It is generally accepted that priming of antitumor CD8+ cytotoxic T lymphocytes (CTLs) needs help that can be provided by CD4+ T cells. We show that interactions between dendritic cells (DCs) and natural killer (NK) cells can bypass the T helper arm in CTL induction. Bone marrow–derived DCs caused rejection of the A20 lymphoma and induced tumor-specific long-term memory, although they were not loaded with tumor-derived antigen. Experiments using CD40- knock-out mice and cell depletion showed that this effect did not require CD4+ cells. Both primary rejection and long-term CTL memory were the result of NK cell activation by DCs. NK cytotoxicity, which was necessary for primary rejection, was dependent on expression of natural killer group 2 D (NKG2D) ligands on tumor cells. Blocking of these ligands using NKG2D tetramers abrogated tumor killing in vitro and in vivo. The long-term response was due to CTLs directed against antigen(s) expressed on A20 and in vitro–differentiated DCs. The mechanism leading to CD4+ helper cell–independent CTL responses was elucidated as a cascade that was initiated by NK cell activation. This pathway was dependent on inter-feron-γ expression and involved priming endogenous DCs for interleukin-12 production. Our data suggest a novel pathway linking innate and adaptive immunity.
Introduction
Induction of efficient immune responses requires a coordinated interplay between innate and adaptive immune effector systems. Dendritic cells (DCs) are components of the innate immune system that activate specific effectors of adaptive immunity.1,2 In an immature state, DCs are able to ingest antigen (Ag). Following a maturation process that involves migration to lymphoid tissues, down-regulation of Ag uptake and upregulation of major histocompatibility complex (MHC) and costimulatory molecules, DCs present antigenic peptides to T lymphocytes.1 Exogenous proteins are taken up and processed by DCs and presented to CD4+ cells in association with MHC class II molecules, whereas intracellular Ags are presented by MHC class I molecules to CD8+ cytotoxic T lymphocytes (CTLs). There is, however, emerging evidence that exogenous proteins can also be directed to the endogenous presentation pathway, thus leading to CTL induction, a process referred to as cross-presentation. Efficient generation of CTLs from naive CD8+ T cells needs help from CD4+ T cells.3-5 This help involves secretion of cytokines and CD40/CD40L interactions that lead to increased expression of costimulatory molecules on DCs and to induction of interleukin-12 (IL-12).6 As shown in mouse models, CD4+ T cells are pivotal for protection against tumors and can even mediate tumor rejection independently of CD8+ T lymphocytes, if they are biased toward a T helper 1 (Th1) response.7 Expression of CD40 by DCs is crucial for the production of Th1 cytokines such as IL-12 and for tumor protection.7,8
Natural killer (NK) cells are effector cells of the innate immune system that exert direct cytotoxic functions.9 These are determined by a finely tuned balance of signals delivered by inhibitory and activating receptors.10-12 Inhibitory receptors bind to MHC class I molecules and down-regulate NK cell cytotoxicity and cytokine production. Thus, missing or low self-MHC expression activates NK cells13 and initiates target cell lysis.14,15 Activating receptors are families of immunoglobulin-like or lectinlike molecules that initialize signaling cascades that are similar to those in T cells. A well-characterized activating receptor on human and murine NK cells is natural killer group 2D (NKG2D) that interacts with H60, RAE1, and MULT1 molecules in the mouse.16-18 Triggering of NKG2D was shown to induce NK cell–mediated tumor rejection.19-22
Interestingly, NK cell activation against tumors was associated with the induction of specific T-cell responses in several mouse models, a finding that was not confirmed19,22 in all studies. CTLs against tumors were induced when NK cells were activated by NKG2D ligand binding,23 by CD70- or CD80-expressing RMA-S cells24,25 or by MHC class I–suppressed tumor cells.26 The involvement of CD4+ T cells in NK cell–mediated CTL induction is controversially discussed. In one study, CD8+ T-cell–dependent tumor rejection initiated by NK cells was attributed to a type 1 switch in Ag-presenting cells, but the participation of T helper cells remained open in this investigation.27 In another study, it was shown that type 1 DCs may have contributed to the development of a Th1 response,28 whereas a recent report demonstrated that NK cell–mediated tumor rejection and tumor-specific CTL induction require a Th1-independent CD4+ T-cell pathway.23
Here, we present evidence that the interplay between DCs and NK cells can completely replace CD4+ T-cell help in the induction of CD8+ CTLs. We make use of a system in which rejection of MHC class I–expressing tumors and T-cell memory responses are initiated by unpulsed DCs. We show that rejection and memory are tumor specific, dependent on CD8+ CTLs but entirely independent of CD4+ T cells. Our results reveal that DC-NK cell interactions provide a novel alternative pathway for CTL induction that circumvents the T helper arm of the immune system.
Materials and methods
Cell culture
The B-cell lymphoma cell lines A20 and MPC11 and the colon carcinoma cell line CT26 were cultured in RPMI 1640 supplemented with 5% fetal calf serum, 2 mM glutamine, nonessential amino acids, antibiotics, and 50 μM 2-mercaptoethanol. All cells were maintained at 37°C in a humidified 5% CO2 atmosphere. For preparation of DCs, bone marrow cells were harvested from BALB/c wild-type or CD40-/- mice or from C57BL/6 mice and cultured in standard medium in the presence of 100 ng/mL recombinant murine granulocyte-macrophage colony-stimulating factor (GM-CSF).29 Medium was replaced every 2 days, and a maturation step was performed at day 8 by adding 1 μg/mL lipopolysaccharide (LPS) for 16 hours. If not otherwise indicated, only mature DCs were used in the experiments. DCs were characterized by fluorescence-activated cell sorter (FACS) analyses using monoclonal antibodies (mAbs) against MHC I, MHC II, CD80, CD86, and CD40. mAbs were purchased from Pharmingen (San Diego, CA). No phenotypical differences were seen when DCs were cultured in medium containing IL-4 in addition to GM-CSF.
Animal experiments
BALB/c and C57BL/6 mice were purchased from Bommice (Ry, Denmark), CD40-/- mice were from The Jackson Laboratories (Bar Harbor, ME), and severe combined immunodeficient (SCID) mice were bred in our own animal facility. All animal experiments had been approved by the Regierung von Oberbayern and were in accordance with relevant regulations. Groups of at least 6 female animals received DCs twice subcutaneously in a 1-week interval, and after another 7 days an intravenous challenge of 8 × 105 A20, 105 MPC11, or 105 CT26 cells. In a therapeutic situation, DCs were delivered 5 days following tumor challenge. In rechallenge experiments, mice were injected intravenously with a lethal A20 dose 75 to 150 days after DC vaccination. Mice were killed when showing signs of tumor growth. In some experiments, DCs or A20 cells were injected that were coated with NKG2D tetramer in a 1:5 or 1:100 dilution, respectively. For depletion of NK cells, the IL-2 receptor β chain-specific mAb TMβ1 was given intraperitoneally 4 days before and 2 and 14 days after tumor challenge.26 Because of their lower expression density of IL-2 receptor β chain, T cells were not eliminated by this mAb (not shown). Alternately, mice received rabbit anti–asialo GM1 antiserum (Wako, Neuß, Germany) intravenously 3 days before and 4 and 11 days after tumor injection. Depletion with TMβ1 and anti–asialo GM1, respectively, yielded the same results in all experiments. Depletion of T cells and T-cell subsets was done using mAb MmT1, RmCD4-2, or RmCD8-2, as described elsewhere.26 To neutralize IL-12, 0.3 mg of the mAb C17.830 was injected intraperitoneally 4 hours before and 1, 3, and 7 days after DC treatment. Interferon γ (IFN-γ) was neutralized by injecting 0.5 mg XMG-1.2 mAb intraperitoneally 24 and 4 hours prior to DC delivery.7 In adoptive transfer experiments, mice received 5 × 105 T cells intravenously along with a lethal A20 challenge. To stimulate NK cells in SCID mice, 10 nMol cytidine phosphate guanosine (CpG)–oligonucleotide 1668 (MWG, München, Germany) was injected intraperitoneally 1 day before and after each DC treatment. All animal experiments were performed twice to 5 times. The figures show typical results. Statistical analysis was done using the log-rank test. All results shown were between P less than .01 and P less than .005.
Tetramer staining
NKG2D multimers were generated as described previously.31 Briefly, the extracellular domain of NKG2D was fused at the N-terminus to a biotinylation sequence and expressed as inclusion bodies in Escherichia coli. Purified protein was efficiently refolded in an arginine-rich buffer as it has been described for refolding of MHC molecules.32 Multimerization was achieved by in vitro biotinylation and consecutive incubation with phycoerythrin (PE)–conjugated streptavidin (Molecular Probes, Leiden, The Netherlands). Tumor cells were labeled with PE-conjugated NKG2D tetramer and analyzed in the FACS.
T-cell analyses ex vivo
Four days after injection of mice with DCs, spleens were isolated. If necessary CD8+ T cells were enriched by negative selection over Biotex T-cell columns (TEBU, Frankfurt, Germany) to 98% purity. T cells were stimulated in vitro with irradiated A20 cells, MPC11 cells, DCs, or medium. After 24 hours, interferon-γ (IFN-γ) in the supernatant was measured by enzyme-linked immunosorbent assay (ELISA). Part of the cells was further expanded for 9 days with 5 U/mL IL-2 (Chiron, Ratingen, Germany) in the presence of irradiated A20 cells or DCs, and reactivity was tested against A20, MPC11, CT26, and DCs as outlined above.
Cytotoxicity assays
NK cells from naive animals or from mice injected with DCs 7 days before were isolated from spleens by immunomagnetic separation (Miltenyi, Bergisch-Gladbach, Germany) using positive selection with the NK cell–specific mAb DX5 or negative selection with the NK Cell Isolation Kit (Miltenyi). FACS analyses showed that CD3-DX5+ cells were enriched to about 90% homogeneity and that no CD3+ T cells were present. Cytotoxicity was determined against A20, MPC11, and YAC cells in a standard chromium release assay. Target cells were incubated for 1 hour at 37°C with
Cytokine detection
IFN-γ was detected in culture supernatants by ELISA using the mAb pairs R4-6A2 and XMG-1.2 and by following the manufacturer's instructions (Pharmingen). For the quantitation of IFN-γ–expressing cells in vivo, splenocytes were isolated and subjected to an IFN-γ capture assay (Miltenyi). The cells were counterstained with mAb against CD3 and CD122 (to identify CD3- NK cells) or CD3 and CD8 (to identify CD8+ T cells) and analyzed by FACS. IL-12–expressing DCs were recovered by intracellular FACS staining. After staining with fluorescein isothiocyanate–conjugated CD11c and PE-coupled CD40 mAbs, cells were fixed in paraformaldehyde and permeabilized in saponin. IL-12 was detected with cyanine 5 (Cy5)–labeled C17.8 mAb.
Results
Unpulsed DCs activate NK cells to reject the A20 B-cell lymphoma
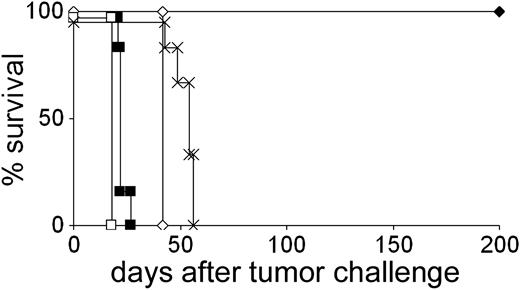
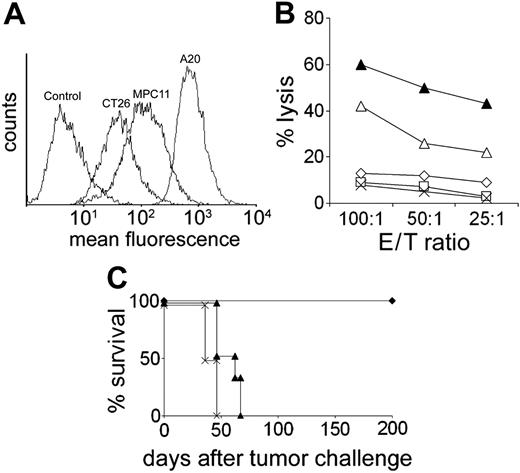
A20 is an aggressive BALB/c-derived B-cell lymphoma frequently used as a model system to investigate tumor vaccination protocols.33 In our attempt to modify ex vivo–generated DCs to present tumor-derived antigenic peptides, we noticed that syngeneic bone marrow–derived DCs protected 100% of mice against a subsequent challenge with a lethal dose of A20 cells even when the DCs were not loaded with tumor-derived Ags (Figure 1). This robust and reproducible protection was not due to priming against culture medium components and was not observed using the syngeneic lymphoma MPC11 (Figure 1) or the colon carcinoma CT26 (not shown). Experiments using NK cell–depleting antibodies showed that rejection of A20 was strictly dependent on NK cells (Figure 1). The ability of DCs to activate NK cells to eliminate tumor cells has been well established.34 It was unclear, however, why NK cell–dependent tumor rejection was confined to A20 in our experiments. To shed light on the mechanism underlying the differential rejection of A20, MPC11, and CT 26 after injection of unpulsed DCs, we compared these tumor cell lines with regard to their expression of ligands for NKG2D, which has been identified as a natural cytotoxicity receptor on NK cells.22,23 FACS analyses using an NKG2D tetramer demonstrated that expression of ligands for NKG2D was up to 18 times higher on A20 cells as compared with MPC11 and CT26 cells (Figure 2A).
To study the functional consequence of this finding, we performed cytotoxicity experiments in vitro using NK cells that were isolated from spleens of naive or DC-treated mice. The cytotoxic activity against A20 and YAC cells was markedly elevated in NK cells derived from DC-immunized mice compared with NK cells from untreated animals (Figure 2B). Thus, NK cells had been activated in vivo by injection of unpulsed DCs. In contrast, cytotoxicity directed against MPC11 and CT26 could never be generated (Figure 2B). This result exactly paralleled the differential NK cell–dependent tumor elimination observed in vivo. To examine whether A20 lysis was due to NKG2D/NKG2D-L interactions, we performed cytotoxicity assays using A20 targets whose NKG2D-L surface molecules were blocked with saturating amounts of NKG2D tetramer. Indeed, lysis ofA20 cells was reduced nearly to background levels (Figure 2B). We then asked whether NKG2D/NKG2D-L interactions were also relevant for killing in vivo. To preclude a possible direct activation of T cells by DCs we used mature CD40-/- DCs that could not directly interact with T cells but conferred robust protection against A20 (see “NK cell–mediated, CD4+ T-cell–independent CTL induction”). DCs were no longer able to prevent A20 growth when the NKG2D-L molecules on the tumor cells were blocked with NKG2D tetramer (Figure 2C). The results show that DCs activate NK cells to kill only A20 cells, even though NK cell activation by DCs is an unspecific event. The data suggest that the expression level of NKG2D-L determines whether or not DC-activated NK cells will recognize and kill tumor cells in vivo. In contrast, blocking of NKG2D ligands on DCs had no effect on tumor protection in vivo, suggesting that NKG2D/NKG2D-L interactions were not relevant for NK cell activation by DCs in this system (not shown).
Rejection of A20 but not of MPC11 lymphoma cells after immunization with unpulsed DCs. Mice received 2 injections of unpulsed DCs and a subsequent challenge with A20 or MPC11 cells. Mice remained tumor-free life long. For NK cell depletion, mAb TMβ1 was used. In all NK cell–depletion experiments, the same results were obtained using anti–asialo GM1 antiserum.  indicates DC + A20; ⋄, A20 control; ▪,DC + MPC11;□, MPC11 control; and ×,DC + A20 + NK cell depletion.
indicates DC + A20; ⋄, A20 control; ▪,DC + MPC11;□, MPC11 control; and ×,DC + A20 + NK cell depletion.
Rejection of A20 but not of MPC11 lymphoma cells after immunization with unpulsed DCs. Mice received 2 injections of unpulsed DCs and a subsequent challenge with A20 or MPC11 cells. Mice remained tumor-free life long. For NK cell depletion, mAb TMβ1 was used. In all NK cell–depletion experiments, the same results were obtained using anti–asialo GM1 antiserum.  indicates DC + A20; ⋄, A20 control; ▪,DC + MPC11;□, MPC11 control; and ×,DC + A20 + NK cell depletion.
indicates DC + A20; ⋄, A20 control; ▪,DC + MPC11;□, MPC11 control; and ×,DC + A20 + NK cell depletion.
Lysis of tumor cells in vitro and in vivo is dependent on NKG2D ligands expressed on the tumor cells. (A) Staining of tumor cells by PE-labeled NKG2D tetramer. As a control, cells were incubated with PE-conjugated streptavidin. (B) Cytotoxicity of NK cells isolated from naive or DC-treated mice against A20, MPC11, and YAC cells. Cytotoxicities against CT26 and against MPC11 were identical. In some experiments, A20 cells were blocked with NKG2D tetramer. □ indicates naive mouse, A20 target; ▵, DC mouse, A20 target; ▴, DC mouse, YAC target; ⋄, DC mouse, A20 target + tetramer; and ×, DC mouse, MPC11 target. E/T ratio indicates effector-target ratio. (C) Preincubation of A20 cells with NKG2D tetramer abrogates the protective effect of DC.  indicates DC + A20; ▴, DC + A20 + tetramer; and ×, tumor control.
indicates DC + A20; ▴, DC + A20 + tetramer; and ×, tumor control.
Lysis of tumor cells in vitro and in vivo is dependent on NKG2D ligands expressed on the tumor cells. (A) Staining of tumor cells by PE-labeled NKG2D tetramer. As a control, cells were incubated with PE-conjugated streptavidin. (B) Cytotoxicity of NK cells isolated from naive or DC-treated mice against A20, MPC11, and YAC cells. Cytotoxicities against CT26 and against MPC11 were identical. In some experiments, A20 cells were blocked with NKG2D tetramer. □ indicates naive mouse, A20 target; ▵, DC mouse, A20 target; ▴, DC mouse, YAC target; ⋄, DC mouse, A20 target + tetramer; and ×, DC mouse, MPC11 target. E/T ratio indicates effector-target ratio. (C) Preincubation of A20 cells with NKG2D tetramer abrogates the protective effect of DC.  indicates DC + A20; ▴, DC + A20 + tetramer; and ×, tumor control.
indicates DC + A20; ▴, DC + A20 + tetramer; and ×, tumor control.
Unpulsed DCs induce a tumor-specific T-cell–dependent memory
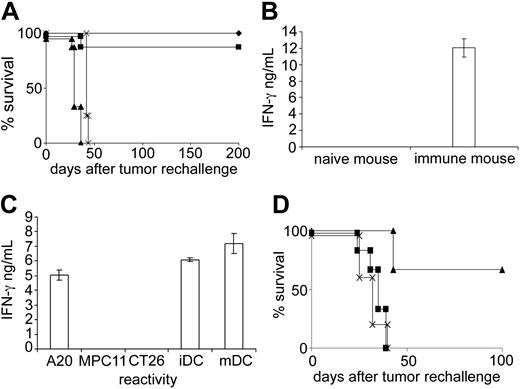
Injection of unpulsed DCs not only gave rise to protection against a subsequent A20 challenge but also induced long-lasting immunologic memory, as mice rejected an A20 rechallenge even 80 or 150 days after DC treatment (Figure 3A). The memory was A20 specific and could not be abolished by eliminating NK cells at the time of tumor rechallenge, which underscores the contention that NK cells do not provide memory functions. Treatment with a T-cell–depleting mAb, however, completely abrogated long-term protection (Figure 3A). Thus, NK cell activity was necessary for primary rejection, whereas a T-cell response appeared responsible at least for long-term protection. Importantly, the A20-specific T-cell–mediated memory was also induced when animals did not receive tumor cells after DC immunization so that cross-priming by components of lysed A20 cells was excluded (not shown). This suggested that unpulsed DCs induced T cells capable of recognizing A20. To test this hypothesis, we isolated CD8+ T cells from spleens of DC-immunized and naive mice and compared their reactivity against A20. Indeed, we could detect T cells that secreted IFN-γ in response to A20 in spleens of DC-treated mice, but not in spleens from naive animals (Figure 3B). When T cells were further cultured in the presence of either A20 or DCs for 9 days, it was even possible to derive T-cell lines. Cells showed specificity for A20 as well as for autologous, unpulsed DCs but not for MPC11 or CT26 (Figure 3C). Comparable T-cell lines could not be derived from spleens of untreated animals. The A20 reactivity of DC-induced T cells in vitro was also confirmed in vivo. Although the T-cell lines were derived from animals that were only immunized with unloaded DCs, they were able to eradicate the A20 tumor upon adoptive transfer (Figure 3D). This was not possible using T cells derived from naive or tumor-bearing mice. Thus, it is possible that DCs present Ag(s) which give rise to T cells cross-reacting with A20. The immunizing potential of DCs was dependent on the in vitro differentiation step in the presence of GM-CSF because precursor cells isolated ex vivo did not induce immunity (not shown).
A20-specific T-cell memory following immunization with unloaded DCs. (A) Mice that had rejected a primary A20 challenge after DC injection were rechallenged with a lethal dose of A20 lymphoma 150 days later ( ). Other groups were additionally treated with the NK cell–depleting mAb TMβ1 (▪) or the anti–T-cell mAb MmT1 (▴) during the effector phase. × indicates tumor control. (B) CD8+ T cells were isolated from spleens of naive or immune mice 4 days after DC injection. IFN-γ secretion was determined in the presence of irradiated A20 cells. Results were corrected for IFN-γ secretion in the absence of A20. (C) T cells were further stimulated for 9 days with DCs, and reactivity was determined against A20, MPC11, CT26, and bone marrow–derived DCs that were or were not treated with LPS (mDC and iDC, respectively). IFN-γ secretion in medium alone was subtracted. Error bars in panels B and C indicate standard deviation. (D) T cells were isolated from DC-immunized (▴) or untreated (▪) mice, stimulated in vitro, and adoptively transferred to naive mice together with a lethal A20 challenge. × indicates tumor control. T cells from mice that were only injected with A20 had no effect.
). Other groups were additionally treated with the NK cell–depleting mAb TMβ1 (▪) or the anti–T-cell mAb MmT1 (▴) during the effector phase. × indicates tumor control. (B) CD8+ T cells were isolated from spleens of naive or immune mice 4 days after DC injection. IFN-γ secretion was determined in the presence of irradiated A20 cells. Results were corrected for IFN-γ secretion in the absence of A20. (C) T cells were further stimulated for 9 days with DCs, and reactivity was determined against A20, MPC11, CT26, and bone marrow–derived DCs that were or were not treated with LPS (mDC and iDC, respectively). IFN-γ secretion in medium alone was subtracted. Error bars in panels B and C indicate standard deviation. (D) T cells were isolated from DC-immunized (▴) or untreated (▪) mice, stimulated in vitro, and adoptively transferred to naive mice together with a lethal A20 challenge. × indicates tumor control. T cells from mice that were only injected with A20 had no effect.
A20-specific T-cell memory following immunization with unloaded DCs. (A) Mice that had rejected a primary A20 challenge after DC injection were rechallenged with a lethal dose of A20 lymphoma 150 days later ( ). Other groups were additionally treated with the NK cell–depleting mAb TMβ1 (▪) or the anti–T-cell mAb MmT1 (▴) during the effector phase. × indicates tumor control. (B) CD8+ T cells were isolated from spleens of naive or immune mice 4 days after DC injection. IFN-γ secretion was determined in the presence of irradiated A20 cells. Results were corrected for IFN-γ secretion in the absence of A20. (C) T cells were further stimulated for 9 days with DCs, and reactivity was determined against A20, MPC11, CT26, and bone marrow–derived DCs that were or were not treated with LPS (mDC and iDC, respectively). IFN-γ secretion in medium alone was subtracted. Error bars in panels B and C indicate standard deviation. (D) T cells were isolated from DC-immunized (▴) or untreated (▪) mice, stimulated in vitro, and adoptively transferred to naive mice together with a lethal A20 challenge. × indicates tumor control. T cells from mice that were only injected with A20 had no effect.
). Other groups were additionally treated with the NK cell–depleting mAb TMβ1 (▪) or the anti–T-cell mAb MmT1 (▴) during the effector phase. × indicates tumor control. (B) CD8+ T cells were isolated from spleens of naive or immune mice 4 days after DC injection. IFN-γ secretion was determined in the presence of irradiated A20 cells. Results were corrected for IFN-γ secretion in the absence of A20. (C) T cells were further stimulated for 9 days with DCs, and reactivity was determined against A20, MPC11, CT26, and bone marrow–derived DCs that were or were not treated with LPS (mDC and iDC, respectively). IFN-γ secretion in medium alone was subtracted. Error bars in panels B and C indicate standard deviation. (D) T cells were isolated from DC-immunized (▴) or untreated (▪) mice, stimulated in vitro, and adoptively transferred to naive mice together with a lethal A20 challenge. × indicates tumor control. T cells from mice that were only injected with A20 had no effect.
If in vitro–generated DCs express A20-related Ag, it would be predicted that they directly stimulate CD4+ and CD8+ T lymphocytes through presentation of antigenic peptides by MHC class II and class I molecules, respectively. Yet this classic T-cell activation pathway was obviously not sufficient to secure primary rejection, as NK cells were absolutely required for tumor protection. Like-wise, the classic pathway alone was not able to elicit long-term protection because memory was not induced when mice were depleted of NK cells during the initiation phase (not shown). This indicates that the T-cell Ag(s) of DCs and A20 were weak immunogens. Nevertheless, potent Ag-specific CTL responses could be mounted if activation occurred via a DC-NK cell axis.
NK cell–mediated, CD4+ T-cell–independent CTL induction
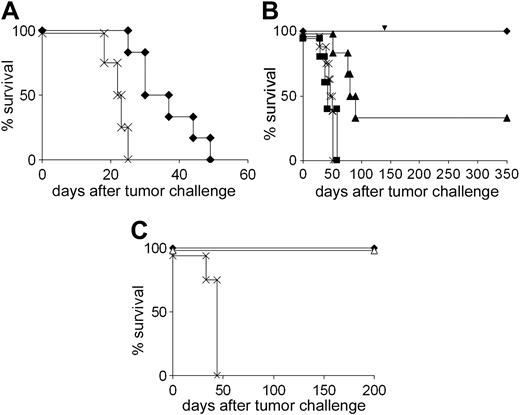
Since CTL induction was at least partly due to DC-NK cell interaction and not or not only to direct priming, we set out to dissect the contribution of these 2 pathways to the immune response. First, we asked whether NK cells alone would be capable of eliminating A20 if their activation was strong enough. To this end, SCID mice that have no functional B and T cells were treated with DCs along with CpG oligonucleotides, which are known to stimulate NK cells.35 The combined NK cell–activating effect of both DCs and CpG-DNA was sufficient to provide a modest but statistically significant survival benefit (Figure 4A).
To analyze the specific role of NK cell activation for CTL induction and development of memory, it was necessary to use immunocompetent mice and to disrupt direct interactions between DCs and tumor-specific T cells. This was done by using DCs from CD40-deficient animals which cannot directly stimulate T cells. In this setting, up to 100% of the animals rejected an A20 challenge (Figure 4B). CD8+ T-cell–dependent long-term protection was also generated (Figure 4B). As CD40-/- DCs could not directly stimulate T cells, the data suggest that the NK cells received stimulation signals from DCs, which were sufficient to promote direct NK cytotoxicity as well as CTL induction.
The ability of CD40-/- DCs to mediate primary tumor rejection was totally abrogated by NK cell depletion and partially inhibited by depleting the CD8+ T cells (Figure 4B). These depletion experiments as well as the SCID mouse experiments, where only prolonged survival but no life-long protection could be achieved, indicated that apart from NK cells, CD8+ T cells also contribute to primary tumor rejection. In depletion experiments using BALB/c-derived DCs, the role of CD8+ T cells in mediating primary rejection was confirmed (not shown). However, the fact that NK cell depletion completely abolished tumor immunity in contrast to CD8+ T-cell depletion (Figure 4B) indicates a central role of NK cells which exert both a direct cytotoxic function and a stimulatory function in the pathway that leads to CTL priming. On the other hand, the ability of CD40-/- DCs to induce robust tumor protection indicated that CD4+ T-cell help was not required for priming of CD8+ cells. To directly demonstrate that NK cell–induced CTL memory was independent of the T helper arm, mice were immunized with BALB/c-derived DCs and depleted of CD4+ cells in the priming phase and were challenged with A20 10 weeks later. All animals survived (Figure 4C). In summary, the data show that activation signals delivered by DCs to NK cellsare strong enough to bypass the need of CD4+ T-cell help for CTL induction.
Contribution of T cells and NK cells to DC-mediated protection against A20. (A) SCID mice received DC with ( ) or without (×) CpG oligonucleotide as described in “Materials and methods” and were challenged with A20 lymphoma. (B) DCs from CD40-/- mice were used for vaccination of BALB/c mice that were challenged with A20 1 week after the second immunization.
) or without (×) CpG oligonucleotide as described in “Materials and methods” and were challenged with A20 lymphoma. (B) DCs from CD40-/- mice were used for vaccination of BALB/c mice that were challenged with A20 1 week after the second immunization.  indicates without depletion; ▪, NK cell depletion; ▴, CD8+ T-cell depletion; and ×, tumor control. The arrow denotes the time point of tumor rechallenge. (C) Long-term memory is independent of CD4+ T cells. BALB/c-derived DCs were used for vaccination of mice that received the CD4+ cell-depleting mAb RmCD4-2 during the priming phase. A20 cells were given 70 days later.
indicates without depletion; ▪, NK cell depletion; ▴, CD8+ T-cell depletion; and ×, tumor control. The arrow denotes the time point of tumor rechallenge. (C) Long-term memory is independent of CD4+ T cells. BALB/c-derived DCs were used for vaccination of mice that received the CD4+ cell-depleting mAb RmCD4-2 during the priming phase. A20 cells were given 70 days later.  indicates without depletion; ▵, CD4+ T-cell depletion; and ×, tumor control.
indicates without depletion; ▵, CD4+ T-cell depletion; and ×, tumor control.
Contribution of T cells and NK cells to DC-mediated protection against A20. (A) SCID mice received DC with ( ) or without (×) CpG oligonucleotide as described in “Materials and methods” and were challenged with A20 lymphoma. (B) DCs from CD40-/- mice were used for vaccination of BALB/c mice that were challenged with A20 1 week after the second immunization.
) or without (×) CpG oligonucleotide as described in “Materials and methods” and were challenged with A20 lymphoma. (B) DCs from CD40-/- mice were used for vaccination of BALB/c mice that were challenged with A20 1 week after the second immunization.  indicates without depletion; ▪, NK cell depletion; ▴, CD8+ T-cell depletion; and ×, tumor control. The arrow denotes the time point of tumor rechallenge. (C) Long-term memory is independent of CD4+ T cells. BALB/c-derived DCs were used for vaccination of mice that received the CD4+ cell-depleting mAb RmCD4-2 during the priming phase. A20 cells were given 70 days later.
indicates without depletion; ▪, NK cell depletion; ▴, CD8+ T-cell depletion; and ×, tumor control. The arrow denotes the time point of tumor rechallenge. (C) Long-term memory is independent of CD4+ T cells. BALB/c-derived DCs were used for vaccination of mice that received the CD4+ cell-depleting mAb RmCD4-2 during the priming phase. A20 cells were given 70 days later.  indicates without depletion; ▵, CD4+ T-cell depletion; and ×, tumor control.
indicates without depletion; ▵, CD4+ T-cell depletion; and ×, tumor control.
CTL induction involves endogenous DCs, IFN-γ, and IL-12
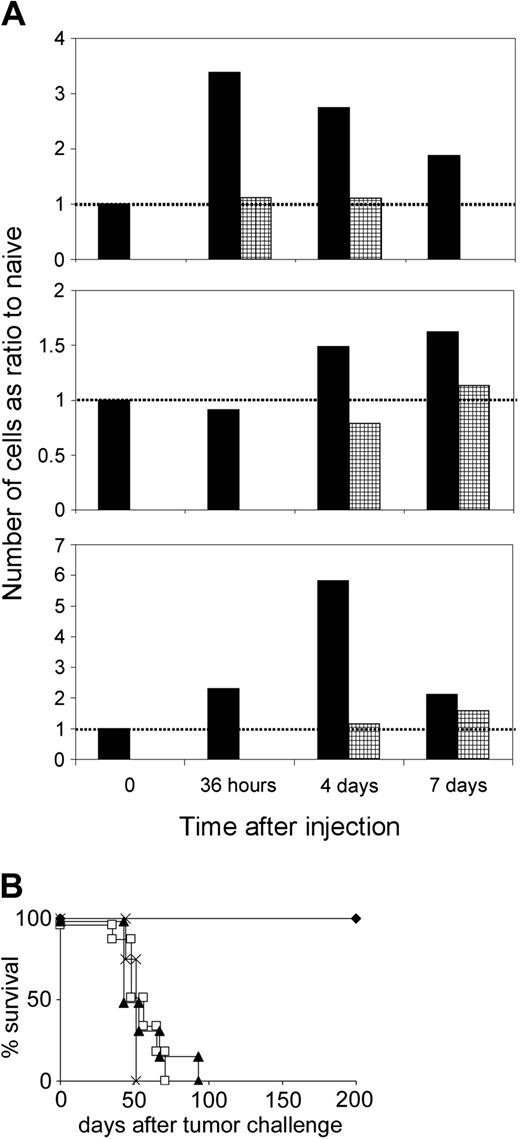
We hypothesized that NK cells, which are activated by DCs to secrete IFN-γ, might induce IL-12 expression in DCs, which then prime a potent CTL response. Because NK cells, which have been activated by transferred DCs, will also lead to CTL priming via IFN-γ stimulation of endogenous DCs, this concept would provide an explanation for CTL induction by CD40-/- DCs, which are not capable of stimulating CTLs per se. Therefore, we determined the IFN-γ and IL-12 expression in different lymph node cell populations at various time points following injection of CD40-/- DCs using flow cytometry. A comparison of naive and DC-immunized mice showed that there was an increase in the number of IFN-γ–secreting NK cells in the draining lymph nodes 36 hours after immunization, whereas the number of IFN-γ–expressing T-cell blasts peaked at day 4 (Figure 5A). At this time point, there was also an activation of endogenous DCs, as detected by an increase of IL-12–producing CD11c+CD40+ cells. Treatment of mice with an IFN-γ–neutralizing mAb abolished the induction of cytokine-producing cells after DC immunization (Figure 5A). Obviously, IFN-γ was necessary for NK cell activation, activation of endogenous DCs, and CTL induction. Injection of DCs leads to a rapid activation of NK cells, followed by production of IL-12 in endogenous DCs, and activation of CD8+ cells.
Unpulsed DCs activate NK cells to induce IL-12 in endogenous DCs and to prime CTL responses. (A) Mice were treated with CD40-/- DCs without (▪) or with ( ) anti–IFN-γ mAb. Draining lymph nodes were taken at different time points, the cells counted, and the different cell populations analyzed by flow cytometry. The total number of cytokine-positive cells was calculated and expressed as a ratio compared with the number calculated for naive mice at each time point (value = 1 for naive mice, indicated as dotted line). Typical result from 2 independent experiments with identical outcome. (B) Role of IFN-γ and IL-12 in vivo. Mice treated with anti–IFN-γ (□), anti–IL-12 mAb (▴), or irrelevant isotype-matched mAb (
) anti–IFN-γ mAb. Draining lymph nodes were taken at different time points, the cells counted, and the different cell populations analyzed by flow cytometry. The total number of cytokine-positive cells was calculated and expressed as a ratio compared with the number calculated for naive mice at each time point (value = 1 for naive mice, indicated as dotted line). Typical result from 2 independent experiments with identical outcome. (B) Role of IFN-γ and IL-12 in vivo. Mice treated with anti–IFN-γ (□), anti–IL-12 mAb (▴), or irrelevant isotype-matched mAb ( ) received 2 immunizations with CD40-/- DCs and were challenged with A20 lymphoma 1 week after the second vaccination. × indicates tumor control.
) received 2 immunizations with CD40-/- DCs and were challenged with A20 lymphoma 1 week after the second vaccination. × indicates tumor control.
Unpulsed DCs activate NK cells to induce IL-12 in endogenous DCs and to prime CTL responses. (A) Mice were treated with CD40-/- DCs without (▪) or with ( ) anti–IFN-γ mAb. Draining lymph nodes were taken at different time points, the cells counted, and the different cell populations analyzed by flow cytometry. The total number of cytokine-positive cells was calculated and expressed as a ratio compared with the number calculated for naive mice at each time point (value = 1 for naive mice, indicated as dotted line). Typical result from 2 independent experiments with identical outcome. (B) Role of IFN-γ and IL-12 in vivo. Mice treated with anti–IFN-γ (□), anti–IL-12 mAb (▴), or irrelevant isotype-matched mAb (
) anti–IFN-γ mAb. Draining lymph nodes were taken at different time points, the cells counted, and the different cell populations analyzed by flow cytometry. The total number of cytokine-positive cells was calculated and expressed as a ratio compared with the number calculated for naive mice at each time point (value = 1 for naive mice, indicated as dotted line). Typical result from 2 independent experiments with identical outcome. (B) Role of IFN-γ and IL-12 in vivo. Mice treated with anti–IFN-γ (□), anti–IL-12 mAb (▴), or irrelevant isotype-matched mAb ( ) received 2 immunizations with CD40-/- DCs and were challenged with A20 lymphoma 1 week after the second vaccination. × indicates tumor control.
) received 2 immunizations with CD40-/- DCs and were challenged with A20 lymphoma 1 week after the second vaccination. × indicates tumor control.
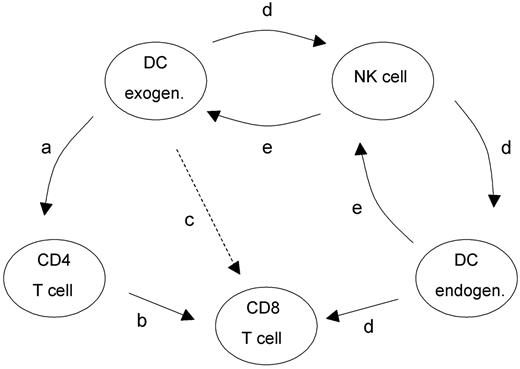
Proposed mechanism of T helper cell–independent CTL induction initiated by exogenously delivered unpulsed DCs. For details, see “Discussion.”
Proposed mechanism of T helper cell–independent CTL induction initiated by exogenously delivered unpulsed DCs. For details, see “Discussion.”
The central role of IFN-γ and IL-12 in the NK cell–dependent induction of T-cell immunity was confirmed in vivo. Neutralization of cytokines with specific anti–IFN-γ or anti–IL-12 mAb substantially decreased the rate of tumor rejection after immunization with CD40-/- DCs (Figure 5B). This demonstrates that IL-12 secretion is also mandatory for CTL induction.
Discussion
Our results assign a new role for DC-NK cell cross talk in the immune response. We show that the interaction between DCs and NK cells obviates the need for CD4+ T-cell help in the induction of CTL responses. Furthermore, we show that unpulsed DCs can lead to A20-restricted T-cell activation and memory without the requirement for exogenous Ag.
Two activation pathways are likely to give rise to CTL priming (Figure 6): The classic pathway3-5 involves presentation of peptides by DCs to CD4+ T cells (a in Figure 6), provision of T-cell help (b in Figure 6) and cross-presentation of Ag to CD8+ lymphocytes (c in Figure 6). The novel mechanism (d in Figure 6) proposed in this paper is mediated by NK cells that are activated after transfer of DCs. By secreting IFN-γ, the activated NK cells stimulate DCs, including endogenous DCs, to produce IL-12, leading to induction of CD8+ lymphocytes. This conclusion is supported by experiments whereby neutralization of IFN-γ inhibited IL-12 production by DCs as well as CTL induction (Figure 5A). In vivo experiments showed that apart from IFN-γ, IL-12 but not IL-10 played a pivotal functional role in the induction of T-cell immunity (Figure 5B and data not shown). Thus, IFN-γ and IL-12 are instrumental in the T helper cell–independent pathway that links the DC-NK cell cross talk to CTL immunity.
Primary rejection of A20 cells following immunization with unpulsed DCs required NK cells as well as CD8+ T cells, whereas long-term memory only needed CD8+ T cells. DCs were able to support the CD8+ T-cell response and the generation of long-term memory without CD4+ T-cell help. A DC-NK cell axis (d in Figure 6) was sufficient to induce CTLs. Whereas CD4+ T-cell depletion did not ablate CTL activity, DC-mediated CTL priming was abrogated by depleting the NK cells. The question as to how DCs activate NK cells was not addressed in detail in our paper. The reciprocal stimulation of DCs and NK cells (d and e in Figure 6) has been well documented34,36-38 and is thought to play an important role in the regulation of innate and adaptive immunity.39 Thus, DCs can stimulate NK cells to exert lytic functions and to secrete IFN-γ.34,40,41 Conversely, NK cells can contribute to further maturation of DCs.36-38 IFN-γ, IL-12, and direct cell contacts were shown to be instrumental in this bidirectional cross talk.34,37,40 In this context, we assume that the cytokine profile and the expression of surface molecules in DCs are instrumental for the cross talk between DCs and NK cells. In contrast to MHC class I–low targets,26 unpulsed DCs seemed to require expression of IFN-γ for activation of NK cells because neutralization of IFN-γ inhibited NK cell activation (Figure 5A). CD40 expression by DCs was obviously not necessary for NK cell activation, since CD40-deficient DCs were as effective as CD40+/+ DCs in vivo. The need for CD40-CD40L ligation in DC-NK cell interactions8 may be obviated by the up-regulation of other molecules during the DC maturation step, which compensate for the lack of CD40.
The redundancy of CD4+ cells after immunization with DCs was supported by experiments using CD40-/- DCs (Figure 4B) or allogeneic DCs (not shown), both of which prevent the cognate interactions of CD4+ T cells with DCs. Stimulation of antitumor CTLs and immunologic memory were achieved in both cases, and additional depletion of CD4+ cells showed that no CD4+ T-cell cytokine secretion was necessary for NK cell activation or CTL induction (not shown). The possibility that CTL induction by allogeneic DCs might be dependent on cytokine milieus provided by alloreactive CD4+ cells can be ruled out by these depletion experiments.
NK cells that were activated by DCs exerted cytotoxicity against A20 but not against the other tumor cells tested. While MHC class I expression was identical on all tumor cell lines, there was a differential expression of NKG2D ligands16-18 that interact with NKG2D-activating receptors expressed by NK cells. MPC11 and CT26 expressed NKG2D ligands to a much lower extent than was seen on A20 cells. We assume that there is a threshold of NKG2D ligand expression below of which tumor cells cannot be killed by NK cells. The nature of the NKG2D ligands involved is not yet clear and will be addressed in future studies. However, in addition to NK cell recognition, CTL memory was also specific for the A20 tumor. Lysis of A20 cells by DC-activated NK cells, leading to release of tumor-associated Ags, could explain the early induction of a specific T-cell response after administration of the A20 cells. Because DCs were able to induce specific CTLs in the absence of A20 cells, we concluded that unloaded DCs express Ag(s) that also give rise to A20-directed CTLs. This contention was supported by in vitro experiments demonstrating the presence of T cells capable of responding to A20 and to autologous in vitro differentiated DCs but not to the other tumors tested (Figure 3C), and by adoptive transfer whereby these T cells conveyed protection against A20 (Figure 3D).
Such T cells against A20 Ag(s) were only induced by immunization with in vitro–differentiated DCs and could not be generated in animals solely injected with A20 or with DCs which were not subjected to in vitro differentiation in the presence of GM-CSF. Therefore, we speculate that Ag(s) are up-regulated on in vitro–differentiated DCs that can elicit T cells cross-reacting with other Ag(s) expressed on A20. It is also possible that there exist Ag(s) shared between DCs and A20 and that the Ag-presenting functions of in vitro–differentiated DCs but not of A20 cells are sufficient to induce CTLs. A bystander effect involving DC-specific CD8+ cells which provide help to A20-specific CD8+ cells can also not completely be excluded. However, this is unlikely because it is difficult to explain why DC stimuli in vivo or in vitro should provide an environment for stimulation of A20-specific T cells but not for T cells reacting against other tumor cell lines. A possible bystander effect exerted by CD4+ cells was excluded by our depletion experiments.
It is unclear what the nature and source of the specific Ag(s) is. Although DC-induced immunity was directed against the A20 B-cell lymphoma, it was not B cell specific because protection was not generated against the syngeneic MPC11 B-cell tumor. The Ag(s) involved seemed to be weak immunogen(s). Accordingly, therapeutic immunization with unpulsed DCs, which is more difficult than vaccination prior to tumor inoculation, was not successful (not shown). The potent stimulation by DCs described in this paper could provide a mechanism to overcome peripheral tolerance or ignorance to A20 Ag(s). It is important to note that signs of autoaggressive disease were never observed in our mice.
A recent paper reported a protective effect of unpulsed DCs against the C57BL/6 lung carcinoma LL/2 and the BALB/c colon carcinoma CT26.42 This suggests that antitumor efficacy of unloaded DCs might be broader. The difference in the outcome using CT26 cells may be due to differences in experimental design: We monitored survival, which is a more rigorous read-out system for tumor growth, rather than determining the number of pulmonary metastases. In addition, our CT26 cells were not transgenic for a xenogeneic Ag. Furthermore, the mechanisms defined in our study seem to be different from those described. In the previous study, primary rejection as well as long-term memory was solely dependent on NK and CD4+ cells. In our system, in contrast, primary rejection and long-term memory needed the presence of CD8+ T cells during the effector phase but no CD4+ T cells. Additionally, we did not observe a dependence of NK cell activation on T helper cells, because NK cells from DC-immunized animals showed unimpaired cytotoxicity in vitro when the animals were depleted of CD4+ T cells (data not shown).
Taken together, our results show that CD4+ T cell help is dispensable in the induction of an antitumor CTL response, when an alternative DC-NK cell axis is properly engaged. This suggests that a rational design using DC immunization has the potential to involve several different components of the immune system in order to maximize the priming of a T-cell response. Because the tumor-restricted CTL response in mice observed in this study might be a finding that cannot be generally applied, the use of unloaded DCs as a therapeutic tool in a clinical setting might not be suitable for generating Ag-specific responses. If, however, endogenous DCs present neoantigens generated by genetic changes in a tumor, the potent T helper cell–independent stimulation pathway could help to mount specific CTL responses.
Prepublished online as Blood First Edition Paper, March 15, 2005; DOI 10.1182/blood-2004-09-3775.
Supported by Deutsche Krebshilfe (10-1917).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
Excellent technical assistance by H. Jennen, J. Jasny, and N. Dierkes is gratefully acknowledged. We thank J. Louis, Lausanne, for the XMG-1.2 hybridoma and K. Falk, Berlin, for the C17.8 hybridoma. Furthermore, we are grateful to D.J. Schendel and C.S. Falk for critically reviewing the manuscript.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal