Abstract
Hepatitis C virus (HCV) is a major cause of chronic hepatitis worldwide. Interaction of dendritic cells (DCs) with viral particles may play an important role in the immunopathogenesis of HCV infection. Since the synthesis or purification of infectious virions is limited, we used HCV-like particles (HCV-LPs) to study the interaction of HCV with human DCs. Immature DCs exhibited an envelope-specific and saturable binding of HCV-LPs, indicating receptor-mediated DC–HCV-LP interaction. Confocal microscopy revealed that HCV-LPs were rapidly taken up by DCs in a temperature-dependent manner. Competition experiments demonstrated that C-type lectins such as mannose receptor or DC-SIGN (DC–specific intercellular adhesion molecule 3–grabbing nonintegrin) were not sufficient for mediating HCV-LP binding. HCV-LP uptake was followed by DC activation. DCs pulsed with HCV-LPs stimulated HCV core-specific CD4+ T cells, indicating that uptake of HCV-LPs by DCs leads to antigen processing and presentation on major histocompatibility complex (MHC) class II molecules. Finally, HCV-LP–derived antigens were efficiently cross-presented to HCV core-specific CD8+ T cells. These findings demonstrate that HCV-LPs represent a novel model system to study HCV-DC interaction allowing definition of the molecular mechanisms of HCV uptake, DC activation, and antigen presentation to T cells. Furthermore, HCV-LP–mediated DC activation and efficient antigen presentation may explain the marked immunogenicity of HCV-LPs in vivo.
Introduction
Hepatitis C virus (HCV), a positive strand RNA virus that belongs to the Flaviviridae family, is one of the major causes of chronic hepatitis worldwide.1 Current therapy based on pegylated interferon and ribavirin is characterized by limited efficacy, high cost, and major adverse effects.2,3 Therefore, the development of a preventive and potentially therapeutic vaccine against HCV infection has a very high priority.
Clearance of acute HCV infection is associated with a strong HCV-specific CD4+ and CD8+ T-cell response directed against epitopes of the structural and nonstructural proteins.4-6 Dendritic cells (DCs) play a key role in the induction and maintenance of antiviral T-cell immune responses. As antigen-presenting cells, DCs capture antigens, process them into peptides, and present them on molecules of the major histocompatibility complex (MHC) to T cells.7,8
A pathway by which DCs internalize antigens for presentation of T cells is receptor-mediated endocytosis, in which C-type lectins play an important role. A growing number of C-type lectins with specificity for mannosylated antigens have been found to be expressed by DCs such as Langerin (CD2079 ), mannose receptor (CD20610 ), DEC-205 (CD20511 ), and DC-SIGN (DC–specific intercellular adhesion molecule 3–grabbing nonintegrin; CD20912 ). Interestingly, DC-SIGN has been shown to play a role in infection of DCs by Ebola virus13 and dengue virus,14,15 another member of the Flaviviridae family. Furthermore, recent studies using recombinant HCV envelope glycoprotein 2 (E2) and HCV pseudotype particles (HCVpp's) have provided evidence that DC-SIGN or liver/lymph-node SIGN (L-SIGN) can bind HCV E2 on DCs or liver sinusoidal endothelial cells.16-18
Several studies have suggested that DCs can be infected with HCV.19-21 Furthermore, defects in DC function such as impaired maturation or allostimulation have been described in HCV-infected individuals by some investigators20,22,23 but not by others.24,25 These contradictory findings indicate that the issue of HCV-induced defects in DC function requires further investigation. Due to the difficulties to synthesize or purify sufficient quantities of infectious virions for the study of virus-host interaction, the study of HCV-DC interaction has been limited to individually expressed recombinant viral proteins as surrogate viral antigens.26 We and others have previously shown that cellular binding and entry of insect cell–derived HCV-like particles (HCV-LPs) represent a convenient and powerful surrogate system for the study of virus–host cell interaction, allowing the definition of the cell surface proteins and viral epitopes required for viral binding and entry into target cells.27-31 HCV-LPs are generated by self-assembly of HCV structural proteins in insect cells and are characterized by morphologic, biophysical, and antigenic properties similar to putative virions from infected humans.27-29,32-34 In vivo, HCV-LPs induce a strong humoral and cellular immune response including HCV-specific helper T cells and cytotoxic T lymphocytes.35-37 The marked immunogenicity of HCV-LPs in animal models strongly suggests HCV-LP uptake and antigen presentation by DCs in vivo.
Virus-like particles (VLPs) of several viruses including human immunodeficiency virus (HIV),38 hepatitis B virus,39 papillomavirus,40 and parvovirus41 have successfully been used to study viral particle uptake, virion-mediated activation, and antigen presentation by DCs. In this study, we used HCV-LPs as a model system to study the molecular interaction of HCV virions with human DCs.
Patients, materials, and methods
Recombinant proteins
HCV-LPs were synthesized as described.27,32 HCV-LP core concentration was determined as previously described27 using purified recombinant core protein C1-12042 as a standard. HCV-LP E2 concentration was determined by an E2-specific enzyme-linked immunosorbent assay (ELISA) as described.33 Recombinant core protein C22-3 was kindly provided by M. Houghton (Chiron, Emeryville, CA). Interleukin 2 (IL-2), IL-3, IL-4, IL-6, and tumor necrosis factor α (TNF-α) were obtained from CellGenix (Freiburg, Germany); IL-1β from R&D Systems (Minneapolis, MN); granulocyte-macrophage colony-stimulating factor (GM-CSF) from Novartis (Basel, Switzerland); CD40 ligand from Leinco Technologies (St Louis, MO); prostaglandin E2, mannan, lipopolysaccharide (LPS; Escherichia coli 026:B6), and poly-L-lysine from Sigma (St Louis, MO); and endoglycosidase H (EndoH) and peptide N-glycosidase F (PNGaseF) from New England Biolabs (Beverly, MA).
Antibodies
Mouse monoclonal anti-E2 (16A6, AP33, and 2F10), anti-E1 (11B7), and antigalactosidase antibodies (monoclonal antibodies [mAbs]) have been described.27 Chimpanzee anti-E2 mAb (49F3) was generated by immunization of a chimpanzee with recombinant E2 protein.43 Anti-E2 antibodies 16A6 and 49F3 bind to an E2 epitope located between HCV amino acids 516 and 530 (E.D., unpublished observations, April 2004). Anti–human DC-SIGN (clone 120507), chemokine receptor 5 (CCR5; clone 45531.111), CCR3 (clone 61828), and CXCR4 mAbs (clone 12G5, from Dr J. A. Hoxie44 ) were generous gifts from the National Institutes of Health (NIH) AIDS Research and Reference Reagent Program (Germantown, MD). Anti–human DC-SIGN mAb (AZN-D1) was a kind gift from Dr T. B. H. Geijtenbeek (Department of Molecular Cell Biology and Immunology, Vrije Universiteit, Amsterdam, The Netherlands). Anti–human DC-SIGN/L-SIGN mAb (clone 120612) was purchased from R&D Systems, anti–human CD205 (DEC-205) and anti–human Toll-like receptor 2 (TLR2) mAb (clone TL2.1) from eBioscience (San Diego, CA), and anti–human langerin mAb (clone DCGM4) from Beckman Coulter (Fullerton, CA). Anti–human lysosomal-associated membrane protein (LAMP-2) mAb, biotinylated anti–human TLR4 (clone HTA125), phycoerythrin (PE)–conjugated anti–human CCR7, PE–conjugated anti–human mannose receptor mAb, and streptavidin-PE were purchased from Becton Dickinson (San Jose, CA). PE–conjugated anti–mouse and PE–conjugated or cyanin 3 (Cy3)–conjugated anti–human immunoglobulin (Ig) G antibodies were obtained from Dako (Glostrup, Denmark). Fluorescein-conjugated anti–mouse IgG was purchased from ICN Biomedicals (Irvine, CA).
Generation of DCs from human peripheral blood monocytes (PBMCs)
DCs were generated from PBMCs of healthy, anti-HCV–negative blood donors as described by Sallusto and Lanzavecchia.45 To obtain monocyte-derived DCs, CD14+ cells were cultured in the presence of IL-4 (1000 U/mL) and GM-CSF (800 U/mL). Immature DCs (imDCs) were collected on day 5 before exposure to the maturation stimuli. For DC maturation, IL-6 (1000 U/mL), IL-1β (10 ng/mL), TNF-α (10 ng/mL), and prostaglandin E2 (1 μg/mL) or LPS (10 μg/mL) were added for the last 2 days (day 5 to 7) of culture.
Isolation of human blood DCs
Blood pre-DCs were isolated from PBMCs of healthy, anti-HCV–negative blood donors using DC isolation kit BDCA-1 for myeloid pre–DCs 1 and kit BDCA-4 for plasmacytoid pre–DCs 2, respectively, according to the manufacturer's protocol (Miltenyi Biotech, Bergisch Gladbach, Germany). ImDCs 1 were generated by short-term culture (48 h) of pre–DCs 1 in DC medium supplemented with IL-4 (1000 U/mL) and GM-CSF (800 U/mL). ImDCs 2 were induced by incubation of pre–DCs 2 with IL-3 (40 ng/mL).
Cytofluorimetric cell-surface phenotyping
Cell staining was performed using fluorescein isothiocyanate (FITC)- and PE-conjugated mAb directed against epitopes of CD80, CD83, CD86, CD123, HLA-DR, CCR7, and mannose receptor. Lineage cocktail (Lin) comprises a combination of anti-CD3, -CD14, -CD16, -CD19, -CD20, and -CD56 antibodies (Becton Dickinson). For staining by flow cytometry, DCs were incubated with the respective antibodies or IgG isotype control antibodies, and cellular fluorescence was monitored using a FACSCalibur flow cytometer with CellQuest 3.11 software (Becton Dickinson). For the detection of C-type lectin and CCR and TLR cell surface expression, DCs were incubated with the respective antibodies or IgG isotype control and stained with PE–conjugated anti–mouse IgG.
Analysis of cellular HCV-LP binding
Cells (0.5 × 105 to 1 × 105 cells/100 μL) were incubated with HCV-LPs or insect cell control preparations (derived from insect cells infected with a recombinant baculovirus containing the cDNA for β-glucuronidase [GUS]32 ), and cell-bound HCV-LPs were detected using anti-E2 antibody (16A6) and flow cytometry as described.27 To assess whether binding of HCV-LPs was envelope dependent, HCV-LPs were preincubated with mouse antienvelope antibodies as described.31 The HCV-LP–antibody mixture was added to imDCs and incubated for 1 hour at 4°C in phosphate-buffered saline (PBS) containing 0.5% bovine serum albumin (BSA). Cellular binding of HCV-LPs was quantified by flow cytometry using chimpanzee anti-E2 mAb (49F3) and PE–conjugated anti–human IgG antibody. Inhibition of HCV-LP binding to imDCs by a human polyclonal anti-HCV antiserum was performed as described.31 To assess the role of HCV-LP envelope protein glycosylation for HCV-LP binding, HCV-LPs and insect cell control preparations were treated with glycosidases, followed by immunoblotting with anti-E1 and -E2 monoclonal antibodies as described.35 To minimize denaturation of HCV-LPs during glycosidase treatment, digestions with glycosidases were performed in the absence of denaturation buffer according to the manufacturer's protocol. Binding of glycosylated (mock treated) and deglycosylated (EndoH and PNGaseF treated) HCV-LPs to imDCs was analyzed side by side as described above.
To characterize the role of cell surface proteins for HCV-LP–DC interaction, DCs were preincubated with antibodies directed against C-type lectins, CCRs, TLRs, or control IgG (antigalactosidase mAb; 50 μg/mL) in PBS–0.5% BSA. Following a 1-hour incubation, HCV-LPs (1 μg/mL HCV-LP E2) were added to the cells and cell-bound HCV-LPs were detected as described earlier in this section using chimpanzee anti-E2 mAb (49F3) and flow cytometry. For inhibition with mannan, DCs were incubated with mannan (3 mg/mL) prior to the addition of HCV-LPs. As a positive control, FITC-dextran uptake was measured in the presence or absence of mannan as described.20
Analysis of HCV-LP entry by immunofluorescence
HCV-LP entry was analyzed by immunofluorescence and confocal laser scanning microscopy as described.30 Briefly, imDCs were incubated with HCV-LPs or insect cell control preparation for 1 hour at 4°C (HCV-LP binding) or 37°C (HCV-LP entry). After removal of nonbound HCV-LPs by washing with ice-cold PBS, imDCs were bound to poly-L-lysine–treated cover slides and fixed with PBS/3.5% paraformaldehyde. DCs were permeabilized in PBS–0.1% Triton X-100 and stained for HCV-LP binding and entry using chimpanzee anti-E2 antibody 49F3 (dilution 1:200 in PBS, 1.5-h incubation) and Cy3-conjugated anti–human IgG (dilution 1:500 in PBS, 45 minutes). DCs were counterstained with mouse anti–human LAMP-2 antibody (dilution 1:700 in PBS, 1.5-h incubation) and FITC-conjugated anti–mouse IgG (dilution 1:700, 45 minutes). Prior to analysis by confocal microscopy, cover slides were mounted in antifade reagent with DAPI (4′, 6-diamidino-2-phenylindole; Molecular Probes, Eugene, OR). Stained cells were analyzed using a Leica TCS SP2 AOBS laser scanning confocal microscope (Leica Microsystems, Heidelberg, Germany) equipped with an HCX PLAN Apo 63×/1.4 oil objective lens and with argon (488 nm) and helium (543 nm) lasers. Leica confocal software TCS SP2 version 2.5.1227a was used for image acquisition and processing (Leica Microsystems). For staining of cell nuclei, DAPI was excited with a diode laser (405 nm). Digitalized images were analyzed using Zeiss-LSM Image Browser version 2.8 (Carl Zeiss, Jena, Germany).
Analysis of HCV-LP–induced activation
To investigate DC activation, imDCs were replated on day 6 at a density of 1 × 105 cells/well in 96-well plates. DCs were then exposed to insect cell control preparations (10 μg/mL), native or denatured HCV-LPs (total protein concentration 10 μg/mL), or LPS (10 μg/mL). Denaturation of HCV-LPs was performed by heat treatment (95°C for 15 minutes) as described.27 DCs were then incubated for 16 hours in DC medium containing IL-4 (1000 U/mL) and GM-CSF (800 U/mL). Finally, DC activation was analyzed by flow cytometry using HLA-DR–, CD86-, CD80-, and CD83-specific antibodies.
Mixed lymphocyte reaction
Monocyte-derived imDCs from healthy, anti-HCV–negative individuals were incubated with HCV-LPs or insect cell control preparations (total protein concentration 10 μg/mL) for 16 hours. DCs were then exposed for 24 hours to maturation stimuli. Following maturation, DCs were collected, washed, irradiated (30 Gy), and used as stimulators for allogeneic PBMCs. Irradiated mature DCs (mDCs; 101-104 cells) were then coincubated with PBMCs (2 × 105 cells) for 6 days. During the last 16 hours of incubation, cells were pulsed with [3H]thymidine (1 μCi/well [0.037 MBq/well]; Amersham, Little Chalfont, United Kingdom) and harvested, and the amount of radiolabel incorporated into the responder cell DNA was determined using a beta counter (LKB, Pharmacia, Uppsala, Sweden). For cytokine analysis, mDCs were coincubated with allogeneic CD4+ T cells at a DC/T-cell ratio of 1:10. Cytokine secretion was quantified by ELISA on days 1 (for IL-4) and 5 (for interferon-γ [IFN-γ]) as described.46
Analysis of HCV core-specific CD4+ T-cell clone stimulation by HCV-LPs
A core-specific CD4+ T-cell clone (recognizing an epitope in the HCV core protein comprising amino acids [aa's] 21-44) was generated from a patient with acute hepatitis C as described.47 HLA restriction for the clone was defined as HLA-DQ3 using a panel of homozygous lymphoblastoid cell lines.48 For expansion, the T-cell clone was restimulated with irradiated allogeneic PBMCs, phytohemagglutinin (2 μg/mL), and IL-2 (50 U/mL). DCs generated from allogeneic HLA-DQ3–positive PBMCs were incubated with different concentrations of recombinant core protein, HCV-LPs, or insect cell control preparation for 3 hours at 37°C. After washing, irradiated DCs (5 × 104 cells/well) were incubated with the core-specific CD4+ T-cell clone (5 × 104 cells/well) for 3 days. On day 3, CD4 cells were labeled by incubation for 16 hours with [3H]thymidine (2 μCi/well [0.074 MBq/well]), and the amount of radiolabel incorporated into DNA was measured using a beta counter.
Cross-presentation of HCV-LPs to HCV core-specific CD8+ T cells
HCV-specific CD8+ T-cell responses were studied in a 75-year-old chronically HCV-infected patient (HCV genotype 1) by a comprehensive enzyme-linked immunospot assay using 441 overlapping peptides (18-mers, overlapping by 11 aa's) spanning the whole HCV genotype 1 polyprotein (NIH AIDS Research and References Reagent Program) similarly as described.49 This method led to the identification of 3 intrahepatic HCV-specific CD8+ T-cell responses in this patient, with the strongest response against a core peptide comprising aa's 36-53 (LLPRRG-PRLGVRATRKTS), peptide 6. An HCV peptide-specific cytotoxic T lymphocyte line was established from PBMCs by stimulation with this peptide as described previously.50 Intrahepatic CD8+ T cells were expanded polyclonally and antigen nonspecifically as described recently (purity confirmed by fluorescence-activated cell sorter [FACS] analysis > 95%).50 To study HCV-LP antigen presentation on MHC class I molecules, autologous imDCs were harvested and incubated with HCV-LPs, insect cell control preparation, or without antigen for 40 hours. After 24 hours, medium was replaced and fresh antigen added. During the final 16 hours, CD40 ligand (1 μg/mL) was added to the cultures as a maturation stimulus. DCs were then extensively washed and cocultured with core-specific intrahepatic CD8+ T cells or peripheral core-specific CD8+ T-cell lines at a ratio of 1:2. After 5 hours of incubation, intracellular IFN-γ staining of core-specific CD8+ T cells was performed as described recently.6 As a positive control, unpulsed mature DCs were incubated for 1 hour at 37°C with peptide 6 (10 μg/mL) and added to the CD8+ T cells following extensive washing. Approval was obtained from the University Hospital Freiburg institutional review board for these studies. Informed consent was provided according to the Declaration of Helsinki.
Results
Binding of HCV-LPs to human DCs
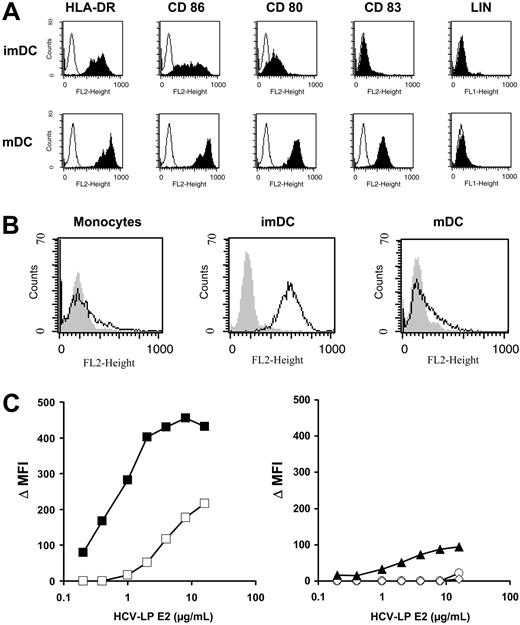
The induction of a broad humoral and cellular immune response against HCV structural proteins by HCV-LPs in mice in vivo35-37,51 has suggested that HCV-LPs are efficiently presented by antigen-presenting cells (APCs). In this study we aimed to analyze the interaction of HCV-LPs with human professional APCs. For that purpose, human imDCs were generated in vitro from monocytes of healthy donor PBMCs. ImDCs were characterized by moderate expression of HLA-DR and CD86 and low or absent expression of CD80 and CD83 (Figure 1A). In contrast, mDCs generated by addition of proinflammatory cytokines were characterized by expression of high levels of HLA-DR, CD80, CD83, and CD86 (Figure 1A). DC maturation was accompanied by down-regulation of CCR3 and CCR5 and up-regulation of CXCR4 expression (Table 1). CCR7 was exclusively detected on the cell surface of mDCs (Table 1). Surface expression of TLR2 and 4 on immature and mDCs was very low or nondetectable (Table 1).
Binding of HCV-LPs to monocyte-derived DCs. (A) Immunophenotyping of immature (imDC) and mature DCs (mDC) used for HCV-LP binding. DC phenotypes were characterized by flow cytometry of HLA-DR, CD86, CD80, and CD83 surface expression (LIN indicates a combination of anti-CD3, -CD14, -CD16, -CD19, -CD20, and -CD56 antibodies). Histograms corresponding to cell surface expression of the respective cell surface molecules (shaded curves) are overlaid with histograms of cells incubated with the appropriate isotype control antibody (open curves). The absence of CD3, CD14, CD16, CD19, and CD56 expression excluded the presence of B cells, T cells, natural killer (NK) cells, or macrophage/monocytes in the DC preparation. (B) Binding of HCV-LPs to monocytes, immature, and mature DCs. Flow cytometry histograms of HCV-LP binding to target cells (open curves) are shown. Background fluorescence (gray shaded curves) was measured using cells incubated with insect cell control preparations. Cellular binding of HCV-LPs was analyzed using mouse anti-E2 mAb (16A6) and PE-conjugated anti–mouse IgG. (C) Dose-dependent binding of HCV-LPs to target cells. Immature (▪) and mature DCs (□; left graph), monocytes (▴), T (○), and B cells (⋄; right graph) were incubated with increasing concentrations of HCV-LP or insect cell control preparations. On the y-axis, net mean fluorescence intensity (Δ MFI) values for each HCV-LP E2 concentration were calculated by subtracting the MFI of insect cell control preparations from that obtained with the respective HCV-LP E2 concentration (x-axis). Concentration of HCV-LP E2 was quantified by an ELISA as described earlier.33
Binding of HCV-LPs to monocyte-derived DCs. (A) Immunophenotyping of immature (imDC) and mature DCs (mDC) used for HCV-LP binding. DC phenotypes were characterized by flow cytometry of HLA-DR, CD86, CD80, and CD83 surface expression (LIN indicates a combination of anti-CD3, -CD14, -CD16, -CD19, -CD20, and -CD56 antibodies). Histograms corresponding to cell surface expression of the respective cell surface molecules (shaded curves) are overlaid with histograms of cells incubated with the appropriate isotype control antibody (open curves). The absence of CD3, CD14, CD16, CD19, and CD56 expression excluded the presence of B cells, T cells, natural killer (NK) cells, or macrophage/monocytes in the DC preparation. (B) Binding of HCV-LPs to monocytes, immature, and mature DCs. Flow cytometry histograms of HCV-LP binding to target cells (open curves) are shown. Background fluorescence (gray shaded curves) was measured using cells incubated with insect cell control preparations. Cellular binding of HCV-LPs was analyzed using mouse anti-E2 mAb (16A6) and PE-conjugated anti–mouse IgG. (C) Dose-dependent binding of HCV-LPs to target cells. Immature (▪) and mature DCs (□; left graph), monocytes (▴), T (○), and B cells (⋄; right graph) were incubated with increasing concentrations of HCV-LP or insect cell control preparations. On the y-axis, net mean fluorescence intensity (Δ MFI) values for each HCV-LP E2 concentration were calculated by subtracting the MFI of insect cell control preparations from that obtained with the respective HCV-LP E2 concentration (x-axis). Concentration of HCV-LP E2 was quantified by an ELISA as described earlier.33
Cell-surface expression levels of chemokine receptors and TLRs on monocyte-derived imDCs and mDCs
. | Δ MFI, mean ± SD . | . | |
|---|---|---|---|
| Receptor . | imDCs . | mDCs . | |
| CCR3 | 172 ± 2 | 84 ± 1.5 | |
| CCR5 | 314 ± 1.5 | 117 ± 1.5 | |
| CXCR4 | 108 ± 0.5 | 258 ± 2 | |
| CCR7 | 9 ± 1 | 89 ± 1.5 | |
| TLR2 | 23 ± 0.5 | 22 ± 4 | |
| TLR4 | 20 ± 0.5 | 0 ± 0 | |
. | Δ MFI, mean ± SD . | . | |
|---|---|---|---|
| Receptor . | imDCs . | mDCs . | |
| CCR3 | 172 ± 2 | 84 ± 1.5 | |
| CCR5 | 314 ± 1.5 | 117 ± 1.5 | |
| CXCR4 | 108 ± 0.5 | 258 ± 2 | |
| CCR7 | 9 ± 1 | 89 ± 1.5 | |
| TLR2 | 23 ± 0.5 | 22 ± 4 | |
| TLR4 | 20 ± 0.5 | 0 ± 0 | |
Expression of cell surface receptors was determined by flow cytometry. Mean ± SD of a representative experiment performed in triplicate is shown.
Δ MFI indicates net mean fluorescence intensity.
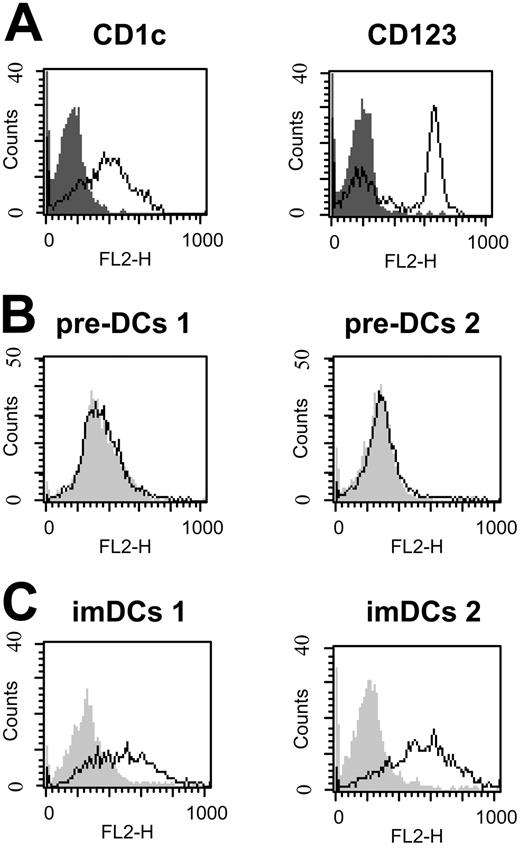
HCV-LP binding to imDCs was dose dependent and achieved saturation (Figure 1B-C). By contrast, mDCs demonstrated a significantly reduced HCV-LP binding that was not saturable at the HCV-LP concentration tested. CD3+ T and CD19+ B cells did not demonstrate any measurable HCV-LP binding. Monocytes (CD14+) showed very low binding at high HCV-LP E2 concentrations (Figure 1B-C). To study whether HCV-LPs interact with human DCs circulating in blood, freshly isolated myeloid (pre–DCs 1) and plasmacytoid pre-DCs (pre–DCs 2) from peripheral blood were analyzed for HCV-LP binding. Pre–DCs 1 strongly expressed CD1c, whereas pre–DCs 2 were characterized by expression of CD123 (Figure 2A). HCV-LPs did not bind to pre–DCs 1 or pre–DCs 2 (Figure 2B). However, following maturation of pre-DCs into imDCs using medium containing defined cytokines, binding of HCV-LPs to blood DCs was induced (Figure 2C).
Interaction of HCV-LPs with blood DCs. (A) Immunophenotyping of freshly isolated myeloid (pre–DCs 1) and plasmacytoid (pre–DCs 2) pre-DCs from peripheral blood used for HCV-LP binding. Phenotypes of pre-DCs were characterized by flow cytometric analysis of CD1c expression for pre–DCs 1 (left) and CD123 expression for pre–DCs 2 (right). Histograms corresponding to cell surface expression of the indicated molecules (open curves) are overlaid with histograms corresponding to cells incubated with the appropriate isotype control antibody (gray shaded curves). (B-C) HCV-LP binding to pre-DCs and imDCs. Freshly isolated pre-DCs as well as myeloid and plasmacytoid imDCs (imDCs 1 and -2) were incubated with HCV-LPs (HCV-LP E2 concentration ≈ 2 μg/mL) and binding of HCV-LPs was analyzed as described in Figure 1. Flow cytometry histograms of HCV-LP binding to pre-DCs (B) and imDCs (C) are shown (open curves). Background fluorescence (gray shaded curves) was measured as described in “Analysis of cellular HCV-LP binding” using cells incubated with insect cell control preparations.
Interaction of HCV-LPs with blood DCs. (A) Immunophenotyping of freshly isolated myeloid (pre–DCs 1) and plasmacytoid (pre–DCs 2) pre-DCs from peripheral blood used for HCV-LP binding. Phenotypes of pre-DCs were characterized by flow cytometric analysis of CD1c expression for pre–DCs 1 (left) and CD123 expression for pre–DCs 2 (right). Histograms corresponding to cell surface expression of the indicated molecules (open curves) are overlaid with histograms corresponding to cells incubated with the appropriate isotype control antibody (gray shaded curves). (B-C) HCV-LP binding to pre-DCs and imDCs. Freshly isolated pre-DCs as well as myeloid and plasmacytoid imDCs (imDCs 1 and -2) were incubated with HCV-LPs (HCV-LP E2 concentration ≈ 2 μg/mL) and binding of HCV-LPs was analyzed as described in Figure 1. Flow cytometry histograms of HCV-LP binding to pre-DCs (B) and imDCs (C) are shown (open curves). Background fluorescence (gray shaded curves) was measured as described in “Analysis of cellular HCV-LP binding” using cells incubated with insect cell control preparations.
HCV-LP–DC interaction is mediated by epitopes in the E1 and E2 N-terminal regions
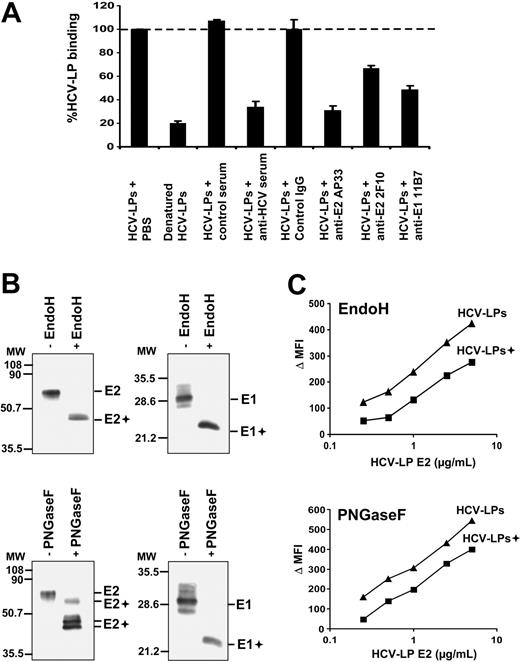
To study whether HCV-LP binding was mediated by the HCV structural proteins, we analyzed cellular HCV-LP binding in the presence of antienvelope antibodies and human polyclonal anti-HCV antiserum.31 As shown in Figure 3A, HCV-LP binding to DCs was markedly inhibited by preincubation with a well-characterized anti-HCV antiserum containing high-titer anti-E2 antibodies31 but not by a control serum. To map viral envelope protein epitopes mediating HCV-LP–DC cell surface interaction, we used a panel of well-characterized anti-E1/E2 mAbs for inhibition of HCV-LP binding. As shown in Figure 3A, HCV-LP binding to imDCs was markedly inhibited by preincubation of HCV-LPs with anti-E2 (AP33 and 2F10) and anti-E1 mAb (11B7). Antienvelope-mediated inhibition of binding was concentration dependent (data not shown) and absent for control mouse monoclonal IgG (Figure 3A). These data suggest that the E2 hypervariable region 1 (aa's 398-403; targeted by mAb 2F10) as well as the E1 and E2 N-terminal regions (aa's 212-224 targeted by anti-E1 mAb 11B7 and aa's 412-423 targeted by anti-E2 mAb AP33) play an important role for docking of HCV-LPs to the cell surface of human DCs. To assess whether cellular binding of HCV-LPs is dependent on native envelope protein conformation, we compared the binding profiles for native and heat-denatured HCV-LPs. Compared with native HCV-LPs, heat-denatured HCV-LPs exhibited a strongly reduced binding to imDCs (Figure 3A). These data suggest that proper conformation of the HCV-LP envelope is crucial for DC binding.
Viral envelope glycoproteins and HCV-LP binding to DCs. (A) Inhibition of HCV-LP binding to imDCs by anti-HCV antibodies. HCV-LPs (1 μg HCV-LP E2/mL) were preincubated with anti-E2 (AP33, 2F10; 50 μg/mL), anti-E1 antibody (11B7; 50μg/mL), or an anti-HCV antiserum (dilution 1:50) containing high-titer antibodies against the HCV envelope glycoprotein E2 for 1 hour at 37°C. Incubation of HCV-LPs with control IgG (50 μg/mL) or anti-HCV–negative serum (dilution 1:50) served as negative controls. HCV-LP binding was analyzed by flow cytometry using chimpanzee anti-E2 mAb (49F3) and PE–conjugated anti–human IgG. Denaturation of HCV-LPs was performed by heat treatment as described.27,35 Mean ± SD of 3 experiments is shown (HCV-LP binding in the absence of antibody = 100%). (B) Analysis of envelope glycoprotein glycosylation. HCV-LPs were digested with EndoH or PNGaseF for 2 hours at 37°C. Glycosidase (+) and mock-digested HCV-LP envelope proteins were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis, followed by immunoblotting with anti-E1 and anti-E2 mAbs as described previously.27,35 Sizes of molecular weight (MW) markers in kilodaltons are indicated on the left (E1+ and E2+ indicate deglycosylated forms of E1 and E2). (C) Impact of envelope glycoprotein glycosylation for HCV-LP binding. ImDCs were incubated with increasing concentrations of glycosidase-digested (+) and mock-digested HCV-LPs, and particle binding was analyzed by flow cytometry as described in Figure 1.
Viral envelope glycoproteins and HCV-LP binding to DCs. (A) Inhibition of HCV-LP binding to imDCs by anti-HCV antibodies. HCV-LPs (1 μg HCV-LP E2/mL) were preincubated with anti-E2 (AP33, 2F10; 50 μg/mL), anti-E1 antibody (11B7; 50μg/mL), or an anti-HCV antiserum (dilution 1:50) containing high-titer antibodies against the HCV envelope glycoprotein E2 for 1 hour at 37°C. Incubation of HCV-LPs with control IgG (50 μg/mL) or anti-HCV–negative serum (dilution 1:50) served as negative controls. HCV-LP binding was analyzed by flow cytometry using chimpanzee anti-E2 mAb (49F3) and PE–conjugated anti–human IgG. Denaturation of HCV-LPs was performed by heat treatment as described.27,35 Mean ± SD of 3 experiments is shown (HCV-LP binding in the absence of antibody = 100%). (B) Analysis of envelope glycoprotein glycosylation. HCV-LPs were digested with EndoH or PNGaseF for 2 hours at 37°C. Glycosidase (+) and mock-digested HCV-LP envelope proteins were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis, followed by immunoblotting with anti-E1 and anti-E2 mAbs as described previously.27,35 Sizes of molecular weight (MW) markers in kilodaltons are indicated on the left (E1+ and E2+ indicate deglycosylated forms of E1 and E2). (C) Impact of envelope glycoprotein glycosylation for HCV-LP binding. ImDCs were incubated with increasing concentrations of glycosidase-digested (+) and mock-digested HCV-LPs, and particle binding was analyzed by flow cytometry as described in Figure 1.
Next, we assessed the impact of envelope glycosylation for HCV-LP binding. Using glycosidase digestion of HCV-LPs, we previously demonstrated that the HCV-LP envelope proteins are extensively N-glycosylated.35 Similar to expression of full-length envelope proteins in mammalian cells,52 the envelope proteins of HCV-LPs do not acquire the complex carbohydrate forms associated with trafficking through the Golgi apparatus.35 Digestion of HCV-LP envelope proteins with glycosidases (Figure 3B) revealed that envelope proteins of HCV-LPs used in this study (H77C strain) are extensively N-glycosylated as shown previously for HCV-LPs derived from the HCV-J strain.35 HCV-LPs digested with EndoH or PNGaseF exhibited a partial decrease in DC binding compared with mock-digested HCV-LPs (Figure 3C). Since we cannot exclude that the reduction of HCV-LP binding was related to changes in HCV-LP envelope conformation following glycosidase treatment, the partial reduction in HCV-LP binding does not support a major role of envelope glycosylation for HCV-LP–DC cell surface interaction.
HCV-LP binding to imDCs requires molecules other than C-type lectins
HCV-LP binding was sensitive to pretreatment of DCs with trypsin, indicating that binding of HCV-LPs is mediated by a cellular protein (Figure 4C). To further characterize the protein interacting with HCV-LPs, we assessed the role of several DC cell surface molecules implicated in the interaction of DCs with microbial pathogens.
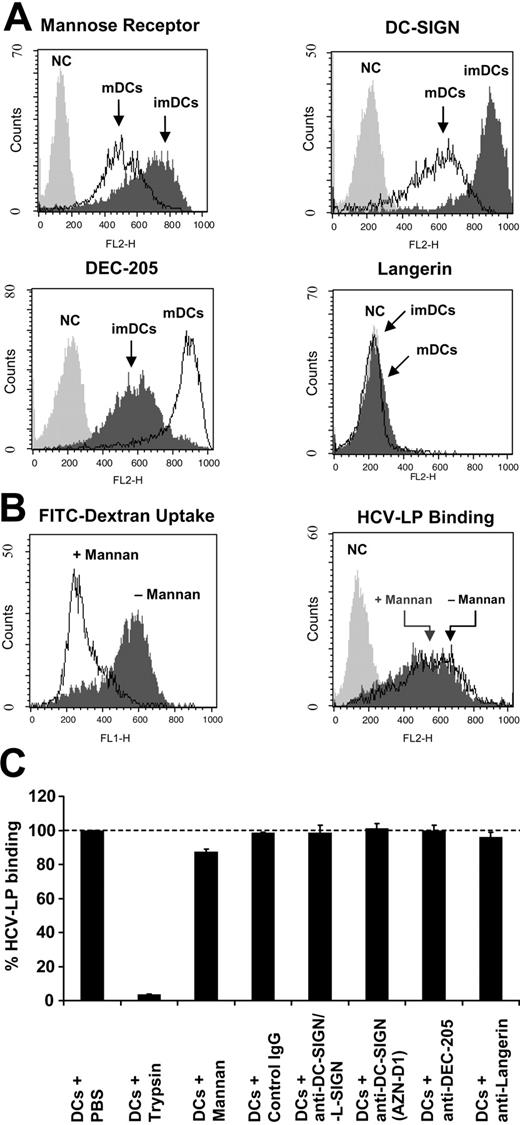
HCV-LP binding to DCs and C-type lectins. (A) Expression of mannose receptor, DC-SIGN, DEC-205, and Langerin on monocyte-derived DCs. C-type lectin expression on imDCs and mDCs was analyzed by FACS using cell surface protein-specific antibodies (NC indicates negative control corresponding to cells incubated with the respective isotype control antibody). (B, left) Inhibition of FITC-dextran binding and uptake into imDCs by mannan. FITC-dextran binding and uptake at 37°C was measured by FACS in the absence or presence of mannan (3 mg/mL). (B, right) Mannan does not inhibit cellular HCV-LP binding to imDCs. HCV-LPs in subsaturating concentrations (1 μg HCV-LP E2/mL) were added to imDCs in the absence or presence of mannan (3 mg/mL) and HCV-LP binding was analyzed as described in Figure 1. (C) Quantitative analysis of cellular HCV-LP binding in the presence of anti–DC-SIGN, anti–DC-SIGN/L-SIGN, anti–DEC-205, anti-Langerin antibodies, or control IgG. DCs were preincubated with mannan (3 mg/mL) or antibody (50 μg/mL) for 1 hour at 25°C. HCV-LPs (1 μg HCV-LP E2/mL) were added to the cells and HCV-LP binding was analyzed by flow cytometry as described in Figure 3A. To study whether trypsin pretreatment of DCs modifies HCV-LP binding, imDCs were exposed to 0.5% trypsin prior to the addition of HCV-LPs as described recently.27 Mean ± SD of 3 experiments is shown (HCV-LP binding in the absence of antibody = 100%).
HCV-LP binding to DCs and C-type lectins. (A) Expression of mannose receptor, DC-SIGN, DEC-205, and Langerin on monocyte-derived DCs. C-type lectin expression on imDCs and mDCs was analyzed by FACS using cell surface protein-specific antibodies (NC indicates negative control corresponding to cells incubated with the respective isotype control antibody). (B, left) Inhibition of FITC-dextran binding and uptake into imDCs by mannan. FITC-dextran binding and uptake at 37°C was measured by FACS in the absence or presence of mannan (3 mg/mL). (B, right) Mannan does not inhibit cellular HCV-LP binding to imDCs. HCV-LPs in subsaturating concentrations (1 μg HCV-LP E2/mL) were added to imDCs in the absence or presence of mannan (3 mg/mL) and HCV-LP binding was analyzed as described in Figure 1. (C) Quantitative analysis of cellular HCV-LP binding in the presence of anti–DC-SIGN, anti–DC-SIGN/L-SIGN, anti–DEC-205, anti-Langerin antibodies, or control IgG. DCs were preincubated with mannan (3 mg/mL) or antibody (50 μg/mL) for 1 hour at 25°C. HCV-LPs (1 μg HCV-LP E2/mL) were added to the cells and HCV-LP binding was analyzed by flow cytometry as described in Figure 3A. To study whether trypsin pretreatment of DCs modifies HCV-LP binding, imDCs were exposed to 0.5% trypsin prior to the addition of HCV-LPs as described recently.27 Mean ± SD of 3 experiments is shown (HCV-LP binding in the absence of antibody = 100%).
For efficient capture of a variety of antigens, DCs use C-type lectins. ImDCs demonstrated high-level expression of mannose receptor, DC-SIGN, and DEC-205 (Figure 4A). Langerin was not expressed on DCs (Figure 4A). Upon maturation of imDCs, expression of mannose receptor and DC-SIGN decreased to lower levels (Figure 4A). In contrast, DEC-205 expression increased during maturation of DCs. To study whether C-type lectins play a crucial role in mediating binding of HCV-LPs to DCs, binding of HCV-LPs was analyzed in the presence or absence of mannan. Incubation of imDCs with mannan abolished binding and uptake of FITC-dextran by C-type lectins (Figure 4B). In contrast, binding of HCV-LPs to imDCs was only marginally inhibited by mannan (Figure 4B). Preincubation of imDCs with anti–human DC-SIGN (AZN-D1) or DC-SIGN/L-SIGN (clone 120612) antibodies that had been previously shown to inhibit binding of recombinant C-terminally truncated E2 to DC-SIGN on imDCs16,17 did not inhibit HCV-LP binding. Similar results were obtained for DEC-205 (Figure 4C), another C-type lectin recognizing mannose-like carbohydrates on microbial pathogens.53 These results suggest that C-type lectins are not sufficient to mediate HCV-LP–DC interaction and that other or additional cell surface molecules are required for HCV-LP binding and entry.
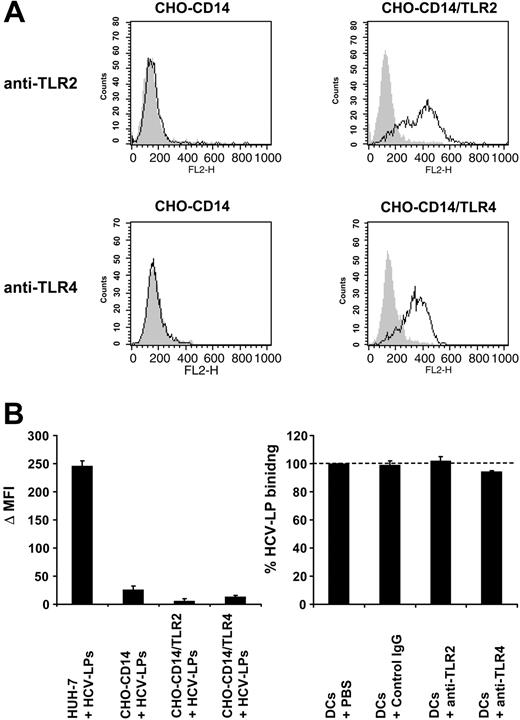
Next, we studied whether defined TLRs or CCRs play an important role for HCV-LP binding. Recent studies have indicated that innate immune responses induced by several viruses are mediated by binding of viral envelope protein to TLR2 or TLR4.54 Preincubation of TLR2 and 4 on imDCs by anti-TLR–specific antibodies did not result in inhibition of HCV-LP binding (Figure 5C). Furthermore, overexpression of either human TLR2 or TLR4 in Chinese hamster ovary (CHO) cells (Figure 5A) was not able to induce cellular binding of HCV-LPs (Figure 5B). These data demonstrate that TLR2 and 4 do not represent cell surface molecules mediating binding of HCV-LPs.
Analysis of HCV-LP binding to cell lines stably expressing TLR2 and 4. (A) Cell surface expression of TLR2 and TLR4 in CHO-CD14, CHO-CD14/TLR2, and CHO-CD14/TLR4 cells. CHO cell lines55 were incubated with anti-TLR2, anti-TLR4, or isotype-matched control mAbs and analyzed for cell surface expression of TLR2 and TLR4 by flow cytometry. Open curves represent TLR-specific staining; gray shaded curves show staining with isotype-matched control Abs. (B) HCV-LP binding to the human hepatoma cell line Huh-731 and TLR-transfected CHO cell lines. Cells were incubated with HCV-LPs for 1 hour at 4°C and HCV-LP binding was quantified by flow cytometry. Data are shown as Δ MFI (mean ± SD) of a representative experiment performed in triplicate. (C) Binding of HCV-LPs to imDCs in the presence of anti-TLR antibodies. ImDCs were preincubated with anti-TLR2, anti-TLR4, or control mAb (50 μg/mL) prior to the addition of HCV-LPs and cellular HCV-LP binding was analyzed as described in Figure 3A. Data are shown as percentage binding relative to the binding of HCV-LPs without antibody preincubation (100%). Mean ± SD of 3 experiments is shown (HCV-LP binding in the absence of antibody = 100%).
Analysis of HCV-LP binding to cell lines stably expressing TLR2 and 4. (A) Cell surface expression of TLR2 and TLR4 in CHO-CD14, CHO-CD14/TLR2, and CHO-CD14/TLR4 cells. CHO cell lines55 were incubated with anti-TLR2, anti-TLR4, or isotype-matched control mAbs and analyzed for cell surface expression of TLR2 and TLR4 by flow cytometry. Open curves represent TLR-specific staining; gray shaded curves show staining with isotype-matched control Abs. (B) HCV-LP binding to the human hepatoma cell line Huh-731 and TLR-transfected CHO cell lines. Cells were incubated with HCV-LPs for 1 hour at 4°C and HCV-LP binding was quantified by flow cytometry. Data are shown as Δ MFI (mean ± SD) of a representative experiment performed in triplicate. (C) Binding of HCV-LPs to imDCs in the presence of anti-TLR antibodies. ImDCs were preincubated with anti-TLR2, anti-TLR4, or control mAb (50 μg/mL) prior to the addition of HCV-LPs and cellular HCV-LP binding was analyzed as described in Figure 3A. Data are shown as percentage binding relative to the binding of HCV-LPs without antibody preincubation (100%). Mean ± SD of 3 experiments is shown (HCV-LP binding in the absence of antibody = 100%).
CCR3, CCR5, and CXCR4 have been shown to act as cofactors for HIV entry.56,57 CCR7 and CXCR4 expression did not correlate with HCV-LP binding (Table 1). Although CCR3 and CCR5 expression partially correlated with HCV-LP binding to immature DCs and mDCs, anti-CCR3 and anti-CCR5 antibodies (recently shown to inhibit HIVgp120-CCR interaction58 ) were not able to inhibit HCV-LP binding (data not shown). These data suggest that these CCRs do not play a major role for HCV-LP binding.
Internalization of HCV-LPs into imDCs
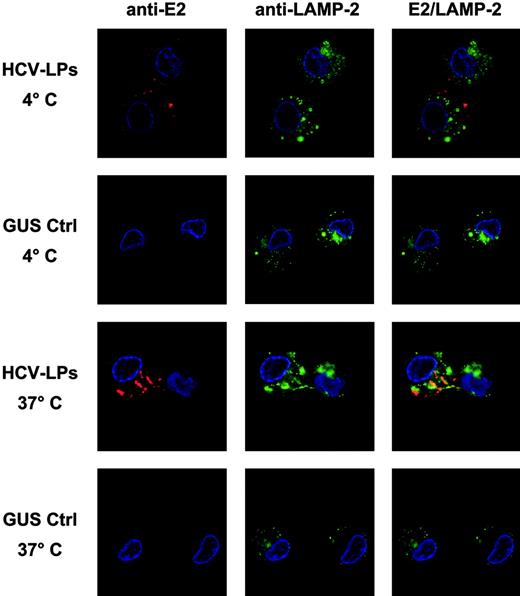
After particle binding, the next step is uptake or entry of particles into the cell. Therefore, temperature-dependent HCV-LP entry into monocyte-derived imDCs was analyzed using HCV-LP–specific immunofluorescence and confocal microscopy. As shown in Figure 6, incubation of imDCs with HCV-LPs at 4°C resulted in the exclusive detection of HCV-LPs on the cell surface, consistent with HCV-LP binding to the cell membrane. By contrast, incubation at 37°C for 1 hour resulted in the translocation of E2 immunoreactivity into the cell consistent with HCV-LP entry. Furthermore, staining of cells with anti-LAMP antibody revealed that HCV-LPs partially colocalized with LAMP-2. In contrast, imDCs incubated with insect cell control preparation did not show any measurable E2-specific immunofluorescence (Figure 6) and PBMC-derived T cells incubated with HCV-LPs did not show any evidence for HCV-LP cell binding and entry (Figure 1C; data not shown). Thus, imDCs specifically bind and rapidly internalize HCV-LPs in a temperature-dependent manner.
Temperature-dependent HCV-LP entry into imDCs. Monocyte-derived imDCs were incubated with HCV-LPs or insect cell control preparation (GUS Ctrl) for 1 hour at 4°C (for HCV-LP binding) or 37°C (for HCV-LP entry). Then, DCs were washed in ice-cold PBS, fixed, and permeabilized. HCV-LPs were detected with chimpanzee anti-E2 mAb (49F3) and Cy3-conjugated anti–human IgG antibody (red fluorescence). For costaining of cytosolic lysosomal protein, cells were incubated with a mouse anti–LAMP-2 mAb and FITC-conjugated anti–mouse IgG antibody (green fluorescence). Nuclear staining was performed using DAPI (4′,6-diamidino-2-phenylindole; blue fluorescence). Comparative analysis of stained imDCs by confocal laser scanning microscopy revealed temperature-dependent HCV-LP internalization.
Temperature-dependent HCV-LP entry into imDCs. Monocyte-derived imDCs were incubated with HCV-LPs or insect cell control preparation (GUS Ctrl) for 1 hour at 4°C (for HCV-LP binding) or 37°C (for HCV-LP entry). Then, DCs were washed in ice-cold PBS, fixed, and permeabilized. HCV-LPs were detected with chimpanzee anti-E2 mAb (49F3) and Cy3-conjugated anti–human IgG antibody (red fluorescence). For costaining of cytosolic lysosomal protein, cells were incubated with a mouse anti–LAMP-2 mAb and FITC-conjugated anti–mouse IgG antibody (green fluorescence). Nuclear staining was performed using DAPI (4′,6-diamidino-2-phenylindole; blue fluorescence). Comparative analysis of stained imDCs by confocal laser scanning microscopy revealed temperature-dependent HCV-LP internalization.
Activation of DCs by native but not by denatured HCV-LPs
DC activation and maturation may be mediated by endogenous cytokines or the antigen itself. To determine whether HCV-LPs can induce DC activation, imDCs were exposed to HCV-LPs or insect cell control preparations. DC activation was then measured by flow cytometry of defined cell surface markers. After exposure to HCV-LPs for 16 hours, CD80 and CD83 were markedly and significantly up-regulated, indicating HCV-LP–mediated DC activation (Figure 7; Table 2). Although this up-regulation was less pronounced than for DCs incubated with LPS, it was highly reproducible for different HCV-LP and DC preparations (Table 2). By contrast, incubation of DCs with insect cell control preparations did not activate DCs. Denatured HCV-LPs (generated by heat treatment of native HCV-LPs) also failed to elicit DC activation (Figure 7; Table 2). This finding suggests that conformation of structural proteins of HCV-LPs is important for DC activation.
DC activation by native HCV-LPs. ImDCs were exposed to insect cell control preparations (GUS Ctrl), native HCV-LPs, denatured HCV-LPs, or LPS at a final concentration of 10 μg/mL. After 16 hours, DC activation was assessed by flow cytometric analysis of HLA-DR, CD80, CD86, and CD83 cell surface expression (dark line). Histograms corresponding to background expression of the respective cell surface molecules in unexposed DCs are shown as a gray line. A representative result of 3 independent experiments is shown.
DC activation by native HCV-LPs. ImDCs were exposed to insect cell control preparations (GUS Ctrl), native HCV-LPs, denatured HCV-LPs, or LPS at a final concentration of 10 μg/mL. After 16 hours, DC activation was assessed by flow cytometric analysis of HLA-DR, CD80, CD86, and CD83 cell surface expression (dark line). Histograms corresponding to background expression of the respective cell surface molecules in unexposed DCs are shown as a gray line. A representative result of 3 independent experiments is shown.
Up-regulation of CD80 and CD83 expression during HCV-LP-induced DC activation
. | Δ MFI, mean ± SD . | . | |
|---|---|---|---|
. | CD80 . | CD83 . | |
| GUS Ctrl | 10 ± 8* | 7 ± 6* | |
| Native HCV-LPs | 41 ± 9*† | 48 ± 19*† | |
| Denatured HCV-LPs | 5.6 ± 4† | 0 ± 0† | |
| LPS | 140 ± 6 | 157 ± 21 | |
. | Δ MFI, mean ± SD . | . | |
|---|---|---|---|
. | CD80 . | CD83 . | |
| GUS Ctrl | 10 ± 8* | 7 ± 6* | |
| Native HCV-LPs | 41 ± 9*† | 48 ± 19*† | |
| Denatured HCV-LPs | 5.6 ± 4† | 0 ± 0† | |
| LPS | 140 ± 6 | 157 ± 21 | |
ImDCs were incubated with insect cell control preparation (GUS Ctrl), native HCV-LPs, denatured HCV-LPs, and lipopolysaccharide (LPS) as described in Figure 7. Cell surface expression of CD80 and CD83 was determined by flow cytometry. Δ MFI (net mean fluorescence intensity) was calculated by subtracting the MFI of DCs incubated without antigen (background) from the MFI of DCs incubated with the respective antigen. Mean ± SD of 3 independent experiments using imDCs from 3 different individuals is shown.
Differences in expression of DC activation markers between native HCV-LPs versus GUS Ctrl-treated DCs (*) as well as DCs incubated with native HCV-LPs versus denatured HCV-LPs (†) were statistically significant (P < .05; two-sided Student ttest).
Immunostimulatory properties of DCs pulsed with HCV-LPs in mixed lymphocyte reaction
DCs in individuals with chronic HCV infection have been shown to display an impaired allostimulatory function.20 To study whether HCV-LPs can modify the allostimulatory function of human DCs, the allostimulatory capacity of DCs pulsed with HCV-LPs was studied in a mixed lymphocyte reaction. As shown in Figure 8, DCs pulsed with HCV-LPs exhibited no quantitative or qualitative difference in their allostimulatory function as assessed by T-cell proliferation (Figure 8A) and cytokine secretion (Figure 8B) compared with DCs pulsed with insect cell control preparations or without antigen. These data indicate that HCV-LPs do not alter the allostimulatory function of human DCs.
Allostimulatory function of DCs pulsed with HCV-LPs. (A) Responder PBMCs (2 × 105 cells/well) were cultured for 6 days in the presence of increasing concentrations of irradiated allogenic stimulator DCs pulsed with HCV-LPs (⬡), insect cell control preparation (▪), or without antigen (▵). T-cell proliferation was measured by [3H]thymidine incorporation during the last 16 hours of culture. The results are expressed as counts per minute (cpm; mean ± SD of an experiment performed in triplicate). (B) IL-4/IFN-γ production by allogeneic CD4+ T cells. CD4+ T cells were cocultured with DCs (T/DCs ratio: 10:1) as described for panel A. For IL-4 and IFN-γ determination, cell culture supernatants were collected on days 1 and 5, respectively. Data are shown as pg/mL (mean ± SD of an experiment performed in triplicate). The thresholds for cytokine detection are indicated as - - -.
Allostimulatory function of DCs pulsed with HCV-LPs. (A) Responder PBMCs (2 × 105 cells/well) were cultured for 6 days in the presence of increasing concentrations of irradiated allogenic stimulator DCs pulsed with HCV-LPs (⬡), insect cell control preparation (▪), or without antigen (▵). T-cell proliferation was measured by [3H]thymidine incorporation during the last 16 hours of culture. The results are expressed as counts per minute (cpm; mean ± SD of an experiment performed in triplicate). (B) IL-4/IFN-γ production by allogeneic CD4+ T cells. CD4+ T cells were cocultured with DCs (T/DCs ratio: 10:1) as described for panel A. For IL-4 and IFN-γ determination, cell culture supernatants were collected on days 1 and 5, respectively. Data are shown as pg/mL (mean ± SD of an experiment performed in triplicate). The thresholds for cytokine detection are indicated as - - -.
DCs pulsed with HCV-LPs induce HCV core-specific CD4+ T-cell responses
Since HCV-LPs can activate imDCs, we also examined whether HCV-LPs are processed by imDCs and whether HCV-LP–derived antigens are presented to CD4+ T cells. DCs pulsed with HCV-LPs, but not with insect cell control preparations, induced a marked and dose-dependent CD4+ T-cell clone proliferation indicating efficient HCV-LP processing and presentation of HCV-LP–derived antigens on MHC class II molecules (Figure 9).
Core-specific CD4+ T-cell clone stimulation by imDCs pulsed with HCV-LPs. Monocyte-derived imDCs were pulsed with corresponding concentrations of recombinant core protein (rCore), HCV-LPs (1-100 μg/mL; equivalent to approximately 0.1-10 μg HCV-LP core/mL), and insect cell control preparations (GUS Ctrl). DCs were then incubated with core-specific CD4+ T cells for 3 days and T-cell proliferation was measured by [3H]thymidine incorporation. Results are shown as counts per minute (cpm; mean ± SD of a representative experiment performed in triplicate).
Core-specific CD4+ T-cell clone stimulation by imDCs pulsed with HCV-LPs. Monocyte-derived imDCs were pulsed with corresponding concentrations of recombinant core protein (rCore), HCV-LPs (1-100 μg/mL; equivalent to approximately 0.1-10 μg HCV-LP core/mL), and insect cell control preparations (GUS Ctrl). DCs were then incubated with core-specific CD4+ T cells for 3 days and T-cell proliferation was measured by [3H]thymidine incorporation. Results are shown as counts per minute (cpm; mean ± SD of a representative experiment performed in triplicate).
Cross-presentation of HCV-LPs to intrahepatic HCV core-specific CD8+ T cells
To study whether HCV-LPs are cross-presented to CD8+ T cells, we assessed presentation of HCV-LP–derived antigens to core-specific intrahepatic CD8+ T cells derived from an HCV-infected individual. Autologous DCs pulsed with HCV-LPs markedly stimulated IFN-γ production by intrahepatic CD8+ T cells (Figure 10). In contrast, DCs pulsed with an insect cell control preparation (GUS Ctrl, Figure 10) or intrahepatic CD8+ T cells incubated with HCV-LPs in the absence of DCs (data not shown) did not result in IFN-γ production of intrahepatic CD8+ T cells. These control experiments rule out that IFN-γ production of CD8+ T cells was due to nonspecific effects related to contaminating insect cell proteins or a direct activation of intrahepatic CD8+ T cells by nonprocessed HCV-LP–derived antigens. Similar results were obtained using peripheral core-specific CD8+ T-cell lines established from the same donor (data not shown). These data demonstrate that human DCs can efficiently cross-present HCV-LPs to HCV-specific CD8+ T cells.
Cross-presentation of HCV-LPs to intrahepatic core-specific CD8+ T cells. Autologous imDCs were loaded with HCV-LPs (50 μg/mL; equivalent to approximately 5 μg HCV-LP core/mL), insect cell control preparation (GUS Ctrl; 50 μg/mL), or core peptide 6 (comprising core aa's 36-53; 10 μg/mL). The pulsed DCs were washed and subsequently cocultured with intrahepatic core-specific CD8+ T cells at a ratio 1:2. After 5 hours of incubation, the cells were stained with antibodies to CD8 and IFN-γ and analyzed by flow cytometry. Percentages of CD8+ T cells that produced IFN-γ in the respective quadrants are indicated on the dot plots. Controls include isotype-control stained CD8+ T cells to determine the quadrant boundaries (not shown).
Cross-presentation of HCV-LPs to intrahepatic core-specific CD8+ T cells. Autologous imDCs were loaded with HCV-LPs (50 μg/mL; equivalent to approximately 5 μg HCV-LP core/mL), insect cell control preparation (GUS Ctrl; 50 μg/mL), or core peptide 6 (comprising core aa's 36-53; 10 μg/mL). The pulsed DCs were washed and subsequently cocultured with intrahepatic core-specific CD8+ T cells at a ratio 1:2. After 5 hours of incubation, the cells were stained with antibodies to CD8 and IFN-γ and analyzed by flow cytometry. Percentages of CD8+ T cells that produced IFN-γ in the respective quadrants are indicated on the dot plots. Controls include isotype-control stained CD8+ T cells to determine the quadrant boundaries (not shown).
Discussion
In this study, we demonstrate for the first time that HCV-LPs interact specifically with human monocyte-derived DCs and defined subsets of blood DCs. DC–HCV-LP interaction is characterized by the following 4 key findings: (1) human DCs bind and rapidly internalize HCV-LPs in a dose-dependent manner; (2) HCV-LP binding and entry require cell surface molecules other than C-type lectins; (3) incubation of DCs with HCV-LPs results in the up-regulation of costimulatory molecules indicating HCV-LP–mediated DC activation; and (4) DCs pulsed with HCV-LPs elicit a strong HCV-specific CD4+ and CD8+ T-cell response demonstrating efficient HCV-LP antigen processing and presentation.
HCV-LPs demonstrated a dose-dependent binding to monocyte-derived imDCs and purified blood imDCs. Maturation of imDCs by proinflammatory cytokines was accompanied by a marked reduction of HCV-LP binding, indicating down-regulation of DC cell surface molecules that bind HCV-LPs during maturation. These observations suggest that imDCs are most likely to be the key antigen-presenting cells interacting with HCV-LPs in humans. Since recent studies have shown that HCV is internalized by imDCs,19,20 our results indicate that HCV-LPs demonstrate a similar tropism for DCs as virions.
ImDCs express several receptors including C-type lectins that mediate recognition and internalization of pathogens.59 Previous studies have demonstrated that recombinant C-terminally truncated HCV envelope glycoprotein E2 and HCVpp can bind to DC-SIGN and L-SIGN.60,61 In contrast to observations using the above-mentioned ligands, blocking of C-type lectins by mannan, as well as preincubation of imDCs with specific DC-SIGN/L-SIGN antibodies, did not result in marked inhibition of HCV-LP binding. Although our results do not exclude binding of HCV-LPs to DC-SIGN, our observations indicate that DC-SIGN is not sufficient to mediate HCV-LP binding to DCs and that other or additional cell-surface receptors are required for HCV-LP binding.
Since glycosylation of HCV-LPs did not appear to play a major role for HCV-LP binding to DCs and HCV-LP binding could be markedly inhibited by antienvelope antibodies directed against nonglycosylated envelope epitopes, it is unlikely that differences in envelope glycosylation play a key role for the observed differences in ligand-cell interaction (recombinant envelope proteins versus HCV-LPs). This conclusion is supported by the observation that both C-terminally truncated E2 purified from the intracellular fraction17 (containing carbohydrate forms sensitive to digestion with EndoH) as well as secreted C-terminally truncated E216,60 (containing EndoH-resistant complex carbohydrate forms associated with trafficking through the Golgi apparatus) similarly interact with DC-SIGN.16,60
Alternatively, differences in envelope protein conformation may play an important role for the interaction of model ligands with DC-SIGN. In contrast to C-terminally truncated recombinant E2 used for interaction studies with DC-SIGN,16,17,60,62 E2 of HCV-LP is expressed in its full length together with glycoprotein E1 as part of an E1/E2 heterodimer.33,35 Several investigators have shown that HCV-LPs contain E2 in a native conformation, which may resemble properly folded E2 in the virion.27,28,33 Thus, it is conceivable that the observed interaction of E2 with cell surface DC-SIGN is predominantly a feature of C-terminally truncated envelope proteins, whereas properly folded E1/E2 heterodimers containing full-length E2 in its native conformation may exhibit a different interaction profile. This hypothesis is supported by two recent studies characterizing the cellular entry of HCV E1 and E2 glycoprotein complexes as part of retroviral HCVpp. Although HCVpp can bind to DC-SIGN,18 DC-SIGN is not able to mediate entry of HCVpp into DCs18 or hepatoma cells.63 Recent evidence suggests that C-type lectins may rather play a role for facilitating transmission of HCV from DCs or L-SIGN–positive liver sinusoidal endothelial cells to neighboring hepatocytes.18,60,61
Since the detailed structural and functional properties of infectious virions are still poorly characterized, it is unknown whether the surface properties of HCV-LPs or other surrogate systems such as HCVpp are identical with the infectious virion. The ongoing development of recombinant infectious virions may ultimately allow investigators to discover the role of DC-SIGN for binding and entry of the native virion in the future.
Several cell surface molecules including CD81,64 scavenger receptor class B type I (SR-BI),65 and heparan sulfate30 have been suggested as mediators of HCV envelope protein binding. Interestingly, preliminary studies from our laboratory indicate that HCV-LP binding to DCs can be partially inhibited by anti-CD81 mAb (H.B. and T.F.B., unpublished observations, April 2004), which was recently reported to block HCV E2–CD81 interaction.66 Further studies analyzing the role of CD81, SR-BI, and heparan sulfate in mediating HCV-LP binding to DCs are in progress.
Following cell surface binding, antigen-receptor complexes are internalized into lysosomal compartments of DCs where proteinases mediate antigen processing.67 Confocal microscopy demonstrated that HCV-LPs partially colocalized with LAMP-2 following HCV-LP uptake, suggesting that HCV-LPs may be targeted into the endosomal-lysosomal pathway. Trafficking and processing of HCV-LPs into the endosomal-lysosomal pathway is corroborated by the finding of efficient MHC class II–restricted HCV-LP antigen presentation to CD4+ T cells. DCs are also capable of transporting antigens from the endocytic compartment to the cytosol, leading to “cross-presentation” on MHC class I molecules to CD8+ T cells. This pathway can be used by particular antigens such as VLPs but not by recombinant soluble proteins (for review see Heath and Carbone68 ). In this study, we show for the first time that human DCs cross-present HCV-LPs to HCV-specific CD8+ T cells.
Incubation of imDCs with HCV-LPs also led to a small but reproducible and significant up-regulation of the costimulatory molecules CD80 and CD83 indicating HCV-LP–induced DC activation. These data are in line with two previous reports on activation of mouse and human monocyte-derived imDCs by papillomavirus VLPs.40,69 Similar to the results using denatured HCV-LPs, nonassembled papillomavirus structural protein L1 failed to activate DCs.40 In contrast to a marked DC activation induced by LPS, HCV-LP–induced activation was of substantially lower magnitude. This finding is not surprising since neither HCV1 nor HCV-LPs (T.F.B. and T.J.L., unpublished observations, July 2002) generally induce a systemic inflammatory reaction in humans (HCV) or mice (HCV-LPs) in vivo.
A growing number of studies have characterized the function of DCs in chronic hepatitis C. Similar to findings in other chronic viral infections,70 two observations have been made: (1) an impaired DC maturation has been described in patients with chronic but not in resolved HCV infection23 ; (2) DCs in chronic HCV infection appear to display an impaired allostimulatory function.20,22,23 In contrast to these findings, two recent studies did not show evidence for an impaired DC function in HCV-infected chimpanzees or humans.24,25 Since incubation of DCs with HCV-LPs resulted in an increased activation of DCs and did not modify the allostimulatory function of DCs, our findings suggest that uptake and processing of the virion do not alter DC function. Therefore, it is conceivable that the observed defects in patients with chronic HCV infection in some20,22,23 but not in other24,25 studies may either require ongoing HCV viral replication or are possible consequences of chronic HCV infection associated with liver disease progression.
In conclusion, the interaction of HCV-LPs with DCs represents a powerful model system to study HCV-DC interaction, allowing the characterization of HCV uptake and activation and presentation of HCV structural proteins on the molecular level. This includes the isolation of DC receptor(s) required for HCV binding and uptake, the exploration of signaling pathways including TLRs in mediating DC activation, the mapping of viral epitopes presented to T cells, and finally the detailed exploration of HCV cross-presentation. Furthermore, our model system may allow evaluation of HCV-specific alterations in dendritic cells postulated in patients with chronic HCV infection.71
Finally, our findings of HCV-LP–mediated DC activation and efficient antigen presentation may explain the marked immunogenicity of HCV-LPs in vivo.35-37,51 Together with similar findings of papillomavirus VLP-DC interaction69 and the successful development of a human papillomavirus VLP-based vaccine for the prevention of chronic papillomavirus infection,72 our findings suggest that HCV-LPs may be a potent vaccine candidate for the induction of antiviral cellular immune responses in humans.
Prepublished online as Blood First Edition Paper, January 18, 2005; DOI 10.1182/blood-2004-05-1952.
Supported by grants from the European Union (Brussels, Belgium; QLK-2-1999-00356, QLK-2-2002-01329, and ViRgiL No. E) and the Deutsche Forschungsgemeinschaft (Bonn, Germany; Ba1417/11-1).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors would like to thank M. Ritter and M. Geißler for support in generation of monocyte-derived DCs; M. Follo for performing confocal laser scanning microscopy; A. Patel, S. Levy, and T. B. H. Geijtenbeek for the gift of antibodies; G. Inchauspé, C. Bain, J. Dubuisson, F.-L. Cosset, and J. McKeating for helpful discussions; and A. Farmer for support in manuscript preparation. P. Schürmann and B. Gissler are gratefully acknowledged for technical assistance.


![Figure 8. Allostimulatory function of DCs pulsed with HCV-LPs. (A) Responder PBMCs (2 × 105 cells/well) were cultured for 6 days in the presence of increasing concentrations of irradiated allogenic stimulator DCs pulsed with HCV-LPs (⬡), insect cell control preparation (▪), or without antigen (▵). T-cell proliferation was measured by [3H]thymidine incorporation during the last 16 hours of culture. The results are expressed as counts per minute (cpm; mean ± SD of an experiment performed in triplicate). (B) IL-4/IFN-γ production by allogeneic CD4+ T cells. CD4+ T cells were cocultured with DCs (T/DCs ratio: 10:1) as described for panel A. For IL-4 and IFN-γ determination, cell culture supernatants were collected on days 1 and 5, respectively. Data are shown as pg/mL (mean ± SD of an experiment performed in triplicate). The thresholds for cytokine detection are indicated as - - -.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/105/9/10.1182_blood-2004-05-1952/6/m_zh80090577560008.jpeg?Expires=1767744997&Signature=ZyvMAJcEx9NMdLhD5eohYP36NtI4c0sSnauD8fdBLpC0Q7oJT7jUZ45y8c39kbQMGQ95bP-oO9qXUbiadlge-l4y5p~ELn2yIbkEjmiKYv~ktIbExZ7WDDqhvWIm7mCg6aLyEL25vWBycEDf-0p7ZuUNvhYZgiuPORrS55g5cIiFscKtLFLtnbmqb6VWn7EO89ULu4K3OnRd~czKoVDkXT1F3yKx7SSdxqQamDhBYzkJnxS-TqTqtFQKWWkD27cSzBWx3k94hea4QW3SBSCCySC6Q2XFr8d3ZFRFV9u1MNqIIDXcLuONiIksaKVslenchx99vnRjOMphVfhF5J6fqQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 9. Core-specific CD4+ T-cell clone stimulation by imDCs pulsed with HCV-LPs. Monocyte-derived imDCs were pulsed with corresponding concentrations of recombinant core protein (rCore), HCV-LPs (1-100 μg/mL; equivalent to approximately 0.1-10 μg HCV-LP core/mL), and insect cell control preparations (GUS Ctrl). DCs were then incubated with core-specific CD4+ T cells for 3 days and T-cell proliferation was measured by [3H]thymidine incorporation. Results are shown as counts per minute (cpm; mean ± SD of a representative experiment performed in triplicate).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/105/9/10.1182_blood-2004-05-1952/6/m_zh80090577560009.jpeg?Expires=1767744997&Signature=XTeGmO6tgUqqpdZM7kbdtjWortk~U-Ef~etNgkbRCGhSlbgB~0SelboFNEp7rbLaBCAz4ix2I0HzgH4OIUB-f5JnYN1eK1GaAp5W58ifvwYXmR8eZvDaDxseKyH9ElKWtjq43VExmJei3ZAIA1hKKy6E7ojo4V31TU3U694xVGkJsubUEAhW~UNxumQO3zKH4zUg0YjeUFxXkAWC5mYAwvfXTtoUwHqv30NSpFgmkVHJfIBG9dHsMW2FWHFNbIma3WZA7oQqLReSs6EBBJJHGRr-rUravYKkuvnxypajDf88-pxU5AdccfJGc-F0noPullSs4ub4VyfDG01zEsSP9w__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal