001).
Introduction
Chromosomal translocations involving the immunoglobulin heavy chain gene (IgH) locus are found frequently in multiple myeloma (MM), a malignancy of terminally differentiated B cells.1-3 One of the most common translocations, t(4;14), seen in 10% to 20% of cases, is associated with a poor prognosis.4-7 The molecular pathogenesis of t(4;14) is thought to involve aberrant immunoglobulin class switching recombination, leading to a reciprocal translocation between chromosomes 14q32 and 4p16.3 that repositions FGFR3 to der(14) and creates a fusion gene with MMSET on der(4) under the influence of strong enhancers from the IgH region.8,9
Fibroblast growth factor receptor 3 (FGFR3) is 1 of a family of 5 tyrosine kinases through which fibroblast growth factors signal. These receptors are characterized by an extracellular domain with 2 or 3 immunoglobulin-like domains, a transmembrane domain, and a cytoplasmic tyrosine kinase domain. On ligand binding, FGFR3 undergoes dimerization and tyrosine autophosphorylation, resulting in cell proliferation and differentiation. In MM, FGFR3 dysregulation appears to occur exclusively as a consequence of translocation.10 We and others have demonstrated that aberrant FGFR3 expression induces lymphoid malignancies in mice and that aberrant expression in myeloma cell lines results in cell proliferation and survival.11,12 Recent studies from our group suggest that the inhibition of FGFR3 by a small molecule induces differentiation and apoptosis of myeloma cell lines and primary MM cells, suggesting FGFR3 is a therapeutic target.13-15
The t(4;14) can be detected by fluorescence in situ hybridization (FISH) or gene expression profiling,16,17 and FGFR3 expression has been assessed at the mRNA level by reverse transcriptase–polymerase chain reaction (RT-PCR).6 However, FGFR3 protein expression, which could be a more clinically applicable diagnostic tool, has not been systematically examined. We therefore evaluated paraffin immunohistochemistry (IHC) for FGFR3 expression and correlated its expression with t(4;14) by FISH and clinical outcome.
Study design
Patients
Eighty-five consecutive MM cases accessioned and diagnosed by Durie-Salmon criteria between 1999 and 2002 were studied. The clinical and laboratory features are summarized in Table 1. The median age of the 53 men and 32 women was 53 years. Forty-three patients had an IgG paraprotein; 23, IgA; 3, IgD; 14, light chain disease; and 2 were nonsecretory. With institutional ethics board approval, bone marrow aspirates and aspirate section/biopsies were obtained from patients with active disease. All patients were treated with melphalan-based high-dose chemotherapy and autologous stem cell transplantation. The median follow-up after stem cell transplantation was 20 months. Approval was obtained from University Health Network (UHN) research reviewer board, and informed consent was provided by the patients in the study in accordance with the Declaration of Helsinki.
Characteristics of patients according to FGFR3 expression by IHC
. | FGFR3 . | . | . | . | |||
|---|---|---|---|---|---|---|---|
| Characteristics . | Total, n = 85 . | Negative, n = 72 . | Positive, n = 13 . | P . | |||
| Female; male (% female) | 32; 53 (37.7) | 24; 48 (33.3) | 8; 5 (61.5) | .067 | |||
| Age, y (range) | 53 (32-71) | 53 (38-71) | 58 (32-67) | .34 | |||
| β2-microglobulin level, mmol/L (range) | 207 (116-2690) | 207 (116-2690) | 291 (128-437) | .93 | |||
| CRP level, mg/L (range) | 3.5 (2.0-122.0) | 3.8 (2.0-122.0) | 2.5 (2.5-144.0) | .45 | |||
| Calcium level, mmol/L (range) | 2.4 (1.6-4.2) | 2.4 (1.6-4.0) | 2.5 (1.7-4.2) | .25 | |||
| Creatinine level, μmol/L (range) | 88 (51-1759) | 88 (51-1759) | 92 (52-216) | .25 | |||
| Hb level, g/dL (range) | 10.7 (5.2-15.8) | 11.3 (5.2-15.8) | 10.0 (5.6-12.9) | .29 | |||
| Albumin level, g/L (range) | 39.5 (20-53) | 40 (22-53) | 35 (20-47) | .62 | |||
| Bone lesions; n = 72 | .72 | ||||||
| None | 25 | 20 | 5 | ||||
| 1-3 | 21 | 18 | 3 | ||||
| More than 3 | 26 | 23 | 3 | ||||
| Stage; n = 83 | .99 | ||||||
| I-II | 31 | 26 | 5 | ||||
| III | 52 | 44 | 8 | ||||
| Isotype; n = 83 | |||||||
| IgA | 23 | 15 | 8 | .0059 | |||
| IgG | 43 | 40 | 3 | ||||
| IgD | 3 | 2 | 1 | ||||
| Light chains | 14 | 13 | 1 | ||||
. | FGFR3 . | . | . | . | |||
|---|---|---|---|---|---|---|---|
| Characteristics . | Total, n = 85 . | Negative, n = 72 . | Positive, n = 13 . | P . | |||
| Female; male (% female) | 32; 53 (37.7) | 24; 48 (33.3) | 8; 5 (61.5) | .067 | |||
| Age, y (range) | 53 (32-71) | 53 (38-71) | 58 (32-67) | .34 | |||
| β2-microglobulin level, mmol/L (range) | 207 (116-2690) | 207 (116-2690) | 291 (128-437) | .93 | |||
| CRP level, mg/L (range) | 3.5 (2.0-122.0) | 3.8 (2.0-122.0) | 2.5 (2.5-144.0) | .45 | |||
| Calcium level, mmol/L (range) | 2.4 (1.6-4.2) | 2.4 (1.6-4.0) | 2.5 (1.7-4.2) | .25 | |||
| Creatinine level, μmol/L (range) | 88 (51-1759) | 88 (51-1759) | 92 (52-216) | .25 | |||
| Hb level, g/dL (range) | 10.7 (5.2-15.8) | 11.3 (5.2-15.8) | 10.0 (5.6-12.9) | .29 | |||
| Albumin level, g/L (range) | 39.5 (20-53) | 40 (22-53) | 35 (20-47) | .62 | |||
| Bone lesions; n = 72 | .72 | ||||||
| None | 25 | 20 | 5 | ||||
| 1-3 | 21 | 18 | 3 | ||||
| More than 3 | 26 | 23 | 3 | ||||
| Stage; n = 83 | .99 | ||||||
| I-II | 31 | 26 | 5 | ||||
| III | 52 | 44 | 8 | ||||
| Isotype; n = 83 | |||||||
| IgA | 23 | 15 | 8 | .0059 | |||
| IgG | 43 | 40 | 3 | ||||
| IgD | 3 | 2 | 1 | ||||
| Light chains | 14 | 13 | 1 | ||||
CRP indicates C-reactive protein; Hb, hemoglobin.
Immunohistochemistry
B5-fixed (EMD Chemicals, Gibbstown, NJ) bone marrow aspirate sections and biopsies were decalcified and embedded in paraffin. Sequential 5 μm–thick sections were cut, mounted, dewaxed, and immunostained for CD138 and FGFR3 by the avidin-biotin-peroxidase method (ABC kit, Vectastain; Vector Laboratories, Burlingame, CA). The mouse monoclonal anti-FGFR3 antibody (sc-13121; Santa-Cruz Biotechnology; Santa Cruz, CA) is raised against the amino acids 792 to 806, mapping at the carboxy terminus of the precursor form of human FGFR3.
Briefly, the sections were boiled in 10 mM citrate buffer, pH 6.0, for 25 minutes, stored overnight in the buffer at room temperature, treated with 2% hydrogen peroxide and 10% methanol for 20 minutes, incubated with 10% goat serum for 1 hour, and then incubated overnight at 4°C with one of the primary antibodies. Antibodies to CD138 and FGFR3 were used at dilutions of 1:100 and 1:800, respectively. This incubation was followed by a 30-minute incubation with a biotinylated linking reagent (ID Labs, London, ON, Canada) and a subsequent 30-minute incubation with horseradish peroxidase–conjugated Ultra Streptavidin labeling reagent (ID Labs). The immunoreaction was visualized with freshly prepared NovaRed Solution (Vector Laboratories); the slides were counterstained with Mayer hematoxylin and evaluated with an Olympus BX50 microscope (Olympus, Melville, NY).
The positive control for aberrant expression of FGFR3 was a xenograft tumor from a t(4;14)-positive myeloma cell-line (KMS11)–injected mouse. As a negative control, nonimmune mouse serum was substituted for the primary antibody. We considered cells FGFR3 positive if the membrane and/or cytoplasm of at least 20% of the plasma cells stained. Ten normal bone marrow biopsies (containing less than 5% plasma cells) and 10 B-cell chronic lymphocytic leukemia (CLL)–infiltrated bone marrow biopsies served as additional controls.
cIg-FISH analysis
Ficoll-gradient centrifugation–enriched mononuclear cells were stained by interphase FISH combined with immunofluorescent cytoplasmic light chain staining (cIg-FISH) to optimize the scoring specificity as previously described.7
Statistical analysis
Statistical evaluation used the Fisher exact test, χ2 test, and nonparametric Wilcoxon test. Progression-free survival (PFS) and overall survival (OS) were calculated from the transplantation date by the Kaplan-Meier method. Differences between survival curves were analyzed by the log-rank test.
Results
Immunocytochemistry
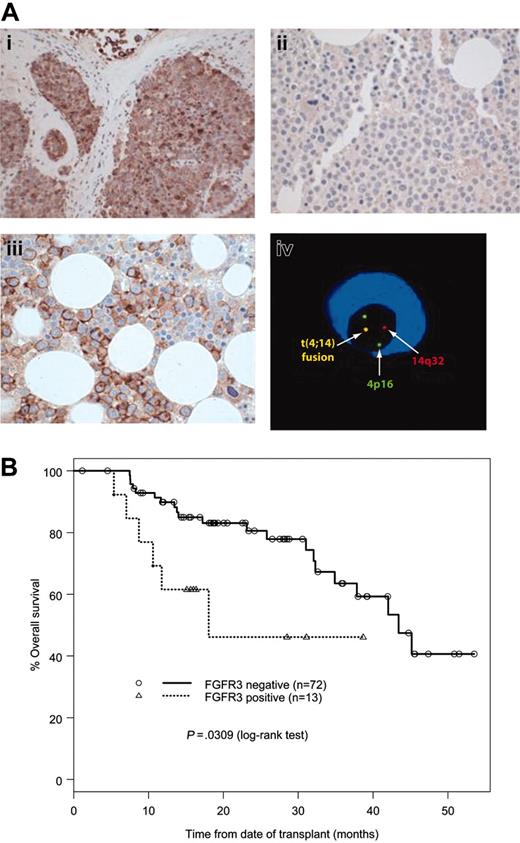
Anti-CD138 stained all myeloma cells in the evaluated cases. In 13 (15%) of 85 cases, more than 50% of the myeloma cells had moderate to strong membrane and/or cytoplasmic FGFR3 immunoreactivity (Figure 1A). The bone marrow hematopoietic cells were FGFR3 negative as were all 10 normal and 10 CLL bone marrow controls. Plasma cells (less than 5%) from normal marrows or CLL marrows were CD138 positive but negative for FGFR3.
Correlation of FGFR3 expression, t(4;14), and clinical features
In 16 (19%) of 85 cases, cIg-FISH detected t(4;14) fusion signals in 54% to 98% of the clonal plasma cells. Twelve (92%) of the 13 FGFR3 IHC-positive cases had a t(4;14), and 12 (75%) of the 16 t(4;14)-positive cases overexpressed FGFR3. The t(4;14) and FGFR3 expression were strongly correlated (P < .001). FGFR3 expression was associated with the IgA isotype (P < .001), but there was no correlation with age, sex, disease stage, lytic bone lesions, bone marrow plasmacytosis, albumin, creatinine, or β2-microglobulin levels (Table 1). Patients with FGFR3 expression by IHC had a significantly shorter OS than those without expression (median, 19.2 versus 46.3 months; P < .001) (Figure 1B). The PFS of FGFR3 IHC-positive patients was also significantly shorter (median, 11.5 versus 25.8 months; P < .001).
Discussion
The t(4;14) is an independent prognostic factor in MM and, because it is predictive for an adverse outcome, a simple, robust, and rapid method to detect t(4;14) would be clinically useful. In this study, we found IHC can detect FGFR3 expression in myeloma cells and is strongly correlated with t(4;14) detected by interphase cIg-FISH. To our knowledge, this is the first report of aberrant expression of FGFR3 detected by IHC in a large MM series. Furthermore, FGFR3 expression in MM is associated with the IgA isotype and a shorter OS or PFS, consistent with the features of t(4;14) detected by interphase FISH4,7
FGFR3 immunoreactivity and OS. (A) Detection of FGFR3 and t(4;14) in MM. Immunohistochemistry study with anti-FGFR3 antibody showed a positive reaction with the tumor tissue from the t(4;14) KMS11 cell line–injected mouse (i), a negative reaction with a bone marrow biopsy from a t(4;14)-negative myeloma patient (ii), and a positive reaction with a bone marrow biopsy from a t(4;14)-positive myeloma patient (iii). cIg-FISH showed a fusion signal indicating a t(4;14) translocation in a myeloma cell (iv). (B) OS of MM patients according to FGFR3 expression by IHC. The image in panel iv was obtained with an Axioscop microscope equipped with UPlanFl 20 ×/0.40 and 40 ×/0.75 objective lenses (Zeiss, Jena, Germany).
FGFR3 immunoreactivity and OS. (A) Detection of FGFR3 and t(4;14) in MM. Immunohistochemistry study with anti-FGFR3 antibody showed a positive reaction with the tumor tissue from the t(4;14) KMS11 cell line–injected mouse (i), a negative reaction with a bone marrow biopsy from a t(4;14)-negative myeloma patient (ii), and a positive reaction with a bone marrow biopsy from a t(4;14)-positive myeloma patient (iii). cIg-FISH showed a fusion signal indicating a t(4;14) translocation in a myeloma cell (iv). (B) OS of MM patients according to FGFR3 expression by IHC. The image in panel iv was obtained with an Axioscop microscope equipped with UPlanFl 20 ×/0.40 and 40 ×/0.75 objective lenses (Zeiss, Jena, Germany).
In this series, 80% of the MM cases were negative for both FGFR3 expression and t(4;14); 14% were positive for both FGFR3 and t(4;14); 4.7% were negative for FGFR3 but were t(4;14) positive; and 1.5% were positive for FGFR3 but were t(4;14) negative. Therefore, the sensitivity of IHC is 75% and the specificity is 98% in predicting t(4;14). We found that 25% of the t(4;14)-positive cases are FGFR3 negative by IHC, in agreement with reports by Keats et al6 and Santra et al16 who identified that 26% and 32% of their MM cases, respectively, lacked FGFR3 expression at the mRNA level while expressing the IgH/multiple myeloma suppressor of var-enhancer of zeste and trithorax domain (MMSET) transcript. An interstitial FGFR3 deletion could explain this finding but, in this series, t(4;14) FISH analysis did not detect an FGFR3 deletion in cases lacking FGFR3 expression. Other cryptic mechanisms that block FGFR3 expression at transcriptional or translational levels may account for this discrepancy.
That 1 (7.7%) of our 13 cases with FGFR3 expression was t(4;14) negative is unexpected and contrasts with a previous study in which t(4;14) was detected in all cases expressing FGFR3 at the mRNA level.18 Although rare, it is possible that FGFR3 activation may be due to gene mutations19 or other mechanisms not involving the IgH locus. A recent study by gene expression profiling to analyze t(4;14)-positive and t(4;14)-negative MM cases found 25% of t(4;14)-negative MM patients overexpressed MMSET.17 Furthermore, a small percentage (about 4%) of t(4;14)-negative patients expresses FGFR3 at low level on gene expression profiling (P. L. Bergsagel, personal communication). It is also possible that our FGFR3-positive, t(4;14)-negative case is an artifact due to nonspecific staining by the anti-FGFR3 monoclonal antibody. However, this is unlikely because, by using Western blot in 8 human myeloma cell lines, we found the anti-FGFR3 monoclonal antibody only reacted with the t(4;14)-positive cell lines but not with the t(4;14)-negative cell lines (data not shown). Our findings suggest that in a subset of MM, alternative pathways activate FGFR3.
In conclusion, paraffin immunohistochemistry reliably detects for aberrant expression of FGFR3 in MM and is strongly correlated with the presence of the t(4;14) translocation and an adverse clinical outcome. Because paraffin immunohistochemistry is routinely available, robust, and inexpensive, we suggest FGFR3 immunocytochemistry of myeloma cells become a panel of routine evaluation because, if present, FGFR3 represents a potential target for kinase inhibitor therapy.
Prepublished online as Blood First Edition Paper, March 10, 2005; DOI 10.1182/blood-2005-01-0033.
Supported in part by the Leukemia Research Fund of Canada.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors thank K. So for technical advice and B. Patterson for helpful comments.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal