The antiviral compound azidothymidine (AZT), alone or in combination with other agents, induces apoptosis in early-passage, Epstein-Barr virus–positive Burkitt lymphoma (EBV+ BL) lines and has clinical activity in EBV+ BL. We report here a mechanism of AZT's antitumor activity. The nuclei of these cells contain activated nuclear factor-κB (NF-κB) subunits p50, c-Rel, RelB, and p52, but not p65. Treatment of primary EBV+ BL lines with AZT inhibited NF-κB within 1 to 2 hours. This was followed by up-regulation of EBV gene expression including viral thymidine kinase (vTK) and apoptosis. Subclones of EBV+ BL cells that demonstrated activated p65 were resistant to AZT. In EBV+ BLs, AZT but not ganciclovir (GCV) was highly phosphorylated to its monophosphate form (AZT-MP). Phosphorylation, as well as apoptosis, was markedly enhanced in the presence of hydroxyurea. AZT inhibits NF-κB and up-regulates EBV gene expression in primary EBV+ BLs. AZT with hydroxyurea may represent an inexpensive, targeted regimen for endemic BL.
Introduction
In recent work, we have demonstrated that the thymidine nucleoside analog azidothymidine (AZT), alone and in combination with other antiviral agents, has in vitro and in vivo activity against lymphomas associated with Epstein-Barr virus (EBV) and human herpes virus type 8 (HHV-8).1 In particular, AZT induces apoptosis in primary early-passage EBV+ Burkitt lymphoma (BL) cell lines.1,2 Viral and cellular gene expression varies in different forms of EBV-associated malignancies and in primary tumors versus long-established lines.3,4 In EBV+ BL only EBV nuclear antigen 1 (EBNA I) and EBV-encoded RNA (EBERs) are expressed although recent studies have indicated that a substantial heterogeneity in EBV gene expression and promoter usage exists among endemic BLs.5,6 In contrast, EBV+ immunoblastic lymphomas (IBLs) express high levels of latent membrane protein 1 (LMP-1), which enforces latency through constitutive activation of the transcription factor complex nuclear factor-κB (NF-κB) and in particular the p65 subunit.7,8
NF-κB refers to a group of transcriptional regulatory proteins involved in inflammatory, immune, and pro- and antiapoptotic cellular responses.9,10 NF-κB–mediated transactivation occurs through assembly of monomeric subunits at κB binding sites. In the canonical pathway, inhibitor of κB (IκB) segregates NF-κB (p50, p65) in the cytoplasm, thereby preventing its entry to the nucleus. In response to a variety of stimuli, including cytokines and viral infection, IκB is phosphorylated by the IκB kinase complex (IKK), typically IKKβ, at specific serine sites and degraded by the ubiquitin proteosome pathway, allowing for nuclear localization and DNA binding by NF-κB subunits.9,10 Other investigators have demonstrated that inhibition of NF-κB in gamma herpes virus–associated lymphomas results in induction of apoptosis.11,12
Recently, blockade of NF-κB has been demonstrated to disrupt gamma herpes viral latency.13 Induction of the lytic cycle should render EBV+ lymphomas susceptible to antivirals that can be converted to cytotoxic compounds and is potentially a highly specific therapeutic approach.14
We have previously demonstrated that AZT inhibits NF-κB in HHV-8–associated primary effusion lymphoma (PEL).1 To understand the molecular basis for the activity of AZT in EBV+ BL we characterized the pattern of NF-κB expression in early-passage BL (both EBV+ and EBV-) cells in the presence and absence of AZT.
By using several methods we demonstrate that in EBV+ BL, AZT alters activation/expression of NF-κB, induces expression of the virus lytic cycle, and causes efficient apoptosis, whereas the guanine nucleoside analog ganciclovir (GCV) is relatively inactive in these assays. Rapid conversion of AZT, but not GCV, to its monophosphate form suggests that both cellular and EBV thymidine kinases may contribute to the accumulation of this toxic metabolite and enhance tumor cell killing in a virus-specific manner. Both phosphorylation and the proapoptotic effect of AZT were markedly enhanced in the presence of hydroxyurea.
This novel antilymphoma activity of AZT might easily be incorporated into therapeutic regimens for EBV-associated BL.
Materials and methods
Cell lines
This study was approved by the University of Miami Institutional Review Board. Informed consent was obtained from patients prior to obtaining clinical samples. BL-8 and BL-5 are primary cell lines derived from patients with EBV+ BL and carry the typical t8:14 c-MYC translocation.2 BL-9 is an early-passage EBV- line derived from a patient with sporadic BL and L3 acute leukemia. BL-10 was derived from primary cells from an AIDS-related EBV-, L3 leukemia. Ramos is an established EBV- BL line obtained from the American Type Culture Collection (ATCC; Manassas, VA). BCBL-1 and PEL-5 are HHV-8+ EBV- lines derived from PEL tumors. BCBL-1 was obtained from the ATCC and PEL-5 has been submitted to the AIDS and Cancer Specimen Resource (ACSR; San Francisco, CA). Cells were maintained in suspension culture in Iscove modified Dulbecco medium (IMDM; GIBCO-BRL, Carlsbad, CA) supplemented with 10% fetal bovine serum (FBS), 1% penicillin and streptomycin, 1% glutamine, and 1 mM Na-pyruvate at 37°C in a 5% CO2-humidified atmosphere.
Antibodies and reagents
For electrophoretic mobility shift assay (EMSA) supershift experiments the following antibodies were used: anti-p50 (H-119), anti-p65 (F-6), anti–c-Rel (N), p52 (C-5), and RelB (C-19) (all from Santa Cruz Biotechnology, Santa Cruz, CA). Anti–Ea-D (East-Coast Biologics, North Berwick, ME) was used for Western blot analysis.
Apoptosis measurement
5.0 × 105 cells were treated with 10 μg/mL AZT or GCV for the indicated times before being washed twice with cold phosphate-buffered saline (PBS). Cells were then incubated with annexin V–fluoroscein isothiocyanate (FITC; BD Pharmingen, San Diego, CA) and propidium iodide (PI) as previously described.2 Cells were then analyzed using a FACScan flow cytometer (Becton Dickinson, San Jose, CA).
EMSA
Nuclear extracts were prepared as described previously.1 Cells (5 × 106) were washed twice with cold PBS and the cell pellet suspended in 400 μL of hypotonic buffer A (10 mM HEPES [N-[2-hydroxyethyl]piperazine-N′-[2-ethanesulfonic acid]), pH 7.9, 10 mM KCl, 1.5 mM MgCl2, 1 mM dithiothreitol (DTT), 0.5 mM phenylmethylsulfonyl fluoride, and protease inhibitor cocktail. After 15 minutes of incubation on ice, 25 μL of 10% Nonidet P-40 was added and the sample vigorously vortexed. The nuclei were collected by centrifugation at 3000g for 5 minutes at 4°C and washed once with buffer A. The nuclear pellet was then suspended in 75 μL buffer B (20 mM HEPES [pH 7.9], 400 mM NaCl, 1.5 mM MgCl2, 0.2 mM EDTA [ethylenediaminetetraacetic acid], 1 mM DTT, 5% glycerol, and protease inhibitor cocktail) and incubated for 30 minutes at 4°C after brief mixing. The mixture was centrifuged at 10 000g for 5 minutes at 4°C and the nuclear extract was stored at -80°C. Protein concentrations were measured using the Bradford assay (Bio-Rad, Hercules, CA).
Double stranded NF-κB consensus oligonucleotide (Santa Cruz Biotechnology) was end-labeled with γ-[32P]adenosine triphosphate (ATP) using T4 polynucleotide kinase. Unincorporated ATP was removed with G-50 spin columns (Amersham Biosciences, Piscataway, NJ) and centrifugation. Before the addition of oligonucleotide probe, 10 μg nuclear protein was incubated with binding buffer (2 μg poly(dI-dC), 12% glycerol, 20 mM HEPES [pH 7.0], 1 mM DTT, 1 mM EDTA, 50 mM NaCl) for 10 minutes at room temperature. A radiolabeled oligonucleotide (20 000-50 000 counts per minute [cpm]) was incubated with reaction mixture for 20 minutes at room temperature. DNA-protein complex was resolved by electrophoresis in 5% nondenaturing polyacrylamide gel electrophoresis (PAGE) in × 0.5 Tris (tris(hydroxymethyl)aminomethane)–borate-EDTA (TBE) buffer. The gels were dried and analyzed by autoradiography. Supershift was performed by adding antibodies to the incubation mixture of nuclear extract (p50, p52, p65, RelB, and c-Rel; Santa Cruz Biotechnology) and incubating for 45 minutes on ice before the addition of radiolabeled probe.
ELISA
The level of NF-κB protein p50 in the nuclear extracts was measured using Clontech Mercury Transfector enzyme-linked immunosorbent assay (ELISA) kits (Clontech, Palo Alto, CA) according to the manufacturer's instructions. Wells were precoated with NF-κB double stranded DNA (dsDNA; wild) or mutant DNA (negative control).
Real-time quantitative PCR for EBV
RNA was isolated as previously described15 using RNAzol (Tel-Test, Friendswood, Texas). Polymerase alpha (PolyA)–mRNA was prepared using deoxythymidine (dT) beads (Qiagen, Valencia, CA) and reverse-transcribed using Superscript-II reverse transcriptase (Life Technologies, Rockville, MA) according to the manufacturer's recommendations. The procedures for real-time quantitative polymerase chain reaction (QPCR) analysis have been previously described.15,16 The EBV primers used in this analysis are available from the authors (D.W., D.P.D.) upon request; a manuscript detailing their development is in preparation. The final PCR reaction contained 2.5 μL primer mix (final concentration, 166 nM), 7.5 μL 2xSYBR PCR mix (Applied Biosystems, Foster City, CA), and 5 μL sample. To guard against contamination and handling errors, all real-time QPCR reactions were assembled in a segregated clean room using a CAS-1200 robot (Phoenix, Hayward, CA) with 0.1 μL accuracy, liquid-level sensing, and filtered pipette tips. Real-time PCR was performed using an ABI PRIZM5700 machine (Applied Biosystems, Foster City, CA) and universal cycle conditions.
Measurement of AZT and GCV phosphorylation
Cells (5.0 × 106) were grown in 5 mL media in the presence of 5 μg/mL [methyl-3H]-AZT (specific activity, 4.44 × 1010 Bq/mM [1.2 Ci/mM]) and 5 μg/mL [8-3H]-GCV (specific activity, 4.44 × 1010 Bq/mM [1.2 Ci/mM]) (Moravek Biochemicals, Brea, CA) for the indicated times. Cells were harvested, washed 3 times with ice-cold PBS, and extracted in 0.5 mL of 65% ice-cold methanol. AZT-MP and GCV-MP were separated and quantified by high-performance liquid chromatography (HPLC) on a column (Whatman Partisil 5 SAX; Whatman, Clifton, NJ) upon elution with (10-750 mM) ammonium phosphate (pH 3.8) plus 7% methanol gradient. The radioactivity of the eluted fractions was counted by liquid scintillation. To study the effect of hydroxyurea on AZT phosphorylation, cells were treated with 25 μM hydroxyurea and 1.25 μg/mL [methyl-3H]-AZT, and HPLC was performed as described earlier in this paragraph.
Western blot analysis
After the indicated treatment, whole cell extracts were made using lysis buffer (150 mM NaCl, 50 mM Tris-HCl [pH 7.5]) 1 mM DTT, and Triton X-100 (0.1%) supplemented with protease inhibitor cocktail (Sigma, St Louis, MO). Forty micrograms of total protein was fractionated on 12% sodium dodecyl sulfate (SDS)–PAGE and transferred by electroblotting onto nitrocellulose membrane (Bio-Rad, Hercules, CA). Western blot was performed as previously described1 and proteins were visualized by enhanced chemiluminescence (ECL) developing kit (Amersham Pharmacia Biotech).
Results
AZT transiently inhibits NF-κB in early-passage EBV+ BL cells
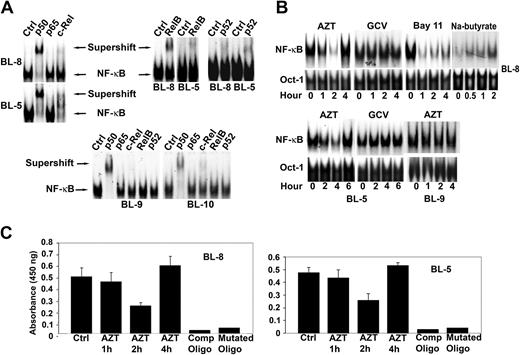
To investigate the effect of AZT in EBV+ lymphomas we first characterized the subunit composition of NF-κB in these tumors. Nuclear extracts from 2 EBV+ BL lines (BL-5, BL-8) contained p50, p52, c-Rel, and RelB subunits as evidenced by both band shift and a decrease in the major band intensity in the presence of specific antibodies, while p65 was not detected. Extracts from the EBV- BLs (BL-9, BL-10) were both p65 and RelB negative (Figure 1A). We next examined the effect of AZT on NF-κB localization by EMSA on nuclear extracts prepared from drug-treated cells. AZT, but not GCV, inhibited NF-κB in BL-8 and BL-5 within 1 to 2 hours, after which NF-κB activity increased again (Figure 1B). This contrasted with the NF-κB inhibitor, Bay 11-7082, which caused a more sustained inhibition of the transcription factor for up to 4 hours (Figure 1B). Treatment of EBV+ BL cells with sodium butyrate (2.5 mM), an agent known to induce apoptosis in EBV+ BL via induction of the viral lytic program, also resulted in an increase in NF-κB DNA binding (Figure 1B). AZT had no effect on NF-κB in EBV- BL-9 cells (Figure 1B). These primary tumor lines were not amenable to transfection with reporter constructs. Therefore, to confirm the EMSA results, AZT-treated and -untreated nuclear extracts were assayed with a p50-specific ELISA. Both BL-8 and BL-5 showed similar transient suppression of p50 protein (2 hours) followed by induction, thus confirming our EMSA data (Figure 1C).
AZT, but not GCV, inhibits the binding of nuclear proteins to oligonucleotides containing NF-κB sites. (A) Purified nuclear extracts (10 μg) prepared from EBV+ BL cell lines (BL-8, BL-5) and EBV- BL cell lines (BL-9, BL-10) were preincubated with the indicated antibodies to the NF-κB components before the addition of radiolabeled probes. The composition of the NF-κB complex in EBV+ and EBV- BL cells was characterized by supershift analysis. Supershift indicates the binding of antibody and protein-DNA complex. (B) Nuclear extracts prepared from AZT (10 μg/mL), GCV (10 μg/mL), Bay 11-7082 (2.5 μM), or sodium butyrate (2.5 mM) treated cells (BL-5, BL-8, BL-9) for the indicated times were assayed by EMSA using either NF-κB (top blot) or Oct-1 (bottom blot) specific consensus oligonucleotides as probes. The results are representative of 3 independent experiments. Oct-1 was used as a loading control for nuclear proteins. (C) BL-5 and BL-8 cells were treated with AZT (10 μg/mL) for the indicated times and p50 was detected in nuclear extracts by ELISA. Specificity of binding was confirmed by using both wild-type and mutated NF-κB oligonucleotides. Experiments were performed in triplicate. Results are shown as the mean ± standard deviation.
AZT, but not GCV, inhibits the binding of nuclear proteins to oligonucleotides containing NF-κB sites. (A) Purified nuclear extracts (10 μg) prepared from EBV+ BL cell lines (BL-8, BL-5) and EBV- BL cell lines (BL-9, BL-10) were preincubated with the indicated antibodies to the NF-κB components before the addition of radiolabeled probes. The composition of the NF-κB complex in EBV+ and EBV- BL cells was characterized by supershift analysis. Supershift indicates the binding of antibody and protein-DNA complex. (B) Nuclear extracts prepared from AZT (10 μg/mL), GCV (10 μg/mL), Bay 11-7082 (2.5 μM), or sodium butyrate (2.5 mM) treated cells (BL-5, BL-8, BL-9) for the indicated times were assayed by EMSA using either NF-κB (top blot) or Oct-1 (bottom blot) specific consensus oligonucleotides as probes. The results are representative of 3 independent experiments. Oct-1 was used as a loading control for nuclear proteins. (C) BL-5 and BL-8 cells were treated with AZT (10 μg/mL) for the indicated times and p50 was detected in nuclear extracts by ELISA. Specificity of binding was confirmed by using both wild-type and mutated NF-κB oligonucleotides. Experiments were performed in triplicate. Results are shown as the mean ± standard deviation.
AZT is phosphorylated and induces apoptosis
To investigate the intracellular metabolism (phosphorylation) of AZT, cells were incubated with radiolabeled nucleosides (AZT or GCV) and intracellular methanol extracts were prepared at several time points and analyzed by HPLC. In the EBV+ BL lines, the monophosphorylated form of AZT (AZT-MP), but not GCV-MP, was detected and rose rapidly after 4 hours (Figure 2A). By comparison, AZT was phosphorylated to AZT-MP at a far lower level in EBV- BL-9 and Ramos cells. The di- and triphosphate forms ofAZT together made up less than 3% of the total percentage of phosphorylated nucleoside in each line (data not shown). We next determined the sensitivity of each line to AZT by culturing them in the presence of nucleoside analogs and measuring apoptosis by annexin V/PI staining. Both BL-5 and BL-8, but not the control EBV- primary line BL-9 or Ramos cells, underwent apoptosis in the presence of AZT alone. By comparison, the guanine antiviral nucleoside analog GCV was inactive (Figure 2B). Other nonthymidine nucleosides such as dideoxyinosine (ddI) and dideoxycitidine (ddC) also exhibited no cytotoxicity (data not shown).
Activation of p65 in EBV+ BL correlates with inhibition of phosphorylation and apoptosis
We noted that serially passaged EBV+ BL-5 cells (BL-5R) became insensitive to AZT-induced apoptosis. For example, the BL-5 line, after being passaged 12 times (in the absence of AZT) in a period of 6 weeks, became resistant to this antiviral nucleoside (Figure 3A). HPLC analysis demonstrated that phosphorylation of AZT was also much lower in these cells than in the parental line (Figure 3B). Interestingly, supershift analysis demonstrated that these resistant cells (BL-5R), unlike the parental line (BL-5), contained activated p65 (Figure 3C). Therefore, activation of p65 correlated with resistance to AZT mediated phosphorylation and apoptosis. This observation is provocative since previous work has demonstrated that p65 expression inhibits gamma herpesvirus reactivation from latency.13 We cannot exclude that other cellular and viral factors induced via serial passage of the AZT sensitive parental line (BL-5) may have played a role in the acquired resistance to this antiviral.
AZT but not GCV induces apoptosis and is phosphorylated in EBV+ BL cell lines. (A) EBV+ BL (BL-5, BL-8) and EBV- BL (BL-9, Ramos) cells were treated with either [methyl-3H]-AZT (5 μg/mL; A) or [8-3H]-GCV (5 μg/mL; G) for 1, 4, 8, and 16 hours. Methanol extracts were then subjected to HPLC fractionation to purify the different phosphorylated forms of the nucleosides. The figure shows the relative amounts of the monophosphorylated nucleosides. (B) EBV+ BL (BL-5, BL-8) and EBV- BL (Ramos, BL-9) cells were treated with medium, AZT (10 μg/mL), or GCV (10 μg/mL) for 36 hours and analyzed for apoptosis by Annexin/PI staining and FACS analysis. Bars represent the percentage of apoptotic cells in a triplicate experiment. The results are shown as the mean ± standard deviation.
AZT but not GCV induces apoptosis and is phosphorylated in EBV+ BL cell lines. (A) EBV+ BL (BL-5, BL-8) and EBV- BL (BL-9, Ramos) cells were treated with either [methyl-3H]-AZT (5 μg/mL; A) or [8-3H]-GCV (5 μg/mL; G) for 1, 4, 8, and 16 hours. Methanol extracts were then subjected to HPLC fractionation to purify the different phosphorylated forms of the nucleosides. The figure shows the relative amounts of the monophosphorylated nucleosides. (B) EBV+ BL (BL-5, BL-8) and EBV- BL (Ramos, BL-9) cells were treated with medium, AZT (10 μg/mL), or GCV (10 μg/mL) for 36 hours and analyzed for apoptosis by Annexin/PI staining and FACS analysis. Bars represent the percentage of apoptotic cells in a triplicate experiment. The results are shown as the mean ± standard deviation.
AZT-mediated apoptosis and phosphorylation in EBV+ BL cells is inhibited by p65. (A) Serially passaged BL-5R cells were treated with AZT (10 μg/mL) for 40 hours, and apoptosis was measured by FACS analysis. (B) BL-5 and BL-5R cells were treated with [methyl-3H]-AZT for 1, 4, 8, and 16 hours. Methanol extracts were subjected to HPLC analysis. The figure demonstrates the relative amounts of AZT-MP generated in each line. (C) AZT-resistant BL-5 cells (BL-5R) were analyzed for NF-κB subunit usage by supershift analysis. Results are shown as the mean ± standard deviation.
AZT-mediated apoptosis and phosphorylation in EBV+ BL cells is inhibited by p65. (A) Serially passaged BL-5R cells were treated with AZT (10 μg/mL) for 40 hours, and apoptosis was measured by FACS analysis. (B) BL-5 and BL-5R cells were treated with [methyl-3H]-AZT for 1, 4, 8, and 16 hours. Methanol extracts were subjected to HPLC analysis. The figure demonstrates the relative amounts of AZT-MP generated in each line. (C) AZT-resistant BL-5 cells (BL-5R) were analyzed for NF-κB subunit usage by supershift analysis. Results are shown as the mean ± standard deviation.
Hydroxyurea enhances AZT phosphorylation and apoptosis in EBV+ BL
Hydroxyurea can enhance phosphorylation of nucleoside analogs by inhibiting ribonucleotide reductase.17 We therefore investigated the possibility that this might enhance the activity of AZT in EBV+ BL. Both phosphorylation and the apoptotic effect of low-dose AZT (1.25 μg/mL) were markedly enhanced in BL-8 cells in the presence of hydroxyurea (Figure 4A-B). Hydroxyurea also potentiated the inhibition of NF-κB in AZT-treated p65+ and p65- EBV+ BL cells. NF-κB inhibition was still noted at 6 hours in AZT/hydroxyurea (vs AZT alone)–treated BL-8 cells (data not shown). This indicates that this inexpensive agent may enhance the antineoplastic effect of AZT in EBV+ lymphomas.
AZT induces EBV gene expression in primary EBV+ BLs
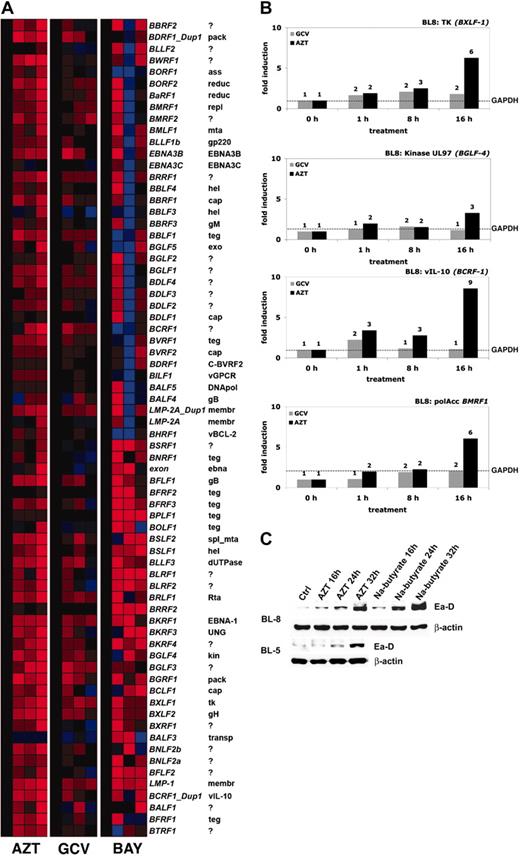
AZT underwent a progressive increase in phosphorylation and induced apoptosis in our primary EBV+ BL lines. High levels of AZT-MP might be generated by cellular or viral thymidine kinase. It has been previously shown that EBV TK (BXLF-1) phosphorylates AZT very efficiently.18 To measure the effect of AZT on EBV gene expression, we performed whole genome real-time quantitative reverse transcriptase (RT)–PCR analysis by a method recently introduced for HHV-8.15,16 BL-8 cells were treated with media, GCV (10 μg/mL), or AZT (10 μg/mL) in a time-course experiment. We also investigated the effect of Bay 11-7082 (2.5 μM) on EBV gene expression in BL-8. After normalizing each mRNA level relative to its level in mock (media)–treated cells, it was apparent that up-regulation of EBV gene expression occurred in the AZT-treated cells shortly after the observed inhibition (2 hours) of NF-κB (Figure 5A). Some up-regulation of EBV expression was noted in the Bay 11-7082 cells. This is in agreement with previously reported data regarding NF-κB inhibition and induction of the lytic cycle.13 As has been reported,12,19 Bay 11-7082 was extremely toxic to these EBV+ lymphomas, and apoptosis also occurred far more rapidly than with AZT (data not shown). Hence for Bay 11-7082, the array analysis likely monitors relative levels of RNA in a rapidly dying culture than a coordinated transcriptional cascade. We compared relative levels of expression of key EBV genes (normalized against the housekeeping gene GAPDH) in BL-8 cells treated with AZT or GCV. AZT-induced viral interleukin-10 (IL-10; BCRF-1) 9-fold, BMRF-1 6-fold, and viral TK (BXLF-1) 6-fold. There was also a lesser (3-fold) induction of the EBV-encoded phosphotransferase BGLF-4. GCV had little effect on the expression levels of the same genes (Figure 5B). EBV+ BL cells were treated with AZT, and expression of the immediate early replication protein Ea-D (BMRF-1) was quantified. AZT induced its expression in a time-dependent manner (Figure 5C). These observations suggest, but do not prove, that phosphorylation of AZT is markedly enhanced through the basal expression and induction of EBV TK.
Discussion
A variety of genotoxic agents, including gamma radiation and chemotherapy, have been shown to be capable of inducing the expression of EBV lytic proteins in lymphomas.20,21 Transit through the entire lytic cycle would itself result in cell death. However, abortive replication may result in expression of early genes, such as the viral kinases, that if used to phosphorylate and convert nucleoside analogs to cytotoxic compounds could also significantly contribute to the selective killing of tumor cells. AZT, although not a dangerous cytotoxic agent, does exhibit a degree of genotoxicity which may be sufficient to induce EBV in LMP-1- BL.22 Therefore, the incorporation of AZT as a therapy for EBV+ BL may have great promise. Our data demonstrate that AZT inhibits NF-κB in EBV+ BLs. The effectiveness of AZT in EBV-associated lymphomas may be determined largely by the histologic subtype of tumor and the expression of viral and cellular antiapoptotic proteins. EBV+ BLs may be uniquely susceptible to AZT-mediated apoptosis due to their intrinsically high level of c-MYC and lack of LMP-1, Bcl-2 expression, and p65 nuclear translocation.3,7,23 Consistent with this, we have observed that serially passaged primary EBV+ BLs that contain activated p65 are relatively insensitive to AZT. This is also consistent with previous observations that have demonstrated that p65 is a potent inducer of viral latency.13 It is still unclear how AZT is initially phosphorylated in these primary EBV+ BL lymphomas. Other investigators have demonstrated that AZT is an excellent substrate for EBV viral TK.18 Rapidly proliferating cells such as BLs express high levels of cellular thymidine kinase. One possibility is that in these cells AZT would be phosphorylated, and in the presence of EBV (and absence of p65) the genotoxic effect of the nucleoside would trigger viral reactivation including viral kinases. It is also possible that EBV+ primary BL contain a population of cells undergoing spontaneous, intermittent reactivation. AZT might be activated in these cells and then further induce the EBV genomic program. The increase in NF-κB at later time points is likely due to a cellular stress response as viral reactivation is up-regulated and the apoptotic program is initiated. This is supported by our observation of an increase in NF-κB in sodium butyrate–treated BL cells. Susceptibility to AZT would be expected to decrease as continued passage in culture selects for tightly latent clones and yields the well-characterized type-I BL lines.
Hydroxyurea synergistically potentiates AZT-mediated apoptosis and phosphorylation in EBV+ BL-8 cells. (A) BL-8 cells were treated with low doses of AZT (1.25 μg/mL), hydroxyurea (25 μM), or both agents combined for 36 hours and apoptosis was determined by PI annexin staining and FACS analysis. (B) BL-8 cells were incubated for 1 hour or 8 hours with [methyl-3H]-AZT (1.25 μg/mL) alone or in combination with hydroxyurea (25 μM). Methanol extracts were separated in HPLC to purify the AZT-MP. The values represent the mean ± standard deviation of triplicate experiments.
Hydroxyurea synergistically potentiates AZT-mediated apoptosis and phosphorylation in EBV+ BL-8 cells. (A) BL-8 cells were treated with low doses of AZT (1.25 μg/mL), hydroxyurea (25 μM), or both agents combined for 36 hours and apoptosis was determined by PI annexin staining and FACS analysis. (B) BL-8 cells were incubated for 1 hour or 8 hours with [methyl-3H]-AZT (1.25 μg/mL) alone or in combination with hydroxyurea (25 μM). Methanol extracts were separated in HPLC to purify the AZT-MP. The values represent the mean ± standard deviation of triplicate experiments.
AZT induces the genomewide expression and lytic cycle associated viral mRNAs (proteins) of EBV in BL cells. (A) Hierarchical cluster analysis using a real-time, quantitative RT-PCR for EBV demonstrates the temporal effects of exposure to AZT (10 μg/mL), GCV (10 μg/mL), or Bay 11-7082 (2.5 μM) in BL-8 cells, where the mRNA signals were normalized to the level in mock (medium)–treated cells (CT). mRNAs that are highly abundant are coded in shades of red, those of intermediate levels in black, and those that are below the detection limit in blue. mRNA from sodium butyrate (2.5 mM)–treated BL-8 cells (for 8 hours) was used as a positive control. (B) BL-8 cells were treated with AZT (10 μg/mL) or GCV (10 μg/mL) for the indicated times (0, 1, 8, or 16 hours) and subjected to real-time quantitative RT-PCR for all EBV genes. After normalization to glyceraldehyde-3-phosphate dehydrogenase (GAPDH) levels (–), fold changes relative to untreated controls were calculated for individual mRNAs. (C) BL-8 and BL-5 cells were treated with AZT (10 μg/mL) or GCV (10 μg/mL) for the indicated times and immunoblot analysis was subsequently performed on harvested lysates to determine the expression of BMRF-1 (Ea-D). Lysate from sodium butyrate (2.5 mM)–treated cells was used as a positive control. β-actin was used as a loading control.
AZT induces the genomewide expression and lytic cycle associated viral mRNAs (proteins) of EBV in BL cells. (A) Hierarchical cluster analysis using a real-time, quantitative RT-PCR for EBV demonstrates the temporal effects of exposure to AZT (10 μg/mL), GCV (10 μg/mL), or Bay 11-7082 (2.5 μM) in BL-8 cells, where the mRNA signals were normalized to the level in mock (medium)–treated cells (CT). mRNAs that are highly abundant are coded in shades of red, those of intermediate levels in black, and those that are below the detection limit in blue. mRNA from sodium butyrate (2.5 mM)–treated BL-8 cells (for 8 hours) was used as a positive control. (B) BL-8 cells were treated with AZT (10 μg/mL) or GCV (10 μg/mL) for the indicated times (0, 1, 8, or 16 hours) and subjected to real-time quantitative RT-PCR for all EBV genes. After normalization to glyceraldehyde-3-phosphate dehydrogenase (GAPDH) levels (–), fold changes relative to untreated controls were calculated for individual mRNAs. (C) BL-8 and BL-5 cells were treated with AZT (10 μg/mL) or GCV (10 μg/mL) for the indicated times and immunoblot analysis was subsequently performed on harvested lysates to determine the expression of BMRF-1 (Ea-D). Lysate from sodium butyrate (2.5 mM)–treated cells was used as a positive control. β-actin was used as a loading control.
Some interesting reports on the biology of endemic BL suggest that in some primary tumors EBV is not strictly latent. The presence of lytic transcripts (BZLF-1 and IR2, 4) was reported in the majority of fresh biopsy specimens of endemic BL in children from Malawi, Africa.5 Most therapies for aggressive B-cell lymphomas are based upon intensive chemotherapeutic regimens, expensive modalities (bone marrow transplant, rituximab), or sophisticated experimental approaches (gene therapy, cytotoxic T-cell infusion) that are difficult, if not impossible, to implement in developing countries. We have recently treated a patient with EBV+ HIV-related BL that had relapsed following rituximab and CHOP (cyclophosphamide/doxorubicin/vincristine/prednisone) chemotherapy with intravenous AZT and hydroxyurea. There was a dramatic regression of the patient's tumor and decrease in the tumor marker lactate dehydrogenase (6 400 U/dL to 690 U/dL) without the addition of any other antilymphoma therapy. Although EBV+ BLs are relatively rare in developed nations, AZT treatment should be applicable to similar tumors that arise in patients with organ transplants, hereditary immunodeficiencies, and AIDS. One could evaluate primary tumor specimens for susceptibility to AZT-mediated induction of the viral lytic program. Resistant EBV+ lymphomas might be induced pharmacologically with inhibitors of NF-κB or other EBV-inducing agents, then treated with nucleoside analogs. Incomplete therapy is a major cause of relapse in endemic BLs.24 In addition, patients that present with large tumor burdens have a significantly poorer prognosis.25 Treatment of these individuals with AZT (perhaps with the addition of hydroxyurea) might increase the effectiveness or shorten the duration of conventional therapy. Through pharmacologic exploitation of EBV, it should be possible to develop highly effective and relatively nontoxic therapies for these aggressive tumors.
Prepublished online as Blood First Edition Paper, March 24, 2005; DOI 10.1182/blood-2004-09-3748.
Supported by the National Institutes of Health grants 1UO1-CA-70058, 1RO1-CA-82274 (S1), and R21-CA-97951.
M.K. and S.K.G. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

![Figure 2. AZT but not GCV induces apoptosis and is phosphorylated in EBV+ BL cell lines. (A) EBV+ BL (BL-5, BL-8) and EBV- BL (BL-9, Ramos) cells were treated with either [methyl-3H]-AZT (5 μg/mL; A) or [8-3H]-GCV (5 μg/mL; G) for 1, 4, 8, and 16 hours. Methanol extracts were then subjected to HPLC fractionation to purify the different phosphorylated forms of the nucleosides. The figure shows the relative amounts of the monophosphorylated nucleosides. (B) EBV+ BL (BL-5, BL-8) and EBV- BL (Ramos, BL-9) cells were treated with medium, AZT (10 μg/mL), or GCV (10 μg/mL) for 36 hours and analyzed for apoptosis by Annexin/PI staining and FACS analysis. Bars represent the percentage of apoptotic cells in a triplicate experiment. The results are shown as the mean ± standard deviation.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/106/1/10.1182_blood-2004-09-3748/4/m_zh80130580510002.jpeg?Expires=1769095071&Signature=sist5Ak5heKJdUaLmhQ--LOBrako36fNKDhSWWXrSOs3ZCul4Ffdr~N5~tnE8vzdD0PfmV8NqVkDbWbeaixUU-Ajmc1FDYk57AQXreX1R6xaPgtXV3vn7W8KCxKc7tPgNfUMpCKjGKQ8oQJp3t1DBPgl7usRLqhyg3-7pLBmRLEh42-Dutc7RbxpmQlDsL4dSz~nU-qcrCcjPnN1ezKo9KbZ-dvVD~0FDBlEUhNeQRK24SmNm2IvkTz~k6P~aDlxLw3DXMOIM7sY98zxGOTpyq5i1BKzwnKSA~0quJF~kCaeba1rQaI--kmJGiufasvm3mGwAuBs1eTQhm1Fbhxz7A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 3. AZT-mediated apoptosis and phosphorylation in EBV+ BL cells is inhibited by p65. (A) Serially passaged BL-5R cells were treated with AZT (10 μg/mL) for 40 hours, and apoptosis was measured by FACS analysis. (B) BL-5 and BL-5R cells were treated with [methyl-3H]-AZT for 1, 4, 8, and 16 hours. Methanol extracts were subjected to HPLC analysis. The figure demonstrates the relative amounts of AZT-MP generated in each line. (C) AZT-resistant BL-5 cells (BL-5R) were analyzed for NF-κB subunit usage by supershift analysis. Results are shown as the mean ± standard deviation.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/106/1/10.1182_blood-2004-09-3748/4/m_zh80130580510003.jpeg?Expires=1769095071&Signature=4pBQbxKAOt4PbOD3wUwuxPpkfmLFhClWtkCRBbOofliO-Lb4pZY-m99vRjmnQ1s~gOfcKMIyja~8TLehNW1h3SQ5XObpW-XOgCJc~jPq9VAcRcO2zutbFaAMjFbHRYsYK4uPWEZC2wl8DIapLa1JDIV~Fl6P8moblZMl8-iBVvOWRk5ss86xtSZgXyM-~12roeYzTRgCLBcRXCRt88ZIGHZdsg3jKLhYoK7ZFl6RWUF4lfci5v9q0vN6HqYOCJhvEZL3e9l1xLFNzwrOKQmBgt5HRM3QwELYXyqt6zexSSxbBIM7omD1P-L1UH0KaAHnwffpTvSyh0PJeARqkPexhg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 4. Hydroxyurea synergistically potentiates AZT-mediated apoptosis and phosphorylation in EBV+ BL-8 cells. (A) BL-8 cells were treated with low doses of AZT (1.25 μg/mL), hydroxyurea (25 μM), or both agents combined for 36 hours and apoptosis was determined by PI annexin staining and FACS analysis. (B) BL-8 cells were incubated for 1 hour or 8 hours with [methyl-3H]-AZT (1.25 μg/mL) alone or in combination with hydroxyurea (25 μM). Methanol extracts were separated in HPLC to purify the AZT-MP. The values represent the mean ± standard deviation of triplicate experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/106/1/10.1182_blood-2004-09-3748/4/m_zh80130580510004.jpeg?Expires=1769095071&Signature=FuKfM5GBIBquaDU8HiIwltQuwsL-uxpFB4sHC3D~GhjwBB9wrrk~Gv6Q5Phn56eMNIQ4s4kDutr5UJAbFaClo~yOxkjxARVP99H7RzO2DVBKhtv9QUJ-AOoFtqg-5Qs-NpuByU4go0txrAVODSM5H2qJxO3L7mpk4ZGgX3w2yIvZtO4mcAcXnPNLlsvmBT9v9-I3bczTPqDd1xwaC~KHAj~mmEesGnI1O2LS7xxiksIpq9dce1VxYAAzU9pBGgNXzlf52-5KPjXUjGGpBg~o6acwVvGZ8KHsuovN1xUJPF~oe5ULPt1E92ghVJHYXT-4bF2bry3BJajKsx57jzNokw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal