Abstract
The pleiotropic cytokine tumor necrosis factor-α (TNF-α) and thrombin lead to increased endothelial permeability in sepsis. Numerous studies demonstrated the significance of intracellular cyclic nucleotides for the maintenance of endothelial barrier function. Actions of cyclic adenosine monophosphate (cAMP) and cyclic guanosine monophosphate (cGMP) are terminated by distinct cyclic nucleotide phosphodiesterases (PDEs). We hypothesized that TNF-α could regulate PDE activity in endothelial cells, thereby impairing endothelial barrier function. In cultured human umbilical vein endothelial cells (HUVECs), we found a dramatic increase of PDE2 activity following TNF-α stimulation, while PDE3 and PDE4 activities remained unchanged. Significant PDE activities other than PDE2, PDE3, and PDE4 were not detected. TNF-α increased PDE2 expression in a p38 mitogen-activated protein kinase (MAPK)–dependent manner. Endothelial barrier function was investigated in HUVECs and in isolated mice lungs. Selective PDE2 up-regulation sensitized HUVECs toward the permeability-increasing agent thrombin. In isolated mice lungs, we demonstrated that PDE2 inhibition was effective in preventing thrombin-induced lung edema, as shown with a reduction in both lung wet-to-dry ratio and albumin flux from the vascular to bronchoalveolar compartment. Our findings suggest that TNF-α–mediated up-regulation of PDE2 may destabilize endothelial barrier function in sepsis. Inhibition of PDE2 is therefore of potential therapeutic interest in sepsis and acute respiratory distress syndrome (ARDS).
Introduction
Sepsis—the systemic inflammatory response to infection—is the most common cause of death among patients in noncoronary intensive care units.1 Laboratory markers of inflammation include high circulating levels of tumor necrosis factor α (TNF-α) and other cytokines, as well as activation of the coagulation cascade.1 Endothelial hyperpermeability is a hallmark of sepsis. TNF-α increases endothelial cell permeability2 with subsequent vascular leakage contributing to severe organ dysfunction such as adult respiratory distress syndrome (ARDS).3
Half of the sepsis patients develop disseminated intravascular coagulation2,4 due to a shift toward a procoagulant state with excessive thrombin and fibrin generation.1
On a cellular level, thrombin changes the shape of endothelial cells and increases endothelial permeability,5 thereby impairing endothelial barrier function synergistically with TNF-α.1,6
Thrombin, the main effector protease of the coagulation cascade, activates endothelial cells directly via protease-activated receptor 1 (PAR1) and PAR4,7 thereby inducing hyperpermeability and promoting adhesion of platelets and leukocytes, as well as secretion of platelet-activating factor (PAF) and inflammatory cytokines.8
After binding to one of the various receptors, TNF-α mediates activation of diverse signaling pathways, such as the p38 mitogen-activated protein kinase (p38 MAPK), JUN N-terminal kinase (JNK), and nuclear factor kappa B (NF-κB). TNF receptor (TNFR) activation requires the binding of one or more TNFR-associated factors (TRAFs), which determine the cellular response following TNF-α stimulation.9
Both TNF-α and thrombin induce cytoskeletal rearrangement by phosphorylation of myosin light chain (MLC) kinase, ultimately leading to cell shape changes and the formation of paracellular gaps.10-12 In previous studies from others and our laboratory, it was shown that high cyclic nucleotide levels were able to prevent a rise in endothelial permeability.10,13-16 High levels of intracellular cyclic adenosine monophosphate (cAMP) or cyclic guanosine monophosphate (cGMP) can be achieved either by activation of the adenylyl/guanylyl cyclases or by inhibition of the cyclic nucleotide degrading phosphodiesterases (PDEs). This heterogenous group of distinct enzymes metabolizes cAMP and cGMP to their biologically inactive nucleotide 5′-monophosphates. PDEs are categorized into 11 families17,18 that are distinguished by a number of criteria, including substrate specificity, kinetic properties, sensitivity to allosteric modulators, response to synthetic inhibitors, and primary amino acid sequence.19,20
The cGMP-stimulated phosphodiesterase (PDE2) is a homodimer of 2 105-kDa subunits. Each monomer contains 2 GAF domains (found in cGMP-phosphodiesterases, adenylyl cyclases, and FhlA [formate hydrogen lyase transcriptional activator]), which allow either dimerization or binding of cGMP.21 In contrast to other PDE isoenzyme families, PDE2 is encoded by one single gene and only one human splice variant has been described so far. This sequence shares high similarity with the bovine PDE2A3. Thus, the human homolog has been named as PDE2A3.22
PDE2 metabolizes both cGMP and cAMP, although affinity for cGMP is slightly higher than for cAMP.22 High PDE2 activity can be found in the heart23 and brain.22 Lower expression of PDE2A3 mRNA was found in the lung, placenta, liver, skeletal muscle, kidney, and pancreas.22,24
In previous work, we identified PDE2, PDE3, and PDE4 activity in endothelial cells derived from human umbilical vein (HUVEC)14 and porcine pulmonary artery endothelium15 and demonstrated the importance of the cAMP/cGMP-PDE system for the integrity of the endothelial barrier function in vitro10,13-15 and in vivo.10,25
In this study, we investigated the hypothesis that TNF-α activates endothelial PDE2 leading to a decline of intracellular cAMP levels, thereby increasing endothelial permeability.
Our results show that TNF-α increased exclusively PDE2 in a p38 MAPK–dependent manner, whereas PDE3 or PDE4 activity remained unchanged. High PDE2 activity sensitized endothelial cells toward thrombin, thereby leading to endothelial hyperperme-ability. Thrombin-induced hyperpermeability was attenuated by PDE2 inhibition in vitro and in isolated mice lungs.
We conclude from our findings that in severe infection, high PDE2 activity may contribute to endothelial barrier dysfunction, which can be antagonized by PDE2 inhibition. For therapeutic purposes, PDE2 inhibition could be a potential target for the therapy of infection-associated endothelial hyperpermeability.
Materials and methods
Preparation of endothelial cells
Reagents
The PDE2 inhibitor 9-(6-Phenyl-2-oxohex-3-yl)-2-(3,4-dimethoxybenzyl)-purin-6-one (PDP) was example 100 from US patent 5861396 (Bayer, Leverkusen, Germany) resynthesized at Altana Pharma. We confirmed that PDP is a highly selective PDE2 inhibitor with a median inhibitory concentration (IC50) of 0.6 nM, whereas all other PDE isoenzymes are inhibited with an IC50 of more than 1 μM (0.5 μM cAMP substrate concentration), according to our measurements. In all experiments, PDP was used at 1 μM, at which the inhibitor completely blocks PDE2 but does not substantially interfere with any other PDE isoenzyme. The PDE3 inhibitor motapizone was a generous gift from Sanofi-Aventis (formerly Nattermann/Rhone-Poulenc/Rorer, Cologne, Germany). PDE4 inhibitor piclamilast (WO9212961) was synthesized by Altana Pharma. BM13505 was a generous gift from Roche (Mannheim, Germany). TNF-α was bought from R&D Systems (Wiesbaden, Germany). MAP kinase kinase (MEK) inhibitor U0126 and p38 MAPK inhibitor SB202190 were purchased from Calbiochem (Bad Soden, Germany); thrombin (from human plasma) and sodium nitroprusside, from Sigma (Deisenhofen, Germany). Sterile Krebs-Henseleit-hydroxyethylamylopectin buffer was obtained from Serag-Wiessner (Naila, Germany). Ketamin was purchased from Curamed (Karlsruhe, Germany) and Xylazin, from Bayer. Human serum albumin 20% was obtained from Grifols (Langen, Germany).
Mice
Female BALB/C mice were obtained from Charles River Laboratories (Sulzfeld, Germany). All animals were used at a body weight between 18 to 24 g and had free access to feed and water before the experiment.
Phosphodiesterase assay
Phosphodiesterase activity using [H3]cAMP as substrate was measured as described previously27-29 and normalized to protein concentrations of the soluble fraction of HUVEC lysates.30 Selective inhibitors and activators of PDE isoenzymes were used to determine activities of PDE families. The increase of cAMP (0.5 μM) degrading activity in the presence of 10 μM motapizone and 1 μM piclamilast (PDE3 and PDE4 inhibitor, respectively) induced by 5 μM cGMP represented PDE2. PDE2 inhibitor PDP (1 μM) completely inhibited this cGMP–induced activity increment, further verifying this activity as PDE2. PDE4 was calculated as the difference of PDE activities at 0.5 μM cAMP in the presence and absence of 1 μM piclamilast. The difference between piclamilast-inhibited cAMP hydrolysis in the presence and absence of 10 μM motapizone represented PDE3.
Determination of HUVEC cyclic nucleotide content
PCR analysis
Expression of PDE2-mRNA in HUVECs was analyzed semiquantitatively by reverse-transcriptase–polymerase chain reaction (RT-PCR; 28-34 PCR cycles) as described previously.28 The primers for PDE2A3 are as follows: forward primer, 5′-GATCCTGAACATCCCTGACG-3′ and reverse primer, 5′-GCAGAAGTGGGAGACAGAAAAG-3′ (base pairs 1583-1602 and 2165-2144, respectively; GenBank U67733). The primers for glyceraldehyd-3-phosphate dehydrogenase (GAPDH) are as follows: forward primer, 5′-CCACCCATGGCAAATTCCATGGCA-3′ and reverse primer, 5′-TCTAGACGGCAGGTCAGGTCC-3′ (base pairs 212-235 and 809-789, respectively; GenBank M33197).
DNA constructs and transfection procedures
KRSPA flag p38 AEF (dominant-negative p38 MAPK), KRSPAS flag MKK6 (constitutively active MKK6), and the empty vector KRSPA were kind gifts from Dr S. Ludwig (Düsseldorf, Germany).31 PDE2A3 cDNA was cloned into the pCDNA3.1/hygro expression vector (Invitrogen, Karlsruhe, Germany). All transfections were performed with Effectene (Qiagen, Hilden, Germany) according to the manufacturer's protocol. Transfection efficiency was assessed by cotransfection of green fluorescent protein (GFP) plasmid pGreenLantern (Promega, Mannheim, Germany) followed by fluorescence-activated cell sorter (FACS) analysis (efficiency up to 30%). In all experiments, mock transfections with empty vectors were performed as negative controls.
Western analysis
Cells were harvested in lysis buffer supplemented with proteinase inhibitors. Protein concentration was determined as previously described.30 Equal amounts of lysates were subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE, 7.5% gel) and blotted onto Hybond enhanced chemiluminescence (ECL) membranes (Amersham, Freiburg, Germany). Membranes were exposed to PDE2A antibody (1:500) purchased from FabGennix (Shreveport, LA) and to rabbit extracellular signal-related kinase 2 (ERK2)–specific antibody (Santa Cruz Biotechnology, Santa Cruz, CA). Secondary antibodies were IRDye 800–labeled antigoat and cyanin 5.5 (Cy5.5)–labeled antirabbit antibody. Proteins were visualized using Odyssey infrared imaging system (LI-COR, Lincoln, NE).
Analysis of endothelial permeability in vitro
Endothelial cell monolayers were grown on polycarbonate filter membranes as previously described.10,13,15,32,33 The confluent monolayer on a filter membrane was mounted in a modified chemotaxis chamber, and hydrostatic pressure of 10 cm H2O was applied to the “luminal” side of the cell monolayer. The filtration rate across the endothelial monolayer was continuously determined, and hydraulic conductivity was calculated and expressed as described.10,13,15,32,33
Isolated perfused mouse lung
Mice lungs were prepared, ventilated, and perfused according to the experimental setup described previously.34 Anesthetized mice were placed into a 37°C chamber (HSE Harvard Apparatus, Holliston, MA), tracheotomized, and ventilated (positive pressure ventilation, room air) with a rotary vane compressor pump (VCM; HSE Harvard Apparatus).
After laparotomy, sternotomy, and cannulation (left atrium, pulmonary artery), lungs were perfused with sterile Krebs-Henseleit-hydroxyethylamylopectine buffer at a nonrecirculating constant flow of 1 mL/min (Reglo peristaltic pump, Ismatec SA, Glattbrugg, Switzerland). Left atrial pressure was adjusted at +2.2 cm H2O. After closing the chamber lid, oscillating negative pressure ventilation was performed, leading to a tidal volume of 150 μL. Artificial thorax chamber pressure and airflow velocity were measured with 2 distinct differential pressure transducers (HSE Harvard Apparatus; Validyne, Irvine, CA). Pulmonary artery and left atrial pressure were continuously monitored. All data were amplified and analyzed with Pulmodyn software (HSE Harvard Apparatus).
Lungs were ventilated and perfused for 45 minutes to establish baseline conditions. Subsequently, thrombin (750 μM) was added for one minute. PDE2 inhibitor PDP (1 μM) or PDE4 inhibitor piclamilast (1 μM) was added to the perfusate 10 minutes before thrombin application. In all experiments, the thromboxane receptor antagonist BM13505 (5 μM) was given 10 minutes prior to thrombin application. At 30 minutes after thrombin application, wet-dry weight ratio and lung albumin permeability were measured.
Wet-dry weight ratio. Lungs were weighed and subsequently dried in a 60°C oven for 48 hours. The ratio of wet weight to dry weight represented tissue edema.35
Lung albumin permeability. Human serum albumin was admixed at a concentration of 0.04% to the perfusate 20 minutes before thrombin administration. Albumin concentration was measured in the bronchoalveolar lavage (BAL) fluid36 after the experiment using an immunoassay (Bethyl, Montgomery, TX) that is insensitive to murine albumin and mouse serum. A sample of the venous effluent was preserved for control of the perfusate albumin concentration. BAL albumin content was expressed as nanogram per milliliter of BAL fluid.
VE-cadherin and filamentous-actin (F-actin) staining
HUVECs were grown on Thermanox cover slides (Nunc, Wiesbaden, Germany), fixed in paraformaldehyde, permeabilized with 1% Triton X-100, and incubated with mouse antihuman vascular endothelial (VE)–cadherin antibody (1:100; Chemicon, Hofheim, Germany), followed by incubation with secondary goat antimouse Alexa 488–conjugated antibody (1:6000) and Alexa 546–conjugated phalloidin (1:200; both from Molecular Probes, Leiden, the Netherlands).
Cells were analyzed with the LSM 5 Pascal confocal microscope (Zeiss, Oberkochen, Germany) equipped with a Plan-Apochromat 63×/1.4 objective lens. Permafluor embedding medium (Beckman-Coulter, Fullerton, CA) and LSM 5 version 2.8 imaging software (Zeiss) were used.10
Statistical methods
Data are presented as means ± standard error of the mean (SEM). Data of Figures 1, 2A, 3B, 4, and 6 were analyzed using Student unpaired t test and Figure 5, using 2-way analysis of variance. Statistical significance was accepted at P values less than .05.
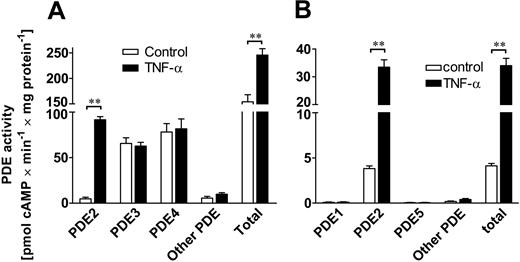
TNF-α increases PDE2 activity but not PDE3 or PDE4 activities. Isoenzyme-specific cAMP-PDE activity for PDE2, PDE3, and PDE4 (A) and cGMP-PDE activity for PDE1, PDE2, and PDE5 (B) were measured for control (□) and TNF-α–stimulated (▪) HUVECs. Cells were stimulated for 18 hours with 10 ng/mL TNF-α. PDE activity was normalized to total cellular protein and expressed as pmol cAMP (or cGMP) × minute-1 × mg protein-1. PDE2 activity for cAMP increased 19-fold in the presence of TNF-α, whereas activity of PDE3 and PDE4 did not change (A). Increase in total cAMP PDE activity of TNF-α–stimulated cells is almost entirely attributable to the rise in PDE2 activity. Other cAMP PDE activities distinct from PDE2, 3, or 4 were detected at very low levels. Using cGMP as substrate, PDE2 was identified as the only significant PDE activity in HUVECs (B). TNF-α stimulation (10 ng/mL) increased PDE2 and hence total cGMP activity more than 8-fold. Data presented are mean ± SEM of 4 independent experiments. **P < .01 versus untreated cells.
TNF-α increases PDE2 activity but not PDE3 or PDE4 activities. Isoenzyme-specific cAMP-PDE activity for PDE2, PDE3, and PDE4 (A) and cGMP-PDE activity for PDE1, PDE2, and PDE5 (B) were measured for control (□) and TNF-α–stimulated (▪) HUVECs. Cells were stimulated for 18 hours with 10 ng/mL TNF-α. PDE activity was normalized to total cellular protein and expressed as pmol cAMP (or cGMP) × minute-1 × mg protein-1. PDE2 activity for cAMP increased 19-fold in the presence of TNF-α, whereas activity of PDE3 and PDE4 did not change (A). Increase in total cAMP PDE activity of TNF-α–stimulated cells is almost entirely attributable to the rise in PDE2 activity. Other cAMP PDE activities distinct from PDE2, 3, or 4 were detected at very low levels. Using cGMP as substrate, PDE2 was identified as the only significant PDE activity in HUVECs (B). TNF-α stimulation (10 ng/mL) increased PDE2 and hence total cGMP activity more than 8-fold. Data presented are mean ± SEM of 4 independent experiments. **P < .01 versus untreated cells.
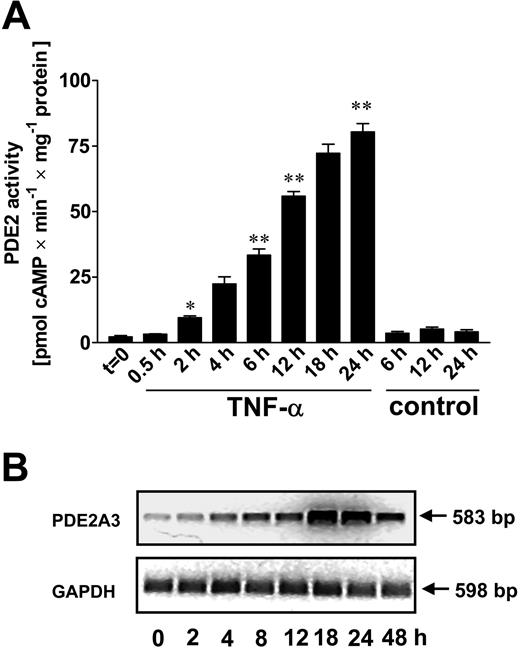
Time course of TNF-α–induced increase of PDE2 activity and mRNA. HUVECs were stimulated with TNF-α (10 ng/mL) and assayed for PDE2 activity (A) and PDE2A3 mRNA (B). PDE2 activity was measured in HUVEC lysates of cells stimulated up to 24 hours, normalized to total cellular protein, and expressed as pmol cAMP (or cGMP) × minute-1 × mg protein-1. Untreated controls were run in parallel for 3 time points (6, 12, and 24 hours). Data presented are mean ± SEM of 4 independent experiments. (B) Semiquantitative RT-PCR analysis of PDE2 mRNA in HUVECs. Cells were stimulated up to 48 hours with TNF-α (10 ng/mL). After RT reaction, PCR was run with 32 cycles (PDE2A3) and 28 cycles (glyceraldehyde-3-phosphate dehydrogenase, GAPDH). Representative ethidium bromide–stained agarose gels are shown. RT-PCR product sizes for PDE2A3 and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were 583 base pair (bp) and 598 bp, respectively. PCR for GAPDH served as internal control. The gels are representative of 4 independent experiments. *P < .05 versus t = 0; **P< .01 versus controls.
Time course of TNF-α–induced increase of PDE2 activity and mRNA. HUVECs were stimulated with TNF-α (10 ng/mL) and assayed for PDE2 activity (A) and PDE2A3 mRNA (B). PDE2 activity was measured in HUVEC lysates of cells stimulated up to 24 hours, normalized to total cellular protein, and expressed as pmol cAMP (or cGMP) × minute-1 × mg protein-1. Untreated controls were run in parallel for 3 time points (6, 12, and 24 hours). Data presented are mean ± SEM of 4 independent experiments. (B) Semiquantitative RT-PCR analysis of PDE2 mRNA in HUVECs. Cells were stimulated up to 48 hours with TNF-α (10 ng/mL). After RT reaction, PCR was run with 32 cycles (PDE2A3) and 28 cycles (glyceraldehyde-3-phosphate dehydrogenase, GAPDH). Representative ethidium bromide–stained agarose gels are shown. RT-PCR product sizes for PDE2A3 and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were 583 base pair (bp) and 598 bp, respectively. PCR for GAPDH served as internal control. The gels are representative of 4 independent experiments. *P < .05 versus t = 0; **P< .01 versus controls.
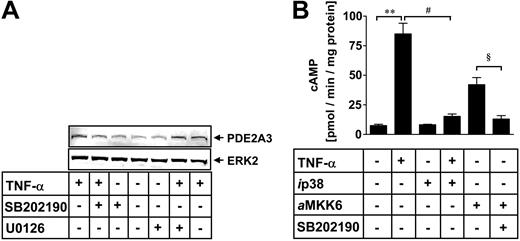
p38 MAP kinase is involved in PDE2A expression. HUVECs were incubated for 6 hours with TNF-α (10 ng/mL). p38 MAPK inhibitor SB202190 (10 μM) or ERK1/2 inhibitor U0126 (1 μM) were coincubated with TNF-α to identify involvement of the respective kinase pathway for TNF-α–mediated PDE2 expression (A). HUVEC protein lysates were denatured and subjected to electrophoresis on 7.5% (wt/vol) SDS–polyacrylamide gels. Proteins were transferred to nitrocellulose and probed simultaneously with anti-PDE2A and ERK2 antibody. The latter antibody detects nonphosphorylated ERK2 protein to control for equal loading. After exposure to the IRDye 800–labeled antigoat and Cy5.5-labeled secondary antirabbit antibody, proteins were visualized using an infrared imaging system. The gels are representative of 5 independent experiments. HUVECs were transfected with a dominant-negative p38 MAPK cDNA construct (ip38) prior to stimulation with TNF-α (10 ng/mL) (B). Constitutively active MKK6 cDNA construct (aMKK6) was transfected into HUVECs and incubated with p38 MAPK inhibitor SB202190 (10 μM). After 18 hours, PDE2 activity was measured and normalized to total cellular protein and expressed as pmol cAMP × minute-1 × mg protein-1 (B). Data presented are mean ± SEM of 4 independent experiments. **P < .01 versus untreated cells; #P < .05 versus TNF-α–stimulated cells; and §P < .05 versus MKK6-transfected cells.
p38 MAP kinase is involved in PDE2A expression. HUVECs were incubated for 6 hours with TNF-α (10 ng/mL). p38 MAPK inhibitor SB202190 (10 μM) or ERK1/2 inhibitor U0126 (1 μM) were coincubated with TNF-α to identify involvement of the respective kinase pathway for TNF-α–mediated PDE2 expression (A). HUVEC protein lysates were denatured and subjected to electrophoresis on 7.5% (wt/vol) SDS–polyacrylamide gels. Proteins were transferred to nitrocellulose and probed simultaneously with anti-PDE2A and ERK2 antibody. The latter antibody detects nonphosphorylated ERK2 protein to control for equal loading. After exposure to the IRDye 800–labeled antigoat and Cy5.5-labeled secondary antirabbit antibody, proteins were visualized using an infrared imaging system. The gels are representative of 5 independent experiments. HUVECs were transfected with a dominant-negative p38 MAPK cDNA construct (ip38) prior to stimulation with TNF-α (10 ng/mL) (B). Constitutively active MKK6 cDNA construct (aMKK6) was transfected into HUVECs and incubated with p38 MAPK inhibitor SB202190 (10 μM). After 18 hours, PDE2 activity was measured and normalized to total cellular protein and expressed as pmol cAMP × minute-1 × mg protein-1 (B). Data presented are mean ± SEM of 4 independent experiments. **P < .01 versus untreated cells; #P < .05 versus TNF-α–stimulated cells; and §P < .05 versus MKK6-transfected cells.
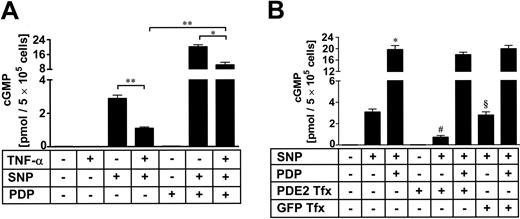
cGMP accumulation is reduced by TNF-α stimulation or PDE2 transfection. HUVECs were incubated with TNF-α (10 ng/mL) for 18 hours followed by PDP incubation (1 μM) for further 20 minutes. SNP (100 μM) was added for 5 minutes with subsequent lysis and measurement of total cellular cGMP levels using an enzyme-linked immunosorbent assay (A). Data presented are mean ± SEM of 5 independent experiments. *P < .05; **P< .01. PDE2A3 cDNA was cloned into the pcDNA3.1 expression vector. Of the pPDE2A3 expression plasmid, 0.1 μg was transfected into approximately 1 million HUVECs and harvested after 18 hours prior to stimulation and subsequent measurement of total cellular cGMP levels using an enzyme-linked immunosorbent assay (B). PDE2 inhibitor PDP (1 μM) was added 20 minutes prior to stimulation with SNP (100 μM) for 5 minutes with subsequent lysis and measurement of cGMP levels. Green fluorescent protein (GFP)–transfected cells were used as control. Data presented are mean ± SEM of 3 independent experiments. *P < .05 versus SNP-treated cells; #P < .05 versus SNP/PDP-treated cells; and §P < .05 versus SNP-treated/PDE2-transfected cells.
cGMP accumulation is reduced by TNF-α stimulation or PDE2 transfection. HUVECs were incubated with TNF-α (10 ng/mL) for 18 hours followed by PDP incubation (1 μM) for further 20 minutes. SNP (100 μM) was added for 5 minutes with subsequent lysis and measurement of total cellular cGMP levels using an enzyme-linked immunosorbent assay (A). Data presented are mean ± SEM of 5 independent experiments. *P < .05; **P< .01. PDE2A3 cDNA was cloned into the pcDNA3.1 expression vector. Of the pPDE2A3 expression plasmid, 0.1 μg was transfected into approximately 1 million HUVECs and harvested after 18 hours prior to stimulation and subsequent measurement of total cellular cGMP levels using an enzyme-linked immunosorbent assay (B). PDE2 inhibitor PDP (1 μM) was added 20 minutes prior to stimulation with SNP (100 μM) for 5 minutes with subsequent lysis and measurement of cGMP levels. Green fluorescent protein (GFP)–transfected cells were used as control. Data presented are mean ± SEM of 3 independent experiments. *P < .05 versus SNP-treated cells; #P < .05 versus SNP/PDP-treated cells; and §P < .05 versus SNP-treated/PDE2-transfected cells.
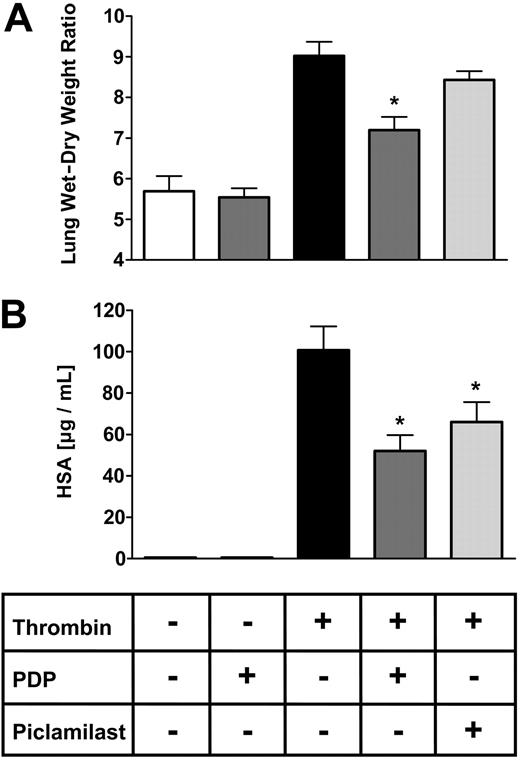
PDP reduced thrombin-related lung edema formation in mice lungs. Isolated mice lungs were perfused and ventilated for 45 minutes before administration of 750 μM thrombin within one minute. After a further 30 minutes, the experiment was terminated and lungs were used to measure wet-dry weight ratio (W/D) (A) or lung permeability for albumin (B). PDE inhibitors were added to the lung perfusate 10 minutes prior to thrombin stimulation. PDE4 inhibitor piclamilast and PDE2 inhibitor PDP were used at a concentration of 1 μM. Lung W/D is the ratio between the weight at the end of the experiment and the weight after drying of the lungs (A). PDE2 (PDP) inhibition reduced lung W/D, while PDE4 inhibition (piclamilast) did not show a significant reduction of lung edema formation. Albumin flux to the bronchoalveolar compartment was measured by admixing human serum albumin (HSA) to the perfusate at a concentration of 0.04% 20 minutes prior to thrombin stimulation and subsequent measurement of the HSA concentration in the bronchoalveolar lavage fluid after termination of the experiment (B). PDE2 (PDP) inhibition and PDE4 inhibition (piclamilast) reduced permeability for albumin. Data presented are mean ± SEM of 5 independent experiments. *P < .05.
PDP reduced thrombin-related lung edema formation in mice lungs. Isolated mice lungs were perfused and ventilated for 45 minutes before administration of 750 μM thrombin within one minute. After a further 30 minutes, the experiment was terminated and lungs were used to measure wet-dry weight ratio (W/D) (A) or lung permeability for albumin (B). PDE inhibitors were added to the lung perfusate 10 minutes prior to thrombin stimulation. PDE4 inhibitor piclamilast and PDE2 inhibitor PDP were used at a concentration of 1 μM. Lung W/D is the ratio between the weight at the end of the experiment and the weight after drying of the lungs (A). PDE2 (PDP) inhibition reduced lung W/D, while PDE4 inhibition (piclamilast) did not show a significant reduction of lung edema formation. Albumin flux to the bronchoalveolar compartment was measured by admixing human serum albumin (HSA) to the perfusate at a concentration of 0.04% 20 minutes prior to thrombin stimulation and subsequent measurement of the HSA concentration in the bronchoalveolar lavage fluid after termination of the experiment (B). PDE2 (PDP) inhibition and PDE4 inhibition (piclamilast) reduced permeability for albumin. Data presented are mean ± SEM of 5 independent experiments. *P < .05.
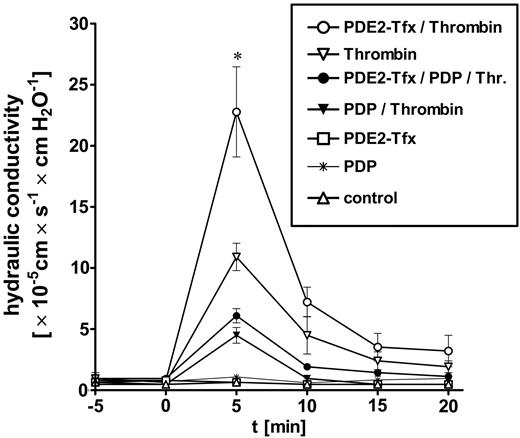
PDE2A3 transfection destabilized endothelial integrity of HUVEC monolayer. HUVECs were grown on polycarbonate filter membranes to confluency before applying hydrostatic pressure (10 cm H2O) and measuring endothelial permeability. Hydraulic conductivity of resting (“sealed”) HUVECs was less than 1 × 10-5 cm × s-1 × cm H2O-1 for both pPDE2A3-transfected and nontransfected HUVECs. Cells were preincubated with PDP (1 μM) for 15 minutes before thrombin stimulation (1 U/mL). pPDE2A3-transfected monolayers responded with a significantly higher increase of hydraulic conductivity compared with control cells. This enhanced sensitivity of pPDE2A3-transfected monolayers was suppressed in the presence of PDP, confirming specificity of PDE2 transfection. Mock-transfected cells (empty pcDNA3.1 vector) did not show differences to untransfected cells. ○ indicates pPDE2A3 + thrombin; ▿, thrombin only; ⬡, pPDE2A3 + PDP + thrombin; ▾, PDP + thrombin; □, pPDE2A3 only; *, PDP only; and ▵, control. Data presented are mean ± SEM of 3 independent experiments. *P < .05 versus thrombin-stimulated cells.
PDE2A3 transfection destabilized endothelial integrity of HUVEC monolayer. HUVECs were grown on polycarbonate filter membranes to confluency before applying hydrostatic pressure (10 cm H2O) and measuring endothelial permeability. Hydraulic conductivity of resting (“sealed”) HUVECs was less than 1 × 10-5 cm × s-1 × cm H2O-1 for both pPDE2A3-transfected and nontransfected HUVECs. Cells were preincubated with PDP (1 μM) for 15 minutes before thrombin stimulation (1 U/mL). pPDE2A3-transfected monolayers responded with a significantly higher increase of hydraulic conductivity compared with control cells. This enhanced sensitivity of pPDE2A3-transfected monolayers was suppressed in the presence of PDP, confirming specificity of PDE2 transfection. Mock-transfected cells (empty pcDNA3.1 vector) did not show differences to untransfected cells. ○ indicates pPDE2A3 + thrombin; ▿, thrombin only; ⬡, pPDE2A3 + PDP + thrombin; ▾, PDP + thrombin; □, pPDE2A3 only; *, PDP only; and ▵, control. Data presented are mean ± SEM of 3 independent experiments. *P < .05 versus thrombin-stimulated cells.
Results
TNF-α increases PDE2 but not PDE3 and PDE4 activity
First, we assessed the TNF-α–dependent regulation of the intracellular cyclic nucleotides in HUVECs by measuring PDE activities for cAMP- and cGMP-metabolizing PDE isoenzymes. In HUVECs, total cAMP-metabolizing activity under resting conditions was mainly due to PDE3 and PDE4 (43% and 51% of total cAMP metabolizing activity, respectively), whereas PDE2 accounted for only 3% of total cAMP-metabolizing activity (Figure 1A). After TNF-α stimulation (10 ng/mL, 18 hours), PDE2 activity rose 19-fold and showed the highest cAMP-degrading activity (37%) compared with PDE3 and PDE4 (26% and 33% of total cAMP PDE activity, respectively). PDE3 and PDE4 activity did not change significantly. Other PDE activities different from PDE2 to PDE4 isoenzymes contributed less than 5% to total cAMP PDE activity in both unstimulated and TNF-treated cells. In HUVECs, PDE2 was found to be the only significant cGMP-degrading activity (Figure 1B). TNF-α stimulation (10 ng/mL, 18 hours) led to a more than 8-fold rise in PDE2 cGMP-hydrolyzing activity. In these cells, contribution of PDE2 to total cGMP-metabolizing activity accounted for more than 98%. Other significant cGMP PDE activities different from PDE2 were not detected in HUVECs. TNF-dependent increase in cAMP PDE2 activity (Figure 2A) was already detectable after 2 hours and continued to rise up to 24 hours (80.4 ± 3.2 vs control 4.1 ± 0.8 pmol cAMP × minute-1 × mg-1 protein). This rise in PDE2 activity was accompanied by higher PDE2A3 mRNA levels, which were found to peak after 18 hours (Figure 2B). In conclusion, we could demonstrate that PDE2 is the predominant PDE isoenzyme activity in HUVECs for both cAMP and cGMP, whereas under resting conditions, cAMP is degraded mainly by PDE3 and PDE4, and cGMP, almost exclusively by PDE2.
TNF-α–stimulated PDE2 expression is mediated by activation of p38 MAPK
To elucidate the role of different MAP kinase cascades in TNF-α–induced PDE2 activation in HUVECs, we studied the effects of the p38 MAPK inhibitor SB202190 and the selective MAP kinase kinase (MEK) inhibitor U0126 on PDE2 expression (Figure 3A).
Incubation of HUVECs for 6 hours with 10 ng/mL TNF-α led to a significant increase in PDE2A protein (Figure 3A), which was reduced in the presence of the p38 MAPK inhibitor SB202190 (10 μM) but not with the MEK1/2 inhibitor U0126 (1 μM). Similar results were obtained when PDE2A mRNA was analyzed by RT-PCR (data not shown). Both Western analysis and PDE2 PCR suggested a predominant role for p38 MAPK for the activation of PDE2 following TNF-α stimulation. In order to corroborate the role of p38 MAPK for PDE2 activation, we transfected dominant-negative p38 (ip38) and aMKK6, the constitutively active upstream activator of p38 MAPK, into HUVECs and measured PDE2 activity after 18 hours (Figure 3B). TNF-α alone (10 ng/mL) increased PDE2 activity to 85 ± 9 pmol cAMP × minute-1 × mg-1 protein (control, 7.5 ± 1.1 pmol cAMP × minute-1 × mg-1 protein). Dominant-negative p38 almost completely suppressed the TNF-α–mediated increase in PDE2 activity (15.3 ± 2 pmol cAMP × minute-1 × mg-1 protein). Transfection of aMKK6 led to a significant rise in PDE2 activity (42.1 ± 6 pmol cAMP × minute-1 × mg-1 protein), which was blocked by the p38 MAPK inhibitor SB202190 (10 μM). Transfection of the empty expression vector did not induce PDE2 expression (data not shown). Our results suggest that in HUVECs, TNF-α increases PDE2 expression via p38 MAPK activation.
TNF-α causes decline in cellular cyclic nucleotide levels
We hypothesized that higher PDE2 activity following TNF-α stimulation would lower cellular cyclic nucleotide levels in HUVECs. Here we present data for intracellular cGMP concentrations (Figure 4). Similar results were obtained analyzing cAMP levels (data not shown). cGMP levels under basal conditions are very low and cGMP in HUVEC is exclusively metabolized by PDE2.13 Therefore other PDE inhibitors besides PDP were not required. HUVECs were incubated with TNF-α (10 ng/mL) for 18 hours followed by PDP (1 μM) for further 20 minutes (Figure 4A). If additional stimulation was performed, the guanylate cyclase activator sodium nitroprusside (SNP, 100 μM) was added for 5 minutes. Basal cGMP level was very low (0.018 ± 0.0064 pmol/5 × 105 cells) and further decreased if the cells were stimulated with TNF-α (0.008 ± 0.0022 pmol/5 × 105 cells). PDP alone (0.022 ± 0.0030 pmol/5 × 105 cells) did not produce a significant increase of cGMP compared with control. SNP elicited cGMP to a 160-fold level (2.88 ± 0.20 pmol/5 × 105 cells). This increase was dampened by 60% when TNF-α–stimulated cells were used (1.09 ± 0.08 pmol/5 × 105 cells), suggesting that higher PDE2 activity impaired cGMP accumulation. We therefore incubated TNF-α–treated cells with PDP for 20 minutes before stimulation with SNP and found that PDE2 inhibition partially restored cGMP concentration (10.24 ± 1.45 pmol/5 × 105 cells), yet did not reach the same cGMP level as cells incubated in the absence of TNF-α (20.03 ± 1.15 pmol/5 × 105 cells).
Transfection of PDE2A3 in HUVECs is as effective as TNF-α stimulation of PDE2 activity
In order to study the role of PDE2 on endothelial permeability, it was a prerequisite to establish high PDE2 activity in HUVECs without the need of TNF-α stimulation, since the pleiotropic cytokine TNF-α itself is known to alter endothelial permeability by various mechanisms.6,37-41 Therefore, we cloned full-length PDE2A3 cDNA into the pCDNA3.1 vector, transfected the expression plasmid pPDE2A3 transiently into HUVECs, and measured PDE2 activity after 18 hours. Transfection of 0.1 μg pPDE2A3 per million cells increased PDE2 activity after 18 hours to a similar level (88%) as 10 ng/mL TNF-α stimulation (data not shown), thereby confirming that transfection produced constitutively active PDE2 protein. The rise in PDE2 protein was also demonstrated by Western blotting (data not shown).
We studied the impact of PDE2A3 transfection on cyclic nucleotide levels by measuring cellular cGMP accumulation in HUVECs. Cells were transfected for 18 hours prior to the experiment with expression plasmid pPDE2A3 or control plasmid green fluorescent protein (GFP).
Before measuring cGMP, cells were stimulated for 5 minutes with sodium nitroprusside (SNP, 100 μM). Where required, PDP (1 μM) was added 20 minutes prior to SNP stimulation.
SNP stimulation produced a cGMP level of 3.077 ± 0.286 pmol/5 × 105 cells. PDE2A3-transfected cells responded to SNP stimulation with a markedly reduced cGMP accumulation (0.703 ± 0.178 pmol/5 × 105 cells). Green fluorescent protein (GFP)–transfected cells (2.769 ± 0.317 pmol/5 × 105 cells) were used as control cells, confirming specificity of PDE2 transfection on cGMP alterations. Furthermore, PDE2-transfected cells displayed no differences compared with untransfected cells in the presence of the PDE2 inhibitor PDP, which also confirmed specificity (17.791 ± 0.882 pmol vs 19.612 ± 1.444 pmol/5 × 105 cells). Therefore, we decided to use 0.1 μg pPDE2A3 per million cells of pPDE2A3 for the subsequent cGMP measurements and permeability studies.
High PDE2 activity destabilizes endothelial monolayer integrity
Sealed HUVEC monolayers displayed a hydraulic conductivity of less than 1 × 10-5 cm × s-1 × cm H2O-1 (Figure 5). Prior to stimulation, we did not observe differences in basal hydraulic conductivity between control cells, PDE2A3-transfected cells, and PDP-pretreated cells. Continuous PDP infusion (1 μM) started 15 minutes before addition of the permeability increasing agent thrombin (1 U/mL). Within 5 minutes after thrombin stimulation, we measured a rapid, more than 10-fold increase of hydraulic conductivity up to 10.9 ± 1.1 × 10-5 cm × s-1 × cm H2O-1 followed by a subsequent decrease to 1.9 ± 0.5 × 10-5 cm × s-1 × cm H2O-1. HUVECs transfected with pPDE2A3 displayed a more than 20-fold increase of hydraulic conductivity to 22.8 ± 3.7 × 10-5 cm × s-1 × cm H2O-1. PDE2 inhibition (PDP 1 μM) attenuated the thrombin-mediated rise in permeability significantly for both nontransfected and PDE2A3-transfected HUVECs (4.5 ± 0.6 and 6.1 ± 0.6 × 10-5 cm × s-1 × cm H2O-1, respectively). In conclusion, PDE2A3 transfection sensitized HUVECs toward thrombin-mediated increase in permeability. PDE2 inhibition was protective for both untransfected and PDE2A3-transfected HUVECs.
PDE2 inhibition reduces wet-dry weight ratio and lung permeability for albumin in mice lungs
We tested whether the PDE2 inhibitor PDP was able to reduce wet-dry weight ratio (W/D) and compared its effects with the PDE4 inhibitor piclamilast (Figure 6A). Under control conditions, PDP did not affect W/D. Pulmonary edema formation was markedly increased after 30 minutes of thrombin stimulation (750 μM) with a lung W/D of 9.024 ± 0.345 (control, 5.69 ± 0.134). PDP reduced edema formation significantly to 7.194 ± 0.325. The PDE4 inhibitor piclamilast attenuated W/D only moderately without reaching statistical significance. Furthermore, we newly established a sensitive method to measure albumin permeability in isolated mice lungs using human serum albumin, which was administered to the lung perfusate at a concentration of 0.04% and was measured in the bronchoalveolar lavage fluid after termination of the experiment. Under control conditions, very low levels of human serum albumin (0.51 ± 0.24 μg/mL) were detected in the bronchoalveolar lavage fluid after the experiment (Figure 6B). The PDE2 inhibitor PDP (1 μM) did not affect lung albumin permeability under these conditions (0.50 ± 0.25 μg/mL). After application of thrombin (750 μM), albumin permeability was dramatically increased 200-fold (100.65 ± 11.57 μg/mL) after 30 minutes. This leakage response was significantly reduced (51.94 ± 7.69 μg/mL) by administration of PDP 10 minutes prior to thrombin stimulation. The PDE4 inhibitor piclamilast (1 μM) reduced hyperpermeability to a lesser extent than PDP.
In control experiments, pulmonary vascular resistance (Rpv) was constant throughout the entire experiment (data not shown). In order to eliminate potential effects of thrombin on pulmonary vasoconstriction potentially contributing to edema formation,42 we administered the thromboxane receptor antagonist BM13505. When lungs were exposed to thrombin, a short rise of Rpv was noted with approximate doubling of Rpv within 2 to 3 minutes. After 10 minutes, Rpv values of thrombin-treated lungs returned approximately to control levels. PDP and piclamilast had no significant effect on the rapidly transient thrombin-induced Rpv increase. Besides thrombin, we also evaluated TNF-α with respect to lung edema formation in mice. No significant changes were observed up to 120 minutes duration of the experiment (10 ng/mL TNF-α, data not shown), which is consistent with earlier reports from rats43 and rabbits.44
PDE2 inhibition reduces TNF-α and thrombin-induced F-actin and VE-cadherin redistribution
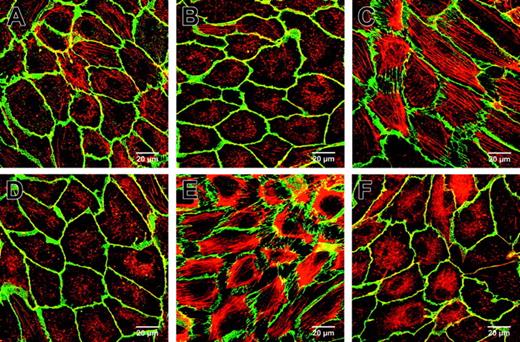
PDP reduced significantly TNF-α and thrombin-mediated alterations of endothelial filamentous actin (F-actin) and redistribution of vascular endothelial cadherin (VE-cadherin), as shown by double immunofluorescence (Figure 7). HUVECs exposed to vehicle or 1 μM PDP for 30 minutes displayed a well-arranged peripheral dense band of F-actin (red) with very few stress fibers and homogenous staining for VE-cadherin (green) with colocalization of these elements appearing as yellow (Figure 7A and Figure 7B, respectively).
PDE2 inhibition reduced TNF-α and thrombin-induced F-actin and VE-cadherin redistribution. HUVECs were grown to confluency on gelatin-coated Thermanox slides, stained for F-actin and VE-cadherin, and visualized by double immunofluorescence (630-fold magnification). Cells treated with vehicle (A) or PDP 1 μM (B) did not show stress fibers or intercellular gap formation as visualized with F-actin–specific phalloidin Alexa 546 (red) and VE-cadherin (green). TNF-α (10 ng/mL, 2 hours) (C) or thrombin stimulation (0.75 U/mL, 15 minutes) (E) caused a massive increase of stress fibers, redistribution of VE-cadherin, and gap formation. Pretreatment with 1 μM PDP for 30 minutes before TNF-α (D) or thrombin (F) stimulation prevented VE-cadherin redistribution and gap formation, reduced stress fibers, and maintained the peripheral dense band. Representative fields of HUVEC monolayers of 3 experiments are shown. Bars = 20 μm.
PDE2 inhibition reduced TNF-α and thrombin-induced F-actin and VE-cadherin redistribution. HUVECs were grown to confluency on gelatin-coated Thermanox slides, stained for F-actin and VE-cadherin, and visualized by double immunofluorescence (630-fold magnification). Cells treated with vehicle (A) or PDP 1 μM (B) did not show stress fibers or intercellular gap formation as visualized with F-actin–specific phalloidin Alexa 546 (red) and VE-cadherin (green). TNF-α (10 ng/mL, 2 hours) (C) or thrombin stimulation (0.75 U/mL, 15 minutes) (E) caused a massive increase of stress fibers, redistribution of VE-cadherin, and gap formation. Pretreatment with 1 μM PDP for 30 minutes before TNF-α (D) or thrombin (F) stimulation prevented VE-cadherin redistribution and gap formation, reduced stress fibers, and maintained the peripheral dense band. Representative fields of HUVEC monolayers of 3 experiments are shown. Bars = 20 μm.
Stimulation with TNF-α (10 ng/mL) for 2 hours (Figure 7C) or thrombin (0.75 U/mL) for 15 minutes (Figure 7E) generated a massive increase in stress fibers with disruption of the peripheral dense band and redistribution of VE-cadherin. At contacting cell borders, VE-cadherin was still detectable but not visible anymore along free cell edges in areas of retraction. In contrast, pretreatment with 1 μM PDP for 30 minutes before TNF-α (Figure 7D) or thrombin (Figure 7F) stimulation reduced stress fiber formation significantly, prevented the complete loss of the peripheral dense band, and prevented VE cadherin loss with intercellular gap formation.
Discussion
Numerous studies have demonstrated the significance of elevated blood TNF-α level in sepsis for the development of multiple organ failure. Of critical importance for the prognosis of sepsis is the occurrence of lung edema formation, which results from increased vascular permeability. TNF-α has been shown to increase vascular permeability in vivo45 and in vitro,41,46,47 by direct activation of endothelial actomyosin-based cytoskeleton.12 TNF-α–induced formation of paracellular gaps involves phosphorylation of myosin light chain kinase.12
Our study supports the hypothesis that TNF-α increases endothelial permeability by inducing endothelial PDE2 expression and that PDE2 activity is linked to a fall of endothelial cyclic nucleotide levels with a subsequent rise in endothelial permeability. We were able to mimic this condition by transfecting PDE2A3 prior to in vitro permeability measurements and found destabilization of endothelial barrier function by high PDE2 activity. In addition, thrombin-induced lung edema formation in isolated mice lungs was markedly reduced in the presence of the PDE2 inhibitor PDP. Our observations highlight a critical role for PDE2 as effector of TNF-α– and thrombin-mediated endothelial injury and describe a beneficial effect of PDE2 inhibition on endothelial barrier dysfunction under inflammatory conditions.
To our knowledge, PDE2 is the first and so far only member of the PDE superfamily that is up-regulated by TNF-α. In HUVECs, we could not detect TNF-α–dependent changes in PDE3 or PDE4 activity, whereas PDE2 activity for cAMP-hydrolysis increased after TNF-α stimulation up to 19-fold to a similar level as PDE3 or PDE4. cGMP hydrolysis increased 8-fold, which is entirely attributable to PDE2 activity since cGMP-specific PDE (PDE5) is not present in HUVECs.13 As a result, total PDE activity for both cAMP and cGMP was increased in TNF-α–stimulated HUVECs.
This resulted in lower levels of cGMP following stimulation with sodium nitroprusside (SNP). We demonstrated that activation of PDE2 is involved in the desensitization toward SNP stimulation by adding PDP, which partially reversed this effect. PDP did not restore cGMP accumulation completely in TNF-α–treated cells, which can be due to lower activity of soluble guanylate cyclase (sGC) activity following TNF-α stimulation.48 Our results are in line with an earlier observation showing decline of cyclic nucleotide content in bovine endothelial cells following stimulation with TNF-α.49 With regard to short-term regulation, PDE2 enzyme activity is directly activated through its GAF domain by cGMP, which may be increased through nitric oxide (NO)–dependent stimulation of sGC. Since PDE2 metabolizes both cGMP and cAMP, it is of considerable significance that PDE2 provides an important link between the NO/cGMP- and cAMP/protein kinase A–dependent signaling pathway. While it is undisputable that cAMP is critical for endothelial permeability, the role for cGMP depends on the vascular bed involved and the local condition of the endothelium.50
The TNF-α–activated signal transduction pathway leading to PDE2 expression is characterized by activation of the p38 MAPK. Inhibition of the p38 MAPK pathway suppressed TNF-α–mediated rise in PDE2 activity, and transfection of MKK6 (upstream activator of p38 MAPK) markedly increased PDE2 expression.
The rapid, p38 MAPK–dependent expression of PDE2 resembles the up-regulation of other proinflammatory effector molecules such as chemoattractant proteins51 and adhesion molecules,52 thereby allowing quick cellular adaptation to TNF-α–induced endothelial injury.
Besides activation of PDE2, the pleiotropic cytokine TNF-α exerts a plethora of cellular changes on vascular endothelial cells.53 Therefore, we decided to investigate the effect of high PDE2 activity on endothelial barrier function independently from other TNF-α–mediated actions on endothelial cells. PDE2 activity was induced by transfecting PDE2A3 into HUVECs.
The in vitro permeability assay is based on the measurement of hydraulic conductivity of HUVEC monolayers exposed to hydrostatic pressure.13-15,32 In this experimental setting, we measured low basal hydraulic conductivity, confirming cellular integrity of both mock-transfected and PDE2A3-transfected HUVECs. Thrombin, an important mediator in sepsis, was used as a rapidly acting and well-established stimulus to increase endothelial permeability.6,10,11
Following thrombin stimulation, hydraulic conductivity increased rapidly with a subsequent decline. PDE2A3-transfected HUVECs responded to thrombin stimulation with a dramatic increment in hydraulic conductivity. The PDE2 inhibitor PDP prevented this markedly increased sensitivity toward thrombin stimulation.
Remarkably, despite the increase of constitutively active PDE2 protein after pPDE2A3 transfection, endothelial barrier function under hydrostatic pressure of 10 cm H2O was maintained in nonstimulated cells. Subsequently, the transient rise of cGMP following thrombin stimulation54,55 may increase PDE2 activity (with a drop of PDE2 Michaelis-Menten constant [Km] for cAMP from 200 μM to 25 μM; our measurements), resulting in a sudden decrease of cAMP with breakdown of endothelial barrier function.
In earlier publications, the significance of human endothelial PDE2 was rather underestimated since basal activity in HUVECs under resting conditions was low and up-regulation under inflammatory conditions was neither shown nor considered.13,14 Moreover, EHNA (erythro-9-[2-hydroxyl-3-nonyl]-adenine), which was the only PDE2 inhibitor available in the public domain, lacked specificity due to its inhibitory effect on adenosine deaminase. In this study, we used the PDE2 inhibitor PDP whose potency and specificity is by far superior to EHNA.
We decided to challenge the hypothesis that PDE2 inhibition could also be beneficial in lung injury. It has to be noted that we chose an experimental model from which we expected lower basal PDE2 activity prior to measurement of endothelial permeability than in septic lungs where TNF-α might have induced PDE2 activity. Nevertheless, results from our isolated, ventilated, and perfused lung model proved to underline the important role of PDE2 for the maintenance of endothelial barrier function.
Lung endothelial permeability increased rapidly following challenge with thrombin, a well-established stimulus in vitro and in vivo.5,13 For both lung wet-to-dry ratio and permeability for human serum albumin (HSA), we found a significant attenuation of lung edema formation in the presence of the PDE2 inhibitor PDP. Remarkably, reduction of lung edema formation by PDE2 inhibition tended to exceed the effect seen with the PDE4 inhibitor piclamilast when measuring lung permeability for HSA. Furthermore, PDP reduced lung wet-to-dry ratio significantly, whereas piclamilast was without significant effect. These findings underline a possible predominance of PDE2 inhibition over PDE4 inhibition on pulmonary edema formation in mice lungs; albeit it is likely that basal PDE2 activity in murine endothelial cells is lower compared with PDE4 activity, as seen with HUVECs. Although in earlier studies it has been shown that PDE3/PDE4 inhibition stabilized the endothelial barrier in mice lungs,10 rabbit lungs,25 and in a mice lung model,56 our study is the first observation placing emphasis on PDE2 over PDE4 on endothelial barrier function in isolated lungs, though PDE4 inhibition might possibly add to the effects seen with PDE2 inhibition.
Thrombin and TNF-α mediate rapid rearrangement of the endothelial microfilament system causing paracellular gap formation with increased paracellular fluid flux.6,10,12,13,15 F-actin staining demonstrated TNF-α– and thrombin-induced disappearance of the peripheral dense band and the massive formation of stress fibers, whereas vascular endothelial cadherin (VE-cadherin) staining showed loss of cell-to-cell contacts and intercellular gap formation. PDP prevented the complete loss of the peripheral dense band and markedly reduced formation of stress fibers and paracellular gaps. These results corroborate the beneficial effects of PDE2 inhibition on endothelial permeability seen in vitro and in isolated mice lungs.
Furthermore, our findings may help to explain the clinical observation that the efficacy of antineoplastic chemotherapy in patients with extremity soft-tissue sarcomas or melanomas is significantly enhanced in combination with TNF-α than chemotherapy alone.57,58 In this situation, TNF-α increases permeability and improves drug penetration into the tumor on top of higher basal permeability of tumor vessels.
In conclusion, we demonstrated that TNF-α rapidly increased PDE2 activity in HUVECs in a p38 MAPK–dependent manner, leading to a decrease in cyclic nucleotide accumulation. High PDE2 activity was shown to destabilize endothelial barrier function toward the action of thrombin. This effect was antagonized by PDE2 inhibition. Furthermore, in isolated mice lungs PDE2 inhibition suppressed thrombin-mediated increase in endothelial permeability. By measuring human serum albumin flux from the vascular to the bronchoalveolar compartment, we were able to demonstrate the superior effect of PDE2 inhibition over PDE4 inhibition in mice lungs. Our findings emphasize the significant role of PDE2, particularly in septic hyperpermeability, and the beneficial effects of PDE2 inhibition on lung edema formation.
Prepublished online as Blood First Edition Paper, January 13, 2005; DOI 10.1182/blood-2004-07-2729.
Supported by the German Federal Research Ministry (BMBF; CAPNETZ/C4 to N.S., BMBF-NBL-3 to S.H.), the Deutsche Forschungsgemeinschaft (DFG; HI 789/5-1 to S.H.), and the Charité University Hospital (to J.S.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The skillful technical assistance of Kerstin Möhr is greatly appreciated. Parts of this work will be included in the MD theses of Şengül Boral, Alexander Bürger, and Dirk Thomas.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal