Exosomes are secreted vesicles formed in late endocytic compartments. Immature dendritic cells (DCs) secrete exosomes, which transfer functional major histocompatibility complex (MHC)–peptide complexes to other DCs. Since immature and mature DCs induce different functional T-cell responses (ie, tolerance versus priming), we asked whether DC maturation also influenced the priming abilities of their exosomes. We show that exosomes secreted by lipopolysaccharide (LPS)–treated mature DCs are 50- to 100-fold more potent to induce antigen-specific T-cell activation in vitro than exosomes from immature DCs. In vitro, exosomes from mature DCs transfer to B lymphocytes the ability to prime naive T cells. In vivo, only mature exosomes trigger effector T-cell responses, leading to fast skin graft rejection. Proteomic and biochemical analyses revealed that mature exosomes are enriched in MHC class II, B7.2, intercellular adhesion molecule 1 (ICAM-1), and bear little milk-fat globule–epidermal growth factor–factor VIII (MFG-E8) as compared with immature exosomes. Functional analysis using DC-derived exosomes from knock-out mice showed that MHC class II and ICAM-1 are required for mature exosomes to prime naive T cells, whereas B7.2 and MFG-E8 are dispensable. Therefore, changes in protein composition and priming abilities of exosomes reflect the maturation signals received by DCs.
Introduction
Dendritic cells (DCs) are key actors in the initiation of immune responses.1,2 Immature DCs present in peripheral tissues constantly sample their microenvironment, thus acquiring antigens. Under steady-state conditions, antigens are eventually processed into peptides, which, when loaded onto major histocompatibility complex (MHC) molecules, can be recognized by specific T lymphocytes. Immature DCs, however, do not induce effective immune responses, but rather are involved in maintaining peripheral T-cell tolerance. By contrast, under inflammatory conditions, DCs gradually acquire high numbers of MHC-peptide complexes and costimulatory molecules, and become the most efficient inducers of T-cell–dependant immune responses.
Immature DCs, like several other cell types, secrete small membrane vesicles called exosomes (50 nm to 100 nm diameter) that form within late multivesicular endosomal compartments (MVBs).3-5 Exosomes are released in the extracellular environment by fusion of MVBs with the plasma membrane. Immature DC–derived exosomes bear functional MHC class I– and class II–peptide complexes, which can induce activation of specific T cells in vivo.6,7 In vitro, exosomes are used as a source of T-cell receptor (TCR) ligands, or of antigens, by recipient DCs which then activate antigen-specific T cells.6,8,9 The costimulatory molecules, such as B7.2, required for T-cell activation, however, are contributed by DCs, and not by exosomes.
These results suggest that exosomes are involved in the spreading of MHC class II–peptide complexes between DCs. Such spreading should increase the number of DCs expressing the ligand for a given antigen-specific T cell. The functional outcome of indirect T-cell activation via exosomes remains, however, unclear. Immature and mature DCs induce different functional T-cell responses (ie, tolerance versus priming). Whether exosomes induce T-cell tolerance, or priming, or none, could therefore depend on the maturation state of DCs producing the exosomes, and/or of DCs receiving the exosomes. We asked here whether exosomes secreted by immature or mature DCs were functionally different.
We have compared exosomes produced by lipopolysaccharide (LPS)–treated (mature) and untreated (immature) DCs. Immature and mature DCs secrete morphologically similar exosomes. In functional T-cell stimulation assays, exosomes from mature DCs induced strong antigen-specific T-cell proliferation both in vitro and in vivo, even when used at very low doses. Exosomes from mature DCs conferred the ability to activate naive T cells to inefficient antigen-presenting cells (APCs) (ie, B cells), whereas exosomes from immature DCs were unable to do so. Exosomes secreted by mature DCs, but not those secreted by immature DCs, were able to prime naive T cells in vivo, as shown by their effect in a minor antigen-bearing skin graft assay. At the molecular level, mature DC–derived exosomes bear increased amounts of MHC class II and costimulatory molecules, and a decreased amount of milk fat globule–epidermal growth factor (EGF)–factor VIII (MFG-E8). We demonstrate that decreased MFG-E8 or increased B7.2 on mature DC–derived exosomes do not account for their stronger activity. By contrast, intercellular adhesion molecule 1 (ICAM-1) is necessary for the immune activity of exosomes secreted by LPS-treated DCs.
Materials and methods
Mice
C57Bl/6 wild-type (WT) and mutant mice were obtained from Charles River (C57Bl/6; L'Arbresle, France), O. Lantz (Marilyn; Institut Curie, Paris, France), CDTA (I-Aβ–/–; Orléans, France), B. Salomon (B7–/–; Pitié-Salpêtrière, Paris, France), Jackson Laboratories (Icam-1–/–; Bar Harbor, ME). Mice were housed in specific pathogen-free conditions. Experiments were done in accordance with the guidelines of the French Veterinary Department.
Cells
The DC line D110 was cultured as previously described11 in 10% fetal calf serum (FCS) complete Iscove modified Dulbecco medium (IMDM; Sigma, St Quentin, France), supplemented with 15% granulocyte macrophage–colony-stimulating factor (GM-CSF)–containing, J558-conditioned medium. Bone marrow–derived DCs (BMDCs) were generated by 10 to 14 days of culture in complete IMDM with 30% J558-conditioned medium,12 and used when fluorescence activated cell sorting (FACS) analysis revealed more than 75% CD11c+ cells. Maturation was induced by a 16-hour treatment with 5 μg/mL LPS (Sigma). DCs were purified from spleens using anti-CD11c–coupled magnetic beads, B cells were purified from the CD11c-negative fraction with anti-CD19 beads (Miltenyi Biotec, Paris, France).
Antibodies and reagents
Fluorophore-coupled antibodies used for FACS analysis were as follows (all from BD PharMingen, Le Pont de Claix, France): anti-CD11c (HL3 clone), I-Ab (AF6-120.1), CD40 (3/23), CD86 (GL1), B220 (RA3-6B2), CD69 (H1.2F3), CD45.1 (A20), CD4 (RM4-5), and the corresponding isotype controls. Tricolor-conjugated streptavidine was from Caltag (Burlingame, CA). Antibodies used for exosome characterization by Western blotting were: rabbit antisera anti–mouse MHC class II α chain C-terminus,12 MHC class I (P8; Dr Ploegh, Boston, MA), MFG-E8 (prepared against LVTLDTQRGDIFTEY peptide from mouse MFG-E8,13 mouse monoclonal anti–mouse clathrin (23 clone; BD Transduction Laboratories, Le Pont de Claix, France), annexin II (Dr Gerke, Münster, Germany), flotillin-1 (18 clone; BD Transduction Laboratories); goat anti–IgG 101 (M-19 clone; Santa Cruz Biotechnology, Santa Cruz, CA); rat monoclonal anti–mouse CD9 (KMC8 clone; BDPharmingen), gp96 (9G10 clone; Stressgen, Victoria, Canada), hsc70 (1B5 clone; Stressgen), and CD86 (GL1 clone; BDPharmingen). Horseradish peroxidase (HRP)–conjugated secondary antibodies were from Pierce (Perbio, Brebieres, France). Carboxyfluorescein diacetate succinimidyl ester (CFSE) was from Molecular Probes (Eugene, OR). HY (NAGFNSNRANSSRSS) and Eα56-73 (ASFEAQGALANIAVDKA) peptides were from Neosystem (Strasbourg, France).
Exosome purification
Exosomes were recovered from the supernatant of cells cultured for 24 hours in depleted medium (ie, medium depleted from FCS-derived exosomes by overnight centrifugation at 100 000g).11 Exosomes were purified by filtration on 0.22-μm pore filters, followed by ultracentrifugation at 100 000g as described.11 In each exosome preparation, the concentration of total proteins was quantified by Bradford assay (BioRad, Marnes la Coquette, France). Exosomal proteins (3 μg to 5 μg) were analyzed by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and Western blotting or coated on beads for FACS analysis as described previously.11 To obtain exosomes bearing MHC-peptide complexes, immature or mature DCs were pulsed for 3 hours in complete medium with 250 nM HY peptide (or 50 μM Eα56-73), before the 24-hour culture period in depleted medium.6
Electron microscopy (EM)
Exosomes fixed in 2% paraformaldehyde (Carlo Erba, Rodano, Italy) were loaded on Formwar/carbon-coated EM grids. Samples were postfixed in 1% glutaraldehyde, contrasted and embedded successively in 2% uranyl acetate, pH 7, and 2% methylcellulose/0.4% uranyl acetate, pH 4. EM samples were observed at 80 kV with a CM120 Twin Phillips electron microscope (FEI company, Eindoven, The Netherlands). EM negatives were scanned at 400 dpi with a HI-SCAN drum scanner (Eurocore, Heidelberg, Germany) and processed with ColorBrain 3.1 software (Eurocore) and Adobe Photoshop 8.0 software (Adobe, San Jose, CA).
Sucrose gradient
Exosomes (30 μg) were separated by a 16-hour centrifugation at 100 000g on a linear 2 M to 0.25 M sucrose gradient as previously described.12 Pellets recovered from 1-mL fractions were analyzed by SDS-PAGE and Western blotting.
Mass spectrometry (MS) analysis
Slices 1-mm wide were cut out from Coomassie blue–stained gels, and were reduced, alkylated, and trypsin digested as previously described.14 Extracted peptides were dried and resolubilized in 95:5 water/acetonitrile. One third of the concentrated protein digests were separated on an LC-Packing system (Dionex, Sunnyvale, CA) coupled to the nanoelectrospray II ionization interface of a QSTAR/Pulsari (Applied Biosystems, Foster City, CA). The MS/MS data were searched using MASCOT software (Matrixscience, London, United Kingdom) against the National Center for Biotechnology Information database (Bethesda, MD). MASCOT files were extracted to a software interface PROMS (Bioinformatics, Institut Curie, Paris, France), and each peptide was validated manually.
In vitro T-cell stimulation assay
As previously described,6 exosomes or HY peptide were incubated in 96-well plates with 2 × 104 recipient cells (ie, LPS-treated BMDCs, purified spleen DCs, or B cells) in 50 μL complete IMDM for 3 to 4 hours at 37°C. Plates were irradiated (5000 rad) and 5 × 104 Marilyn lymph node cells were added in 100 μL complete IMDM. 3H-thymidine (0.5 μCi/well [18.5 KBq/well]) was added 72 hours later for 16 hours. Cells were harvested on glass fiber filters (Tomtec, Hamden, CT), and 3H-thymidine incorporation was counted by liquid scintillation. Alternatively, cells were harvested 24 hours after addition of Marilyn T cells, and stained with anti-CD45.1, anti-CD4, and anti-CD69 antibodies before FACS analysis.
In vivo injection in mice
Female C57Bl/6 mice received intraveneous injections of 106 CFSE-labeled (5 μM in phosphate-buffered saline [PBS]–0.1% bovine serum albumin [BSA], 8 minutes, 37°C) CD45.1+ Marilyn lymph node cells. At 24 hours later, exosomes or DCs were injected in the hindfootpad. Draining lymph nodes were analyzed 4 days later by FACS. The amount of exosomes injected ranged from 1 μgto15 μg of total proteins.
Skin-graft assay
Female C57Bl/6 mice received footpad injections of PBS (50 μL) or 5 μg HY-bearing immature or mature exosomes. The next day, 1-cm2 fragments of belly skin from male C57Bl/6 mice were grafted onto the backs of female recipients. Plasters were removed after 6 days, and from then on, only mice with accepted grafts were analyzed. Grafts were macroscopically examined every other day, and were considered rejected when more than 80% of graft surface appeared necrotic.
Results
LPS-treated DCs secrete exosomes
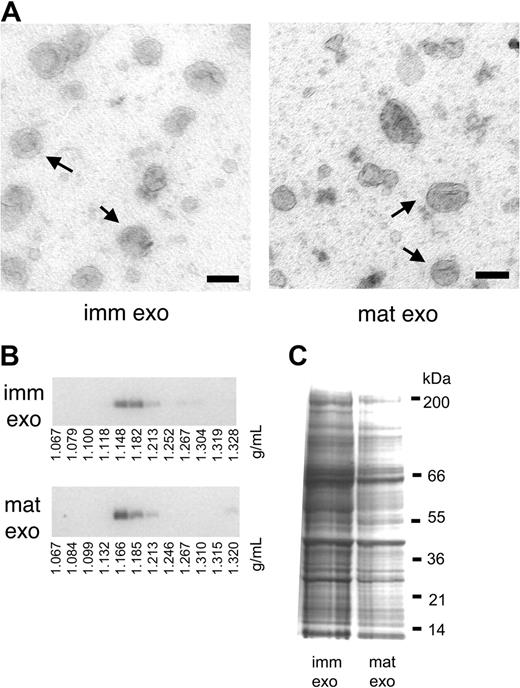
Exosomes produced during 24 hours were purified from supernatants of LPS-treated or untreated DCs (D1 cell line or BMDCs), using the usual filtration and ultracentrifugation-based exosome purification procedure.11 Exosomes from LPS-treated DCs will be called mature exosomes, as opposed to immature exosomes produced by untreated DCs. As described before,12 LPS-treated DCs produced on average 2- to 3-fold fewer exosomes than untreated DCs (0.2 ± 0.1 μg/million mature cells, versus 0.5 ± 0.1 μg/million immature cells). The morphology of mature exosomes observed by electron microscopy (Figure 1A), their density on sucrose gradients (Figure 1B), as well as their overall protein composition, as seen on a coomassie blue–stained gel (Figure 1C) were similar to that of immature exosomes. Thus, although mature DCs lose part of their internal multivesicular endosomes, which become elongated tubular structures,15 the remaining MVBs are still a source of exosome secretion.
Exosomes from mature DCs induce strong T-cell proliferation in vitro
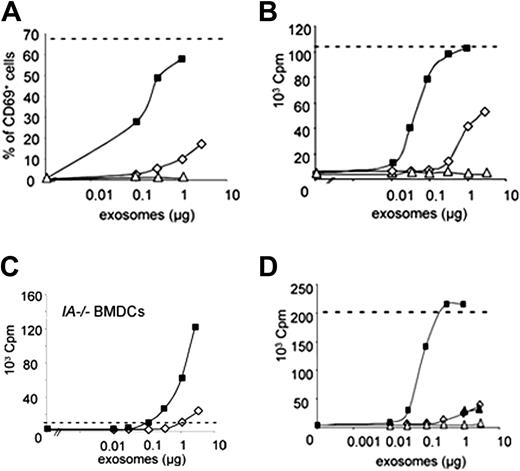
We analyzed whether exosomes from mature DCs would display increased immunogenicity as compared with immature exosomes. A DC-dependent antigen-presentation assay was used, as previously described.6 Immature or LPS-treated DCs were incubated for 3 hours with HY peptide, derived from the male antigen Dby, before the exosome production phase. Purified exosomes were incubated in vitro with mature BMDCs for 3 hours, before addition of HY-specific naive CD4 T cells from Marilyn mice.16 T-cell activation was assessed by measuring up-regulation of the early activation marker CD69 after 24 hours (Figure 2A), and T-cell proliferation after 3 days of culture (Figure 2B). In both assays, mature exosomes induced similar or higher plateau of T-cell activation as peptide directly loaded on DCs, whereas immature exosomes were 50- to 100-fold less efficient. Mature I-Ab/HY–bearing exosomes induced T-cell proliferation when loaded on MHC class II–deficient DCs, which cannot activate T cells in the presence of peptide alone (Figure 2C). In this setting as well, mature exosomes were 100-fold more efficient than immature exosomes. Exosomes from mature DCs that had not been exposed to HY peptide (Figure 2A), or exosomes from mature MHC class II–deficient BMDCs pulsed with HY peptide (Figure 2B) did not induce T-cell proliferation. The weak immature exosome activity was not increased by addition of LPS (Figure S1A, available on the Blood website; see the Supplemental Materials link at the top of the online article) or of mature exosomes that did not bear HY peptide (Figure 2D). We conclude that T-cell activation and proliferation are more efficiently induced by mature than immature exosomes. Strong immunogenicity of mature exosomes is not due to nonspecific contaminants (ie, LPS or free HY peptide) in the mature exosome preparations, but rather to the actual composition of the vesicles secreted by mature DCs.
Mature-DC– and immature-DC–derived exosomes are qualitatively similar. (A) Whole-mount exosomes observed by electron microscopy. Bar represents 100 nm. Arrows indicate some examples of immature exosomes (imm exo) or mature exosomes (mat exo). (B) Immature (imm exo) or mature exosomes (mat exo) were subjected to flotation on a continuous sucrose gradient. Twelve fractions were collected, run on a 10% SDS gel, and analyzed by Western blot. Staining for MHC class II molecules is shown. (C) Coomassie blue staining of 30 μg immature (imm) and mature (mat) exosomes separated on 4% to 12% SDS gel.
Mature-DC– and immature-DC–derived exosomes are qualitatively similar. (A) Whole-mount exosomes observed by electron microscopy. Bar represents 100 nm. Arrows indicate some examples of immature exosomes (imm exo) or mature exosomes (mat exo). (B) Immature (imm exo) or mature exosomes (mat exo) were subjected to flotation on a continuous sucrose gradient. Twelve fractions were collected, run on a 10% SDS gel, and analyzed by Western blot. Staining for MHC class II molecules is shown. (C) Coomassie blue staining of 30 μg immature (imm) and mature (mat) exosomes separated on 4% to 12% SDS gel.
Mature exosomes induce strong T-cell activation and proliferation in vitro. (A) Mature HY-bearing (▪) or HY free (▵) or immature HY-bearing (⋄) exosomes were incubated for 3 hours in vitro with mature BMDCs. Up-regulation of CD69 on Marilyn T cells was measured 24 hours after T-cell addition. Dashes indicate the maximal level of CD69 up-regulation obtained with mature BMDCs incubated with 10 nM HY peptide. One representative of 6 independent experiments is shown. (B) HY-bearing exosomes from mature (▪), immature (⋄), or MHC class II–deficient mature (▵) BMDCs were incubated 3 hours in vitro with mature BMDCs. After irradiation, Marilyn T cells were added in the culture for 3 days and 3H-thymidine incorporation was measured during the last 16 hours. Dashes indicate the maximal level obtained in the presence of 17 nM HY peptide. One representative of 5 independent experiments is shown. cpm indicates counts per minute. (C) 3H-thymidine incorporation by Marilyn T cells cultured with mature MHC class II–deficient (IA–/–) BMDCs and IAb/HY-bearing exosomes from immature (⋄) or mature (▪) DCs. Dashes indicate the level obtained in the presence of 17 nM HY peptide. One representative of 3 independent experiments. (D) 3H-thymidine incorporation by Marilyn T cells cultured with mature BMDCs and mature HY-bearing (▪) or mature HY-free (▵) or immature HY-bearing (⋄) exosomes or both immature HY-bearing and 3 μg of mature HY-free exosomes (▴). One representative of 2 independent experiments. Dashes indicate the plateau obtained in presence of 17 nM HY peptide.
Mature exosomes induce strong T-cell activation and proliferation in vitro. (A) Mature HY-bearing (▪) or HY free (▵) or immature HY-bearing (⋄) exosomes were incubated for 3 hours in vitro with mature BMDCs. Up-regulation of CD69 on Marilyn T cells was measured 24 hours after T-cell addition. Dashes indicate the maximal level of CD69 up-regulation obtained with mature BMDCs incubated with 10 nM HY peptide. One representative of 6 independent experiments is shown. (B) HY-bearing exosomes from mature (▪), immature (⋄), or MHC class II–deficient mature (▵) BMDCs were incubated 3 hours in vitro with mature BMDCs. After irradiation, Marilyn T cells were added in the culture for 3 days and 3H-thymidine incorporation was measured during the last 16 hours. Dashes indicate the maximal level obtained in the presence of 17 nM HY peptide. One representative of 5 independent experiments is shown. cpm indicates counts per minute. (C) 3H-thymidine incorporation by Marilyn T cells cultured with mature MHC class II–deficient (IA–/–) BMDCs and IAb/HY-bearing exosomes from immature (⋄) or mature (▪) DCs. Dashes indicate the level obtained in the presence of 17 nM HY peptide. One representative of 3 independent experiments. (D) 3H-thymidine incorporation by Marilyn T cells cultured with mature BMDCs and mature HY-bearing (▪) or mature HY-free (▵) or immature HY-bearing (⋄) exosomes or both immature HY-bearing and 3 μg of mature HY-free exosomes (▴). One representative of 2 independent experiments. Dashes indicate the plateau obtained in presence of 17 nM HY peptide.
Mature exosomes transfer to B lymphocytes the ability to activate naive T cells
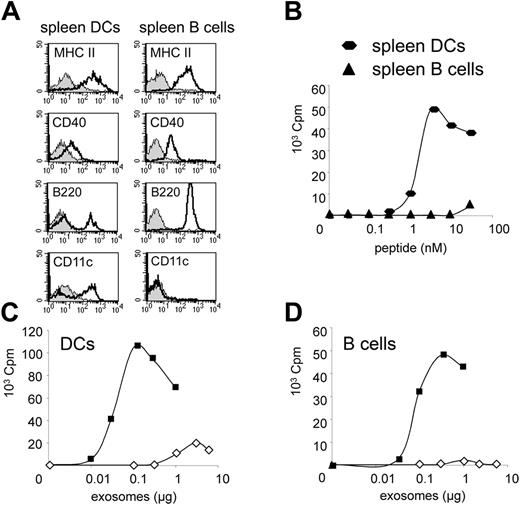
Immature exosomes can be used in vitro by other DCs (BMDCs or spleen-derived DCs) to activate CD4 T cells, but not by other APCs such as B cells or macrophages.6 It is unclear whether this lack of naive T-cell priming is due to the inability of B cells or macrophages to bind exosomes, or to the lack of the appropriate costimulation molecules in these antigen-presenting cells. To determine whether mature exosomes behaved like immature exosomes in this respect, CD11c+ DCs and CD19+ B cells from female mice spleens were used as exosome recipients in the in vitro T-cell activation assay. Both cell types expressed similar levels of MHC class II, and expressed the costimulatory molecule CD40 (Figure 3A). Spleen B cells did not induce naive CD4 T-cell proliferation in the presence of HY peptide (Figure 3B) or immature exosomes (Figure 3D) in conditions where splenic DCs induced efficient T-cell activation (Figure 3B-C). Mature exosomes, by contrast, were readily presented by spleen B cells and induced potent T-cell proliferation (Figure 3D). Thus, mature exosomes can transfer to inefficient APCs (ie, B cells) the unique ability of DCs to activate naive T cells.
Mature exosomes induce CD4+ T-cell priming in vivo
The effects of mature exosomes in vivo were next analyzed as previously described.6 Immature or mature HY-bearing exosomes (15 μg) were injected in the footpads of female C57Bl/6 mice adoptively transferred one day before with CFSE-labeled Marilyn lymph node cells. Draining lymph nodes were harvested 4 days later, and HY-specific T-cell proliferation was evidenced by a 2-fold decrease of CFSE intensity in daughter cells at each division cycle (Figure 4A). Although both immature and mature HY-bearing exosomes induced T-cell divisions, a larger proportion of T cells underwent extensive proliferation (more than 5 division cycles) upon mature exosome injection (Figure 4A). When injected at lower doses, mature exosomes still induced proliferation of Marilyn cells very efficiently, even with as little as 1 μg per mouse, whereas 10- to 20-fold higher concentrations of immature exosomes were needed to induce similar proliferation (Figure 4B). Exosomes from mature DCs that had not been exposed to HY peptide did not induce CD4 T-cell proliferation (Figure 4B). Coinjection of immature exosomes with LPS also did not induce more efficient T-cell activation (Figure S2). Mature exosomes are therefore more efficient than immature exosomes to activate naive T cells in vivo.
B lymphocytes can use mature exosomes to activate naive CD4 T cells. (A) Cell-surface expression of T-cell stimulatory molecules (MHC class II: MHC II, CD40) and phenotypic markers (CD11c, B220) by magnetically purified spleen CD11c+ DCs or CD19+ B cells. Shaded histograms indicate control isotype; open histograms, specific markers. Purified B cells contain 0.1% CD11c+ cells, (B) 3H-thymidine incorporation by Marilyn T cells cultured with magnetically purified spleen CD11c+ DCs or CD19+ B cells in the presence of HY peptide. One representative of 5 independent experiments is shown. (C,D) 3H-thymidine incorporation by Marilyn T cells cultured with magnetically purified spleen CD11c+ DCs (C) or CD19+ B cells (D) in the presence of immature HY-bearing exosomes (⋄) or mature HY-bearing exosomes (▪). One representative of 5 independent experiments is shown. Results shown in A-D were from the same experiment.
B lymphocytes can use mature exosomes to activate naive CD4 T cells. (A) Cell-surface expression of T-cell stimulatory molecules (MHC class II: MHC II, CD40) and phenotypic markers (CD11c, B220) by magnetically purified spleen CD11c+ DCs or CD19+ B cells. Shaded histograms indicate control isotype; open histograms, specific markers. Purified B cells contain 0.1% CD11c+ cells, (B) 3H-thymidine incorporation by Marilyn T cells cultured with magnetically purified spleen CD11c+ DCs or CD19+ B cells in the presence of HY peptide. One representative of 5 independent experiments is shown. (C,D) 3H-thymidine incorporation by Marilyn T cells cultured with magnetically purified spleen CD11c+ DCs (C) or CD19+ B cells (D) in the presence of immature HY-bearing exosomes (⋄) or mature HY-bearing exosomes (▪). One representative of 5 independent experiments is shown. Results shown in A-D were from the same experiment.
Mature exosomes induce strong T-cell activation in vivo and fast skin graft rejection. (A) Exosomes (15 μg) were injected subcutaneously into female C57Bl/6 mice containing adoptively transferred CFSE-labeled, CD45.1+ Marilyn lymph node cells. Draining lymph node cells were analyzed by FACS 4 days after injection. CFSE staining of Marilyn cells, identified as CD4+CD45.1+, from mice treated with 15 μg of HY-free mature exosomes (Control Exo), HY-bearing immature exosomes (Imm HY Exo), or HY-bearing mature exosomes (Mat HY Exo). The percentage of cells that have undergone more than 5 divisions cycles is shown. One representative of 5 independent experiments is shown. (B) Percent of Marylin T cells that underwent more than 5 division cycles, upon injection of various doses of control (▵), immature (⋄), or mature (▪) exosomes. Results obtained from 8 mice per condition, in 3 to 5 independent experiments, were pooled. (C) Male skin grafts were transplanted onto female recipients that had been previously injected with PBS (▴), 5 μg immature HY-bearing exosomes (⋄), or 5 μg mature HY-bearing exosomes (▪). Graft rejection was examined every other day. Results obtained from 9 to 11 mice per group, in 4 independent experiments, are shown. Differences observed between mature exosome–treated mice and control group, and mature exosome–treated mice and immature exosome–treated mice are significant in Log rank test (P<.001 and P=.01 respectively).
Mature exosomes induce strong T-cell activation in vivo and fast skin graft rejection. (A) Exosomes (15 μg) were injected subcutaneously into female C57Bl/6 mice containing adoptively transferred CFSE-labeled, CD45.1+ Marilyn lymph node cells. Draining lymph node cells were analyzed by FACS 4 days after injection. CFSE staining of Marilyn cells, identified as CD4+CD45.1+, from mice treated with 15 μg of HY-free mature exosomes (Control Exo), HY-bearing immature exosomes (Imm HY Exo), or HY-bearing mature exosomes (Mat HY Exo). The percentage of cells that have undergone more than 5 divisions cycles is shown. One representative of 5 independent experiments is shown. (B) Percent of Marylin T cells that underwent more than 5 division cycles, upon injection of various doses of control (▵), immature (⋄), or mature (▪) exosomes. Results obtained from 8 mice per condition, in 3 to 5 independent experiments, were pooled. (C) Male skin grafts were transplanted onto female recipients that had been previously injected with PBS (▴), 5 μg immature HY-bearing exosomes (⋄), or 5 μg mature HY-bearing exosomes (▪). Graft rejection was examined every other day. Results obtained from 9 to 11 mice per group, in 4 independent experiments, are shown. Differences observed between mature exosome–treated mice and control group, and mature exosome–treated mice and immature exosome–treated mice are significant in Log rank test (P<.001 and P=.01 respectively).
To determine whether exosomes induce effector T cells in vivo, we used an autologous skin graft model. DCs loaded with HY peptide (ie, those which activate I-Ab-HY–specific CD4 T cells), have been previously shown to accelerate male skin graft rejection by female mice.17 We thus tested the effect of immature or mature I-Ab-HY–bearing exosomes on male skin graft rejection by autologous female mice. In mice treated with immature exosomes one day before graft, the kinetics of graft rejection was not significantly different from the kinetics in control mice injected with PBS (Figure 4C). By contrast, mature exosomes induced significantly faster skin graft rejection (Figure 4C). This effect was not due to contaminants (for instance residual HY peptide), since exosomes from MHC class II–deficient DCs pulsed with HY peptide did not accelerate the skin graft rejection, as compared with PBS injection (data not shown).
These results show that mature exosomes are more efficient than immature exosomes for the induction of T-cell activation in vivo. For the induction of effector CD4+ T cells, however, only mature exosomes are effective.
Immature and mature exosomes present quantitatively different protein compositions
To identify the molecular bases of the strong immunogenicity of mature exosomes, we analyzed their protein composition. Extensive proteomic analysis by tandem mass spectrometry identified 93 new proteins that had not been evidenced before in DC-derived exosomes11,18 (Table 1). Some of these proteins had also been identified in exosomes from other cell types (Table 1; see Table S1 and corresponding references for exhaustive results). All these proteins were found both in immature DC– and mature DC–derived exosomes. Their respective amounts in each type of exosome could not be assessed by the proteomic approach. Western blotting was therefore performed to compare quantitatively immature and mature exosomes. Gp96, a resident protein of the endoplasmic reticulum used as a control for endoplasmic reticulum contamination, was absent from both types of exosomes (Figure 5A). Several proteins were present in equal amounts in immature and mature exosomes (Figure 5A). On the other hand, several proteins that are modulated during DC maturation were similarly modulated in exosomes (Figure 5B): MHC class II and costimulatory molecules B7.2/CD86 and ICAM-1/CD54 were strongly increased in mature exosomes as compared with immature exosomes. MFG-E8/lactadherin, which is down-regulated in mature DCs19 (P.V., E.S., Gaël Sugano, S.A., and C.T., manuscript submitted, May 2005), was also much less abundant on mature exosomes. Finally, MHC class I and tsg101, which are respectively up-regulated and not affected by LPS treatment, were both slightly down-modulated in mature exosomes (Figure 5B). The protein composition of exosomes produced by DCs incubated with LPS for 3 hours, rather than 16 hours, was intermediate between that of immature and mature exosomes, in terms of MHC class II, CD86, and MFG-E8 content (data not shown).
Newly identified proteins present in immature and mature exosomes
Type . | Name . |
|---|---|
| Chaperones | TCP-1 β,δ,ϵ,θ,ζ |
| cyclophilin A, B | |
| H2-M, la associated invariant chain | |
| Membrane fusion | Annexin 6, 11 |
| Rab 2, 4, 5, 6, 10, 13, 14, 15 | |
| Clathrin, Arp 2/3, Rho A | |
| SNAP 23, syntaxin 7, AP-1 | |
| WD repeat-containing protein 1 | |
| Targeting/adhesion | Thrombospondin 1, Vinculin |
| Plexin A1, C1, Neuropilin 1 | |
| Dectin 1, 2β, 2γ | |
| Integrin β1, CD13, CD166 | |
| Cadherin 1, PGRL, PIRA7 | |
| Cytoskeleton | CAP1, Coronin, advillin, Fascin1, TCTP |
| IQGAP1, Rho GDII, FRL, radixin | |
| Ezrin, Moesin, Talin, Vimentin, myosin heavy chain IX, claudin 1 | |
| dynamin 2, actinin α4, catenin Eα, βcatenin | |
| syntaxin binding protein 3 | |
| Signal transduction | Phospholipase Cα, Fyn, STAT1 |
| Copine 1, c-scr kinase, SHPS-1 | |
| ERK2, Grb 3-3, H-ras | |
| Gi3α, Gsα | |
| Enzymes | Aldehyde reductase, ATP citrate lyase |
| fatty acid synthase, tyk 2, SH2 phosphatase 1 | |
| glucose 6 phosphate isomerase | |
| phosphogluconate deshydrogenase | |
| ATPase Na+/K+ transporting, transketolase | |
| Dipeptidylpeptidase 4, M2 pyruvate kinase | |
| Lipid rafts | Flotillin 1, stomatin, prohibitin |
| Others | Nicastrin, ubiquitine C |
| Ribosomal proteins | |
| MVP, CD98, eEF2 |
Type . | Name . |
|---|---|
| Chaperones | TCP-1 β,δ,ϵ,θ,ζ |
| cyclophilin A, B | |
| H2-M, la associated invariant chain | |
| Membrane fusion | Annexin 6, 11 |
| Rab 2, 4, 5, 6, 10, 13, 14, 15 | |
| Clathrin, Arp 2/3, Rho A | |
| SNAP 23, syntaxin 7, AP-1 | |
| WD repeat-containing protein 1 | |
| Targeting/adhesion | Thrombospondin 1, Vinculin |
| Plexin A1, C1, Neuropilin 1 | |
| Dectin 1, 2β, 2γ | |
| Integrin β1, CD13, CD166 | |
| Cadherin 1, PGRL, PIRA7 | |
| Cytoskeleton | CAP1, Coronin, advillin, Fascin1, TCTP |
| IQGAP1, Rho GDII, FRL, radixin | |
| Ezrin, Moesin, Talin, Vimentin, myosin heavy chain IX, claudin 1 | |
| dynamin 2, actinin α4, catenin Eα, βcatenin | |
| syntaxin binding protein 3 | |
| Signal transduction | Phospholipase Cα, Fyn, STAT1 |
| Copine 1, c-scr kinase, SHPS-1 | |
| ERK2, Grb 3-3, H-ras | |
| Gi3α, Gsα | |
| Enzymes | Aldehyde reductase, ATP citrate lyase |
| fatty acid synthase, tyk 2, SH2 phosphatase 1 | |
| glucose 6 phosphate isomerase | |
| phosphogluconate deshydrogenase | |
| ATPase Na+/K+ transporting, transketolase | |
| Dipeptidylpeptidase 4, M2 pyruvate kinase | |
| Lipid rafts | Flotillin 1, stomatin, prohibitin |
| Others | Nicastrin, ubiquitine C |
| Ribosomal proteins | |
| MVP, CD98, eEF2 |
Forty bands from immature and mature DC exosome preparation separated by SDS-PAGE (shown in Figure 1C) were cut out and analyzed by tandem mass spectrometry. Underlined italicization indicates proteins also identified in exosomes secreted by other cells (see Table S1 for details).
Mature DC– and immature DC–derived exosomes display different quantitative protein compositions. (A,B) Proteins (10 μg) from DC lysate (DCs) or 3 μg of proteins from exosomes (Exo), either immature (i) or mature (m), were loaded on a 10% SDS gel followed by Western blotting using antibodies against proteins indicated on the right. (A) Proteins present in equal amounts in immature and mature exosomes. (B) Proteins up- or down-modulated on mature exosomes.
Mature DC– and immature DC–derived exosomes display different quantitative protein compositions. (A,B) Proteins (10 μg) from DC lysate (DCs) or 3 μg of proteins from exosomes (Exo), either immature (i) or mature (m), were loaded on a 10% SDS gel followed by Western blotting using antibodies against proteins indicated on the right. (A) Proteins present in equal amounts in immature and mature exosomes. (B) Proteins up- or down-modulated on mature exosomes.
Mature exosomes contain 2 to 3 times more MHC II molecules (Figure 5B; Figure S3), and only 1.5 times more specific MHC II–peptide complexes (Figure S3), than immature exosomes. Increased number of MHC-peptide complexes on mature exosomes could therefore not account for their stronger efficiency in activating T cells. Among exosomal proteins known to be involved in immune responses, 3 were strongly modulated in mature exosomes: MFG-E8, B7.2, and ICAM-1. We thus analyzed the role of these proteins in exosome-induced T-cell activation.
Decreased MFG-E8 does not account for mature exosomes enhanced activity
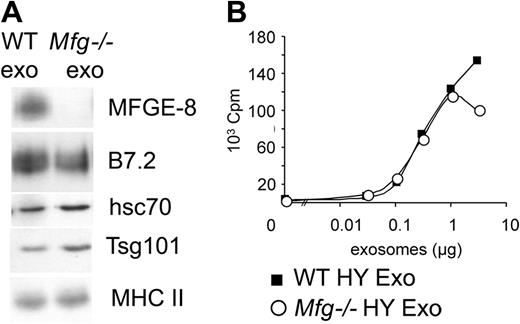
MFG-E8 is involved in phagocytosis and destruction of apoptotic cells by macrophages.20 MFG-E8 present on exosomes could thus mediate targeting of exosomes to DCs. To test this hypothesis, exosomes were purified from MFG-E8–deficient BMDCs. MFG-E8–deficient mice obtained from embryonic stem (ES) cells bearing a genetrap vector in the Mfge8 gene21 are described elsewhere.13 DCs obtained in vitro from BM precursors of Mfge8–/– mice presented identical differentiation kinetics and phenotype as those obtained from WT animals, and induced identical activation of CD4 T cells in the presence of HY peptide (data not shown). Apart from the absence of MFG-E8, exosomes purified from immature Mfge8–/– BMDCs had the same protein composition as exosomes from WT BMDCs (Figure 6A). In the in vitro presentation assay, immature Mfge8–/– exosomes did not induce stronger T-cell proliferation than WT exosomes (Figure 6B). Note that the mature BMDCs used as recipient cells do not express MFG-E819 (P.V., E.S., Gaël Sugano, S.A., and C.T., manuscript submitted, May 2005), and therefore could not compensate for the absence of MFG-E8 on exosomes. Thus, absence of MFG-E8 on mature exosomes does not explain their increased biologic activity.
ICAM-1, but not B7.1 or B7.2, is essential for mature exosomes immunogenicity
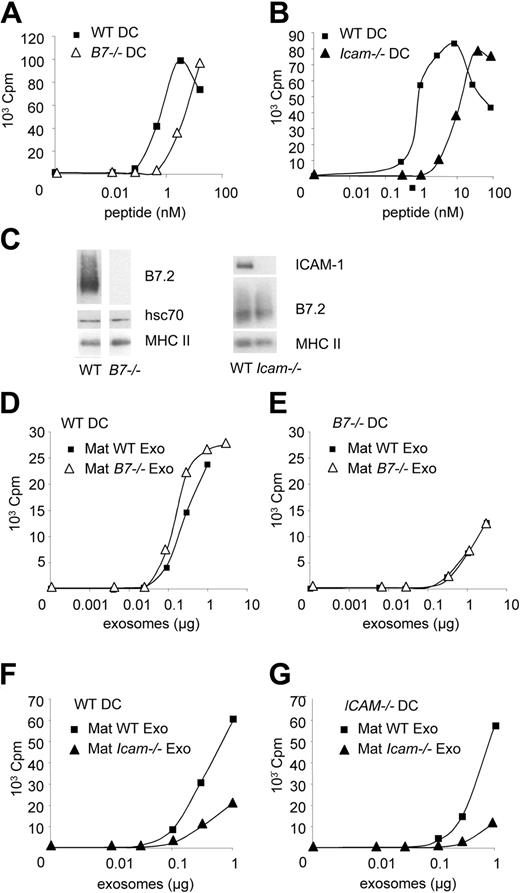
The roles of B7.2 and ICAM-1 on exosomes were analyzed next. As compared with WT DCs, DCs from B7.2/CD86 and B7.1/CD80 double knock-out mice (B7–/– DCs) need 3 times more HY peptide (Figure 7A), and DCs from Icam1/CD54 knock-out mice need 6 times more HY peptide (Figure 7B), to activate Marilyn T cells in vitro. Both molecules thus participate in CD4+ T-cell activation.
Absence of MFG-E8 on mature exosomes does not account for their increased efficiency. (A) Western blot analysis of 3 μg of exosomes purified from immature WT or MFG-E8–deficient (Mfg–/–) BMDCs. (B) 3H-thymidine incorporation by Marilyn T cells cultured with mature BMDCs and HY-bearing immature WT (WT HY Exo) or MFG-E8–deficient exosomes (Mfg–/– HY Exo). One representative experiment out of 4.
Absence of MFG-E8 on mature exosomes does not account for their increased efficiency. (A) Western blot analysis of 3 μg of exosomes purified from immature WT or MFG-E8–deficient (Mfg–/–) BMDCs. (B) 3H-thymidine incorporation by Marilyn T cells cultured with mature BMDCs and HY-bearing immature WT (WT HY Exo) or MFG-E8–deficient exosomes (Mfg–/– HY Exo). One representative experiment out of 4.
Mature exosomes devoid of B7.2 were purified from B7–/– BMDCs. Their protein composition was similar to that of exosomes from WT DCs, except for the absence of B7.2 (Figure 7C). In vitro, in the presence of T cells and recipient WT spleen DCs (Figure 7D), B7–/– exosomes induced similar T-cell proliferation as WT exosomes. When using B7–/– DCs as recipient of WT and B7–/– exosomes, we also observed similar efficiencies of the 2 types of exosomes (Figure 7E), although T-cell stimulation was lower than with WT DCs. Therefore, B7.2 expressed by mature exosomes does not account for their increased immunogenicity as compared with immature exosomes. By contrast, mature exosomes produced by Icam-1–/– BMDCs did not induce efficient T-cell activation, when fed to ICAM-1+ recipient DCs (Figure 7F) or to B cells (Figure S4). ICAM-1 expressed on recipient DCs did not overcome the defect of ICAM-1–deficient exosomes. Expression of ICAM-1 on exosomes is therefore critically required for stimulation of CD4+ T cells.
Finally, B7–/– DCs induced less efficient T-cell activation than WT DCs in the presence of mature WT exosomes (Figure 7D-E), whereas the difference between WT and Icam-1–/– DCs was not as striking (Figure 7F-G). We concluded that recipient DCs can use ICAM-1 present on exosomes, but need their own B7.2 molecules, for efficient T-cell activation.
Discussion
The results presented here show that LPS-treated DCs (ie, mature DCs) secrete bona fide exosomes, as defined by their morphology, qualitative protein composition, and buoyant characteristics. Exosomes from mature DCs, however, are more potent for antigen-specific T-cell activation than exosomes from immature DCs. Three major findings on exosomes produced by mature DCs are reported here: (1) these exosomes induce effector T-cell responses, since they induce rapid rejection of antigen-bearing grafts, (2) they can transfer not only MHC-peptide complexes, but also T-cell–stimulating ability to poorly efficient APCs, such as B cells, and (3) expression of ICAM-1 on mature exosomes is necessary for their immunogenicity.
We have previously reported that immature exosomes induce in vivo antigen-specific CD4 T-cell activation and proliferation.6 However, the functional outcome of this early activation was not evaluated, and could have been either an effector or tolerogenic response. Another group has shown that immature DC–derived exosomes induce efficient CD8-dependent antitumor immune responses, but only when coinjected with an adjuvant.7 In a heart allograft model, repeated intravenous injections of immature exosomes led to tolerance.22 Here, we have used a skin graft assay, which allows detection of tolerogenic and effector responses. In the protocol used here, CD4+ T-cell activation induced by immature exosomes did not give rise to either effector or tolerogenic responses, since skin graft rejection was not significantly affected by these exosomes. By contrast, a single injection of mature exosomes induced effector T-cell generation, and led to fast skin graft rejection in the mouse model system. Using immature exosomes to inhibit immune responses in autoimmune disease or transplantation settings will therefore need careful examination of the treatment conditions, such as injection sites, number of injections, and amount of exosomes.
Since we have first described antitumor immune responses induced by DC-derived exosomes in mice,23 the use of exosomes in clinical antitumor immunotherapy has been a strong focus of interest. As opposed to skin rejection, tumor rejection needs strong CD8+ T-cell response, enhanced by efficient CD4+ T-cell help. Whereas MHC class II was increased on mature exosomes, MHC class I was slightly down-regulated. Experiments are being perfomed to evaluate the impact of mature exosomes on tumor growth in mice. It is also important to stress that human and mouse DC-derived exosomes are not identical. Exosomes purified from monocyte-derived human DCs express ICAM-1 and B7.2,24,25 and probably display an intermediate phenotype between our “immature” and “mature” mouse DC–derived exosomes. Human clinical-grade exosomes,25 loaded ex vivo with peptides, activate both CD4+ and CD8+ T cells in vitro.26 These results suggest that exosomes from human monocyte–derived DCs are efficient to activate T cells.
B7.2 is dispensable, and ICAM-1 is required for increased immunogenicity in mature exosomes. (A,B) 3H-thymidine incorporation by Marilyn T cells cultured with HY peptide and mature BMDCs either WT or deficient for B7.1 and B7.2 (A) or ICAM-1 (B). One representative experiment out of 5 (A) or 2 (B). (C) Western blot analysis of exosomes purified from mature WT, B7.1,2-deficient (B7–/–), or ICAM-1–deficient (Icam–/–) BMDCs. (D-G) 3H-thymidine incorporation by Marilyn T cells cultured with WT (D,F), or B7–/– (E), or Icam-1–/– (G) spleen DCs, and HY-bearing mature exosomes from WT, B7–/– (D,E), or Icam-1–/– (F,G) BMDCs. One representative experiment out of 2. Panels D and E on one side, and F and G on the other, were from the same experiment.
B7.2 is dispensable, and ICAM-1 is required for increased immunogenicity in mature exosomes. (A,B) 3H-thymidine incorporation by Marilyn T cells cultured with HY peptide and mature BMDCs either WT or deficient for B7.1 and B7.2 (A) or ICAM-1 (B). One representative experiment out of 5 (A) or 2 (B). (C) Western blot analysis of exosomes purified from mature WT, B7.1,2-deficient (B7–/–), or ICAM-1–deficient (Icam–/–) BMDCs. (D-G) 3H-thymidine incorporation by Marilyn T cells cultured with WT (D,F), or B7–/– (E), or Icam-1–/– (G) spleen DCs, and HY-bearing mature exosomes from WT, B7–/– (D,E), or Icam-1–/– (F,G) BMDCs. One representative experiment out of 2. Panels D and E on one side, and F and G on the other, were from the same experiment.
The way exosomes induce antigen-specific immune responses is not yet elucidated. We and others have shown that MHC class I– and class II–peptide complexes present on exosomes can be used by recipient DCs to activate antigen-specific T cells.6,9,26 In these studies, other APCs such as B cells or macrophages, which could not activate naive T cells unless high doses of peptide were used, could also not use exosomes as a source of MHC-peptide complexes to activate T cells. A striking property of exosomes produced by LPS-treated DCs is that they endow B lymphocytes with the ability to activate naive T lymphocytes. As described by others,27 we also observed some T-cell activation by exosomes from mature DCs in the absence of recipient APCs (data not shown). This activation, however, was weak and only occurred when using high amounts of exosomes, over 3 μg, equivalent to the amount secreted by about 10 million mature DCs. In the presence of APCs, on the other hand, T-cell activation was strong with as few as 0.1 μg exosomes. These observations suggest that, rather than directly presenting MHC-peptide complexes to naive T cells, exosomes physiologically transfer them to recipient APCs, thus activating T cells. Secretion of exosomes by mature DCs in the lymph node could allow surrounding B cells to acquire MHC-peptide complexes, and so provide chances for a T lymphocyte to encounter its specific ligand and become activated in a broad area of the lymph node (ie, also at the B-cell/T-cell area junction). On the B-cell side, however, the outcome of interaction with a T cell via exosomal MHC-peptide will not necessarily lead to B-cell activation. Indeed, to induce full B-cell maturation and antibody production, repeated stimulation via MHC molecules must occur, especially on daughter B cells resulting from proliferation. Exosomal MHC molecules are probably not available in sufficient amounts to allow this repetitive B-cell activation, and would therefore not result in nonspecific antibody response.
Exosomes secreted by mature DCs are thus small vesicles carrying the exquisite ability of mature DCs to induce T-cell responses. Exosomes do not act as mere adjuvants, since only exosomes bearing both MHC-peptide complexes and other factors from mature DCs induce efficient T-cell activation. Among several molecules differentially expressed by exosomes from immature versus LPS-treated DCs, one molecule was identified as responsible for this increased immunogenicity: ICAM-1. Exosomal ICAM-1 can compensate for a lack of this adhesion molecule on APCs, whereas B7.2 has to be expressed by the APC to efficiently induce T-cell activation. These 2 molecules thus play complementary roles on T-cell activation. As a ligand for the lymphocyte function-associated antigen (LFA-1) and Mac-1 (CD11b/CD18) integrins (which are expressed by DCs, B cells, and T cells), ICAM-1 could either help with capture of exosomes by the recipient APCs, or favor T-cell binding to the recipient APC bearing exosomes at its surface. Endocytosis of immature DC–derived exosomes by immature DCs, and subsequent reprocessing of exosomal antigens by DCs has been shown.18 In the present work, however, recipient APCs that use exosomes to activate T cells are mature DCs and B cells displaying poor endocytosis activity. In addition, presence of preformed MHC-peptide complexes on exosomes allows T-cell activation by recipient APCs without a need for antigen reprocessing. Exosomes from mature DCs could thus be presented to T cells as whole MHC- and ICAM-1–enriched microdomains at the surface of recipient APCs. To do so, exosomes could either fuse with the plasma membrane, or simply stick to the surface of these recipient APCs. Analysis of interaction among exosomes, DCs, and B cells in the presence or not of T lymphocytes, will be performed to clarify this issue.
In any case, our observation that MFG-E8 associated to exosomes does not play a role in their immune function is consistent with a minor role of phagocytosis in exosomal antigen presentation by DCs. MFG-E8 has been proposed to allow phagocytosis, by macrophages and DCs, of phosphatidyl-serine (PS)–containing vesicles, such as apoptotic cells.19,20 Exosomes also expose PS at their surface.18 Here, absence of MFG-E8 on exosomes did not alter in any way the subsequent T-cell activation. We also did not observe either impairment or improvement in T-cell immune responses to antigens associated to apoptotic cells or exosomes in the MFG-E8–deficient mice in vivo (C.T., P.V., unpublished results, September 2004). MFG-E8 is therefore not an essential targeting factor for addressing antigens to DCs in vivo.
The physiologic relevance of MHC-peptide or antigen transfer via exosomes in vivo remains controversial. Existence of exosomes in vivo has now been extensively documented, by purification from various bodily fluids (ie, serum,28 malignant effusions,29,30 bronchoalveolar fluid,31 and urine32 ), and by electron microscopy of human tonsils.33 Exosomes purified ex vivo from malignant effusions are sources of tumor antigens, used by DCs for cross-presentation in vitro.29 Transfer of antigens via exosomes in vivo could take place in the thymus, where DCs acquire antigens from medullary epithelial cells, and thus allow autoreactive T-cell deletion in vivo.34 It could also allow uninfected CD8+ DCs to acquire herpes simplex virus antigens from viable Langherans cells emigrating from infected skin.35 Direct demonstration that antigen transfer via exosomes actually takes place in vivo is still lacking, however. This demonstration would require a way to specifically inhibit exosome production, which is not yet possible. Further understanding of the molecular mechanisms of exosome secretion could make this possible in the future.
Transfer of preformed MHC-peptide complexes by exosomes to other APCs, is specific of exosomes secreted by DCs. Such a transfer could explain how endogenous DCs acquire peptide from injected exogenous DCs, and thus allow efficent T-cell activation.36,37 Recent reports seem to argue against the relevance of such a mechanism. None of them, however, provides a definite proof of the absence of MHC-peptide transfer between DCs. Spörri et al38 have observed that antigen-specific T cells induce full maturation of cognate DCs, but only partial maturation of bystander DCs, which do not bear the TCR-specific ligand. In the herpes simplex virus model, analysis of mixed MHC class II–deficient × MHC class I–mutant hematopoietic chimeras revealed that efficent CD8+ T-cell priming requires presentation of CD4 and CD8 T-cell ligands on the same DC.39 Both observations could be interpreted as a proof of inefficient transfer of MHC-peptide complexes between neighboring DCs in vivo. Another interpretation, however, is that exosomes bound to recipient DCs allow antigen-specific T-cell activation, but do not lead to reverse signaling from the exosomal surface molecules to the recipient DCs, and thus would not sustain full maturation and licensing of the recipient DCs. In another work involving mixed hematopoietic chimeras, Spörri and Reis e Sousa40 have recently shown that deoxycytidylate phosphate deoxyguanylate oligonucleotide (CpG)–matured DCs can induce indirect maturation of neighboring CpG-unresponsive DCs. The indirectly matured DCs, however, did not allow full activation of T cells specific for the MHC-peptide complexes they bore. This observation again suggested that MHC-peptide complexes were not transferred from the CpG-unresponsive to the CpG-responsive DCs, since such a transfer should have allowed full activation of T cells specific for exosomal MHC molecules. But since we show here that exosomes from mature DCs are the efficient MHC-transfer vehicles, it is likely that CpG-unresponsive DCs do not secrete such exosomes, and thus do not lead to T-cell activation in trans. Experiments designed at addressing the 2 questions raised here are currently beeing performed in our laboratory.
In conclusion, we propose that, in vivo, exosomes secreted by mature DCs are sent at medium-range distances in the lymph node, and act as MHC and adhesion molecules microdomains, which, once bound to other APCs, induce T-cell activation. Such a mechanism in vivo remains to be demonstrated. By contrast, the in vivo efficiency of exosomes produced in vitro from mature DCs is clearly established here. Identification of both MHC-peptide complexes and ICAM-1 as major exosomal proteins for induction of immune responses suggests possibilities to produce artifical “exosome-like” liposomes to stimulate immune responses against tumors or infectious agents, which could prove cheaper and as efficient as exosomes produced in vitro by DCs.
Prepublished online as Blood First Edition Paper, March 24, 2005; DOI 10.1182/blood-2005-01-0220.
Supported by Ligue Nationale contre le Cancer, European Community (grant QLRT-2001-00 093), INSERM, Institut Curie. E.S. is a fellow from Ministère de l'Education et de la Recherche.
The online version of the article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Drs B. Salomon (Hôpital Pitié-Salpêtrière, Paris, France) and O. Lantz (Institut Curie, Paris, France) for providing mice; Drs V. Gerke (Institut für Medizinische Biochemie, Münster, Germany) and H. Ploegh (Harvard Medical school, Boston, MA) for providing antibodies; the Laboratory of Mass Spectrometry and Proteomics and the Department of Bioinformatics at Curie Institute for help in proteomic analysis; and Drs O. Lantz and C. Hivroz for helpful comments on the manuscript.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal