Abstract
Idiopathic myelofibrosis (IM) is a disease characterized by marrow fibrosis, abnormal stem/progenitor cell trafficking, and extramedullary hematopoiesis frequently associated with alterations in megakaryocytes (Mks). Mice harboring genetic alterations in either the extrinsic (ectopic thrombopoietin expression, TPOhigh mice) or intrinsic (hypomorphic GATA-1 mutation, GATA-1low mice) control of Mk differentiation develop myelofibrosis, a syndrome similar to IM. The relationship, if any, between the pathobiologic mechanism leading to the development of myelofibrosis in the 2 animal models is not understood. Here we show that plasma from GATA-1low mice contained normal levels of TPO. On the other hand, Mks from TPO-treated wild-type animals (TPOhigh mice), as those from GATA-1low animals, had similar morphologic abnormalities and contained low GATA-1. In both animal models, development of myelofibrosis was associated with high transforming growth factor β1 (TGF-β1) content in extracellular fluids of marrow and spleen. Surprisingly, TPO treatment of GATA-1low mice restored the GATA-1 content in Mks and halted both defective thrombocytopoiesis and fibrosis. These data indicate that the TPOhigh and GATA-1low alterations are linked in an upstream-downstream relationship along a pathobiologic pathway leading to development of myelofibrosis in mice and, possibly, of IM in humans.
Introduction
Idiopathic myelofibrosis (IM) is a myeloproliferative disorder of unknown etiology characterized by marrow fibrosis, abnormal stem/progenitor cell trafficking, and extensive extramedullary hematopoiesis1-3 experimentally transmitted4 and cured5-8 by bone marrow transplantation. It is associated with alterations of megakaryocyte (Mk) differentiation leading to thrombocytopenia,9 and often evolves into leukemia.1-3 Therefore, it is considered a “classic” multistage disorder triggered by a somatic mutation, still to be identified, which confers a proliferative advantage to a multipotent stem cell defective for megakaryocytic differentiation.
Megakaryocytic differentiation is regulated by both extrinsic and intrinsic control mechanisms. The extrinsic control is primarily exerted by thrombopoietin (TPO),10 a growth factor normally produced by liver cells, that binds to a specific receptor, mpl, present on the cell surface. TPO binding to mpl triggers a signaling cascade that will ultimately activate expression of Mk-specific genes.10 On the other hand, a pivotal role in the intrinsic control of megakaryocytopoiesis11 is exerted by GATA-1, in association with its obligatory partner FOG-1 (friend of GATA-1),12 as either inherited or acquired mutations of GATA1 can have severe consequences on Mk differentiation.13 GATA1 expression is activated early in the course of stem cell differentiation and is required for Mk, erythroid, mast, and eosinophil cell differentiation.12 In each of these lineages, GATA1 expression is under the control of specific regulatory sequences. The Mk-specific regulatory sequences are represented by the first DNase hypersensitive site close to the distal promoter, and its deletion, the GATA-1low mutation, almost completely abrogates expression of GATA1 in Mks.14
Mice harboring alterations in either the extrinsic (forced expression of TPO, TPOhigh mice) or intrinsic (reduced GATA-1 expression, GATA-1low mice) control of Mk differentiation develop myelofibrosis, a syndrome very similar to IM. In fact, mice engineered to overexpress TPO in their liver15 and those that received transplants of marrow cells infected with a TPO-containing retrovirus4,5 develop marrow fibrosis and extramedullary hematopoiesis within 2 to 3 months and die from the disease soon thereafter.16 Transient myelofibrosis is also observed in rats receiving recombinant TPO.17 In TPOhigh mice, myelofibrosis is associated with increased platelet counts in the blood and increased frequency of Mks in the marrow.4,5,15 On the other hand, GATA-1low mice are born thrombocytopenic14,18 and anemic19 and, although they recover from the anemia at 3 to 4 months of age,20 remain thrombocytopenic all their life.20,21 All those mutants surviving up to 10 to 12 months develop myelofibrosis with no apparent reduction of their life span.21 Also in GATA-1low mice, the disease is associated with increased frequency of Mks in the marrow.21 However, GATA-1low Mks are abnormal, since the mutation halts their proper progression along the differentiation pathway.14,18,22 The relationship, if any, between the pathobiologic mechanisms leading to development of myelofibrosis in TPOhigh and in GATA-1low mice is not clear. It is also unknown why the disease manifests itself with such different morbidity and mortality in the 2 cases. In fact, little is known as yet on how the TPO signaling pathway interacts with the GATA-1 control machinery in driving Mk differentiation. That such an interaction must exist is inferred by analogy with the control of erythroid differentiation, similarly regulated by extrinsic (erythropoietin, EPO) and intrinsic (GATA-1) mechanisms.23 These mechanisms interact through a positive feedback that links expression of the EPO receptor to presence of GATA-1,24 while expression of GATA-1 is transcriptional and posttranscriptional up-regulated by EPO.25
To clarify the relationship between TPO and GATA-1 in the control of megakaryocytopoiesis and in the pathobiologic mechanisms leading to myelofibrosis in TPOhigh and GATA-1low mice, we compared TPO metabolism and efficacy in wild-type, as well as in GATA-1low, littermates. No difference was found between wild-type and GATA-1low mice in terms of TPO metabolism, and both animals responded to TPO by increasing the number of platelets in circulation. However, the response to TPO of wild-type and GATA-1low mice was mediated, at least partially, through different molecular mechanisms. In GATA-1low mice, TPO treatment increased GATA-1 content in mutant Mks and, consequently, restored their thrombocytopoiesis. In contrast, in wild-type mice, TPO treatment decreased GATA-1 content in Mks. In both GATA-1low and TPOhigh wild-type mice, the development of myelofibrosis was associated with high levels of transforming growth factor β1 (TGF-β1) expression in marrow and spleen. The strict association between occurrence of the disease and low GATA-1 content in Mks here described suggests that TPO and GATA-1 represent elements of a common pathobiologic pathway leading to development of myelofibrosis in mice and, possibly, of IM in humans.
Materials and methods
Mice
GATA-1low mice19 were maintained in the animal facilities of Istituto Superiore Sanità20,26 and are also available from Jackson Laboratories (Bar Harbor, ME). The mice used in this study were 12- to 14-months old and, while presenting some fibrosis, had not progressed to myelofibrosis as yet.21 All the experiments were performed with sex- and age-matched mutant and control littermates under protocols approved by the institutional animal care committee.
Thrombopoietin treatment
Recombinant murine TPO was injected intraperitoneally for 5 consecutive days (100 μg TPO/kg of body weight daily). The mice were killed at 0 (untreated), 7, 10, 14, and 21 days after the first injection and their tissues removed for further analysis, as described in the following sections.
Hematologic parameters
Blood was collected from the retro-orbital plexus into ethylen-diaminotetracetic acid–coated microcapillary tubes (20-40 μL/sampling). Hematocrit, white cell, and platelet counts were determined manually.
Progenitor cell counts
The frequency of colony-forming unit (CFU)–Mks was determined by culturing light density mononuclear cells (0.25-1.0 × 105 cells/plate) in collagen-supplemented cultures (MegaCult-C; Stem Cell Technologies, Vancouver, BC) stimulated with murine interleukin-3 (IL-3, 25 ng/mL), IL-6 (100 ng/mL), and IL-11 (50 ng/mL) and human TPO (50 ng/mL) (Sigma, St Louis, MO). CFU-Mk–derived colonies were counted on acetone-fixed cultures stained for acethyl-colinesterase27 after 7 days of incubation at 37°C in a humidified incubator containing 5% CO2 in air.
Histology and immunohistochemistry
Soft tissues were fixed in 10% (vol/vol) phosphate-buffered formalin and paraffin embedded. Bones were decalcified with acidified EDTA (ethylenediaminetetraacetic acid), according to standard procedures. Consecutive 2.5- to 3-μM sections were stained with hematoxylin-eosin or Gomori-silver (MicroStain MicroKit; Diopath, Bologna, Italy). Microscopic evaluations were performed with a DM RB (Das Microscope Research Biology) microscope (Leica, Heidelberg, Germany) set in a transillumination mode and images were acquired with the Image Manager (IM) 50 system (Leica). The number of Mks was determined at × 40 original magnification in randomly chosen multiple sections to cover an area of 0.33 mm2. For quantitative measurements of fibrosis, images of Gomori-stained sections were acquired at × 40 and processed with Adobe Photoshop (Adobe Systems, San Jose, CA) to calculate the number of fibers–fiber bands intersecting 10 noncontiguous square lines of a 540-μm–length spaced grid in each microscopic section.28 Sections were also incubated with the anti–GATA-1 monoclonal antibody (Santa Cruz Biotechnology, Santa Cruz, CA), and the immunostaining was developed with the Ultraystain polyvalent horseradish peroxidase kit (Ylem, Rome, Italy), using 3.3′-diaminobenzedine (Sigma) as chromogen. Samples were then counterstained with hematoxylin. Samples incubated with a nonimmune immunoglobulin G (IgG) served as negative controls. The sections were analyzed with a light microscope (Leica) equipped with a Coolsnap video camera for computerized images (RS Photometrics, Tucson, AZ).
Transmission electron microscopy
Spleens were fixed in 2.5% glutaraldehyde in 0.1 M cacodylate buffer, pH 7.6, for 2 hours at 4°C and postfixed in osmium tetroxide for 60 minutes at 4°C. The samples were then dehydrated in alcohol at progressively higher concentrations and embedded in Spurr resin (Poliscience, Warrington, PA). Consecutive thin and ultrathin sections were cut using a Reichert ultramicrotome (Reichert, Vienna, Austria). Ultrathin sections were collected on 200 mesh copper grids counterstained with uranyl acetate and lead citrate and observed with an EM (electron microscope) 109 Zeiss (Oberkochen, Germany). At least 50 Mks were analyzed per each experimental group and every group was composed of at least 3 mice.
Immunoelectron microscopy
Spleens were fixed for 3 hours at 4°C in a mixture of 2% paraformaldehyde and 0.1% glutaraldehyde in 0.1 M cacodylate buffer, pH 7.6, dehydrated in alcohol (progressively higher concentrations), and embedded in Bioacryl resin (British Biocell, Cardiff, United Kingdom), followed by UV polymerization, according to standard procedures.22 Ultrathin sections were cut and mounted on 300 mesh nickel grids treated with a blocking buffer composed of phosphate-buffered saline (PBS) supplemented with 0.1% Tween-20, 0.1% bovine serum albumin, and 4% normal rabbit serum, and incubated overnight in the presence of goat anti–von Willebrand factor and antimyeloperoxidase antibodies (sc-6941 and sc-12128; Santa Cruz Biotechnology). The grids were then incubated for one hour with rabbit anti–goat IgG conjugated with 15-nm colloidal gold particles (British Biocell), counterstained in uranyl acetate, and observed with EM 109 Zeiss. Negative controls were represented by cells not exposed to the primary antibody. Twenty-fifty cells were visually analyzed for each experimental point and the number of immunogold particles counted at × 30 000 magnification on 5 randomly selected cells.
Quantification of TPO and TGF-β1 levels in plasma and extracellular fluids
Citrated platelet-poor plasma was prepared according to standard procedures.29 Extracellular fluids were collected from femurs by perfusing the cavity with 0.5 mL cold PBS. The cell-free supernatant, collected after centrifugation at 450g at 4°C for 10 minutes, was stored at -70°C for further use. The levels of TPO and TGF-β1 in these specimens were measured with the murine TPO (detection limit: 62 pg/mL) and human TGF-β1 (detection limit: 31 pg/mL) Quantikine Kits (R&D Systems, Minneapolis, MN), as recommended by the manufacturer. Samples were assayed for TGF-β1 both as untreated (not shown) and acidified specimens, with similar results.
Megakaryocyte purification
Mononuclear spleen (∼ 8 × 108 cells) and marrow (∼ 3 × 107 cells) cells were incubated with the monoclonal fluorescein isothiocyanate (FITC)–labeled rat anti–mouse CD41 antibody (2 μg/106 cells; Pharmingen, San Diego, CA) for 20 minutes at 4°C, washed twice with Ca++- and Mg++-free phosphate-buffered saline containing 2 mM EDTA, and incubated for an additional 15 minutes with anti-FITC coated magnetic microbeads (Miltenyi Biotech, Gladbach, Germany). The cell population recovered from the column was run through a second cycle of immunomagnetic selection. Approximately 4 to 10 × 105 cells, 90% to 95% of CD41+ cells by fluorescence-activated cell sorter (FACS, FACScan flow cytometer; Becton Dickinson, San Diego, CA) reanalysis, were recovered at the end of the second purification.
RNA isolation and semiquantitative and quantitative reverse-transcriptase–polymerase chain reaction (RT-PCR) analysis
Total RNA was prepared from femurs and spleens frozen in liquid nitrogen and ground with mortar and pestle into Trizol (Gibco, Paisley, United Kingdom). RNA (1 μg) was reverse transcribed with 2.5 U Moloney murine leukemia virus reverse-transcriptase and 2.5 μM random hexamers (all from Perkin-Elmer, Jersey City, NJ). Expression of TGFB1, platelet-derived growth factor (PDGF), and β2-microglobulin (as control) was analyzed by semiquantitative PCR, as described.21,26 Positive (RNA from adult marrow) and negative (mock cDNA) controls were included in each experiment (not shown). Aliquots (20 μL) were removed from the amplification mixture after selected cycles and the amplified fragments were separated by electrophoresis on 4% polyacrylamide gel. Gels were dried using the Hercules apparatus (Biorad, Milan, Italy) and exposed to Hyperfilm-MP (Amersham Bioscience Europe, Freiburg, Germany) for 2 hours at -70°C. All procedures were done according to standard protocols.30 For quantitative RT-PCR analysis of GATA-1 expression, mRNA was purified from 2 to 5 × 105 Mks with the μM kit (Miltenyi Biotech) and retrotranscribed with the Superscript kit (InVitrogen, Milan, Italy). GATA-1 cDNA was then quantified with the Taq-Man predeveloped RT-PCR assay (Applied Biosystem, Foster City, CA) using GPDH as housekeeping control gene and the ABI PRISM 7700 Sequence Detection System (SDS; Applied Biosystem) with an initial denaturation/activation step at 95°C for 10 minutes followed by 40 cycles of a 2-step program at 95°C for 15 seconds and 60°C for 60 seconds. The cycle thresholds (CT) were calculated monitoring fluorescent signals with the SDS software. The levels of GATA1 mRNA are expressed as ΔCT (ΔGATA-1CT = GATA-1CT - GPDHCT), as suggested by the manufacturer. Each amplification was assayed in triplicate using concurrently negative (mock cDNA) and positive (cDNA from mouse bone marrow) controls and results are expressed as mean (± SD) ΔGATA-1CT from 3 independent experiments.
Statistical analysis
Statistical analysis was performed by analysis of variance (Anova test) using Origin 3.5 software for Windows (Microcal Software, Northampton, MA).
Results
Normal TPO metabolism in GATA-1low mice
Preliminary experiments had indicated that liver cells from GATA-1low mice express normal levels of TPO mRNA by RT-PCR (not shown). As shown in Table 1, the plasma from untreated GATA-1low mice and those from age- and sex-matched normal littermates contained similar TPO concentrations (≅ 0.75 ng/mL in both cases). Furthermore, in both groups of animals receiving exogenous growth factor intraperitoneally, the TPO plasma concentrations raised to approximately 5.7 to 6.0 ng/mL by day 7 and returned to pretreatment values by day 14 (Table 1). These results indicate that GATA-1low mice have normal TPO metabolism.
TPO and TGF-β1 levels in untreated and TPO-treated (days 7, 14, and 21 after receiving 100 μg TPO/kg of body weight/day for 5 consecutive days, as indicated) wild-type and GATA-1low mice
. | Untreated . | . | Day 7 . | . | Day 14 . | . | Day 21 . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
. | Wild type . | GATA-1low . | Wild type . | GATA-1low . | Wild type . | GATA-1low . | Wild type . | GATA-1low . | ||||
| TPO | ||||||||||||
| Plasma, ng/mL (no. of mice) | 0.76 ± 0.12 (10) | 0.75 ± 0.20 (8) | 6.0 ± 0.5 (10) | 5.7 ± 0.8 (8) | 0.48 ± 0.15 (10) | 0.54 ± 0.16 (8) | 0.58 ± 0.12 (10) | 0.65 ± 0.10 (8) | ||||
| P | - | NS | < .001 | < .001 | NS | NS | NS | NS | ||||
| TGF-1β | ||||||||||||
| Plasma, ng/mL (no. of mice) | 2.1 ± 0.4 (10) | 1.8 ± 0.7 (8) | 16.2 ± 2.7 (10) | 3.5 ± 1.3 (8) | 9.2 ± 2.0 (10) | 2.2 ± 0.8 (8) | 2.0 ± 1.0 (10) | 2.0 ± 1.1 (8) | ||||
| P | - | NS | < .01 | NS | < .01 | NS | NS | NS | ||||
| Extracellular fluids | ||||||||||||
| Bone marrow, ng/mL (no. of mice) | 0.86 ± 0.21 (5) | 1.88 ± 0.33 (4) | ND | ND | 3.45 ± 0.45 (5) | 1.62 ± 0.60 (4) | ND | ND | ||||
| P | - | < .05 | ND | ND | < .01 | NS | ND | ND | ||||
| Spleen, ng/mL (no. of mice) | 1.88 ± 0.39 (5) | 3.20 ± 0.80 (4) | ND | ND | 4.53 ± 1.20 (5) | 3.55 ± 0.65 (4) | ND | ND | ||||
| P | - | < .01 | ND | ND | < .01 | ND | ND | ND | ||||
. | Untreated . | . | Day 7 . | . | Day 14 . | . | Day 21 . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
. | Wild type . | GATA-1low . | Wild type . | GATA-1low . | Wild type . | GATA-1low . | Wild type . | GATA-1low . | ||||
| TPO | ||||||||||||
| Plasma, ng/mL (no. of mice) | 0.76 ± 0.12 (10) | 0.75 ± 0.20 (8) | 6.0 ± 0.5 (10) | 5.7 ± 0.8 (8) | 0.48 ± 0.15 (10) | 0.54 ± 0.16 (8) | 0.58 ± 0.12 (10) | 0.65 ± 0.10 (8) | ||||
| P | - | NS | < .001 | < .001 | NS | NS | NS | NS | ||||
| TGF-1β | ||||||||||||
| Plasma, ng/mL (no. of mice) | 2.1 ± 0.4 (10) | 1.8 ± 0.7 (8) | 16.2 ± 2.7 (10) | 3.5 ± 1.3 (8) | 9.2 ± 2.0 (10) | 2.2 ± 0.8 (8) | 2.0 ± 1.0 (10) | 2.0 ± 1.1 (8) | ||||
| P | - | NS | < .01 | NS | < .01 | NS | NS | NS | ||||
| Extracellular fluids | ||||||||||||
| Bone marrow, ng/mL (no. of mice) | 0.86 ± 0.21 (5) | 1.88 ± 0.33 (4) | ND | ND | 3.45 ± 0.45 (5) | 1.62 ± 0.60 (4) | ND | ND | ||||
| P | - | < .05 | ND | ND | < .01 | NS | ND | ND | ||||
| Spleen, ng/mL (no. of mice) | 1.88 ± 0.39 (5) | 3.20 ± 0.80 (4) | ND | ND | 4.53 ± 1.20 (5) | 3.55 ± 0.65 (4) | ND | ND | ||||
| P | - | < .01 | ND | ND | < .01 | ND | ND | ND | ||||
For statistical analysis, values observed in untreated GATA-1low mice were compared with those observed in untreated wild-type littermates, while values observed in treated animals were compared with those observed in the respective untreated controls.
ND indicates not done; NS, not significantly different (P > .05) from controls; -, not applicable.
TPO treatment increased platelet counts and restored platelet morphology in the blood of GATA-1low mice
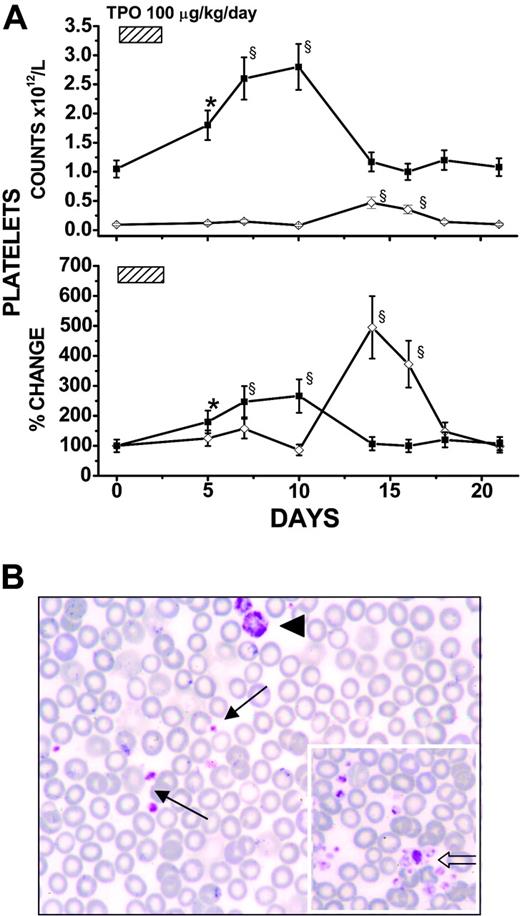
As expected,31 in wild-type mice, TPO treatment increased by 2.3-fold blood platelet counts by days 7 to 10. Platelet counts returned to pretreatment values by day 14 (Figure 1A). In GATA-1low mice, TPO treatment increased by approximately 5-fold platelet counts by days 14 to 16 (from 113 ± 35 to 520 ± 170 ×109/L), and the counts returned to pretreatment levels by day 18 (Figure 1A). Interestingly, blood smears from TPO-treated GATA-1low mice contained at days 14 to 16, in addition to dismorphic megathrombocytes, “normal”-sized platelets that formed aggregated clumps virtually absent on blood smears from untreated mutant mice (Figure 1B). Therefore, both wild-type and GATA-1low littermates responded to TPO treatment by increasing blood platelet counts, albeit with different amplitude and kinetics, suggesting that, at least partially, different mechanisms mediated the response to TPO in the 2 animal groups.
TPO treatment (100 μg/kg per day for 5 days) increased platelet counts and restored platelet morphology in the blood of GATA-1low mice. (A) Platelet counts (as absolute numbers, top; percent change, with respect to values measured in each mouse 3-5 days before TPO treatment, bottom) in wild-type (▪) and GATA-1low (⋄) mice (both 12 months old) treated with 100 μg TPO/kg per day for 5 days, as indicated. Data are presented as the mean (± SD) of independent determinations for a total of 18 GATA-1low and 14 normal littermates analyzed per experimental point. Values statistically higher than those from untreated animals are indicated: * indicates P < .05; §, P < .01. (B) May-Grünwald-Giemsa staining of a representative blood smear from a GATA-1low mouse at the peak (day 14) of its response to TPO, showing the presence in these samples of both megathrombocytes (arrowhead) and normal-sized platelets (arrows). As shown by the open arrow in the insert, normal-sized platelets from TPO-treated GATA-1low mice formed discrete aggregates virtually absent on blood smears from untreated mutant littermates. (Original magnification × 100/1.32 numerical aperture.)
TPO treatment (100 μg/kg per day for 5 days) increased platelet counts and restored platelet morphology in the blood of GATA-1low mice. (A) Platelet counts (as absolute numbers, top; percent change, with respect to values measured in each mouse 3-5 days before TPO treatment, bottom) in wild-type (▪) and GATA-1low (⋄) mice (both 12 months old) treated with 100 μg TPO/kg per day for 5 days, as indicated. Data are presented as the mean (± SD) of independent determinations for a total of 18 GATA-1low and 14 normal littermates analyzed per experimental point. Values statistically higher than those from untreated animals are indicated: * indicates P < .05; §, P < .01. (B) May-Grünwald-Giemsa staining of a representative blood smear from a GATA-1low mouse at the peak (day 14) of its response to TPO, showing the presence in these samples of both megathrombocytes (arrowhead) and normal-sized platelets (arrows). As shown by the open arrow in the insert, normal-sized platelets from TPO-treated GATA-1low mice formed discrete aggregates virtually absent on blood smears from untreated mutant littermates. (Original magnification × 100/1.32 numerical aperture.)
TPO treatment increased the frequencies of Mks and CFU-Mks in hematopoietic tissues of GATA-1low mice
To clarify the mechanism of the different TPO response by wild-type and GATA-1low mice, the number of nucleated cells and the frequencies of Mks and CFU-Mks in the tissues were evaluated in animals killed at different time points after treatment. As reported,21 at 12 months, the femur from GATA-1low mice contained significantly less nucleated cells than that of wild-type animals, while the spleen was bigger and contained 6 to 7 times more cells than that from age- and sex-matched normal littermates. TPO treatment did not induce significant changes in marrow cellularity but increased by approximately 2- to 3-fold the number of nucleated cells observed at day 7 in the spleen from both wild-type and GATA-1low mice. The number of cells in the spleen returned to normal values by day 14 in wild-type animals but remained modestly higher than pretreatment values until day 21 in GATA-1low mice (not shown).
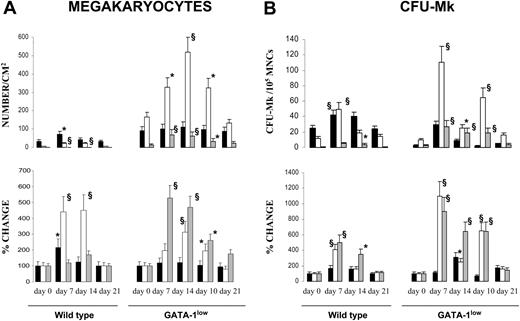
In TPO-treated wild-type animals, the number of Mks/mm2 significantly increased in marrow at day 7 (from 43 ± 9 to 87 ± 19; P < .05) and in spleen at days 7 to 14 (from 19 ± 6 to 90 ± 12-95 ± 17; P < .001), while the increase observed in liver at day 14 was not statistically significant (Figures 2, 3). In TPO-treated GATA-1low mice, the frequency of Mks remained constant in marrow (from 112 ± 21 to 133 ± 29 Mks/mm2), and increased in spleen at days 10 to 14 (from 166 ± 30 to 518 ± 73 Mks/mm2; P < .001) and in liver from days 7 through 21 (from 13 ± 6 to 70 ± 12 Mks/mm2; P < .001). Also the frequency of CFU-Mks increased in the hematopoietic organs from both wild-type and GATA-1low mice after TPO treatment (Figure 3B). In TPO-treated wild-type animals, the CFU-Mk frequency did not change in marrow but increased by approximately 4-fold in spleen at day 7 and in liver at days 7 to 14. In TPO-treated GATA-1low mice, the CFU-Mk frequency modestly (2-fold) increased in marrow at day 10 and massively increased in spleen and liver at days 7 to 14 (≈ 11-fold and 9-fold, respectively) (Figure 3B).
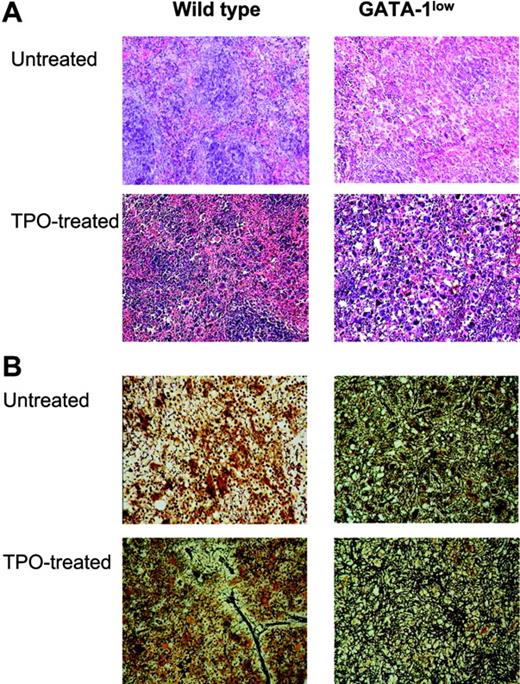
TPO treatment (100 μg/kg per day for 5 days) increased the frequency of Mks in hematopoietic tissues from both wild-type and GATA-1low littermates, but induced fibrosis only in those from wild-type animals. (A) Hematoxylin-eosin staining of spleen sections from untreated and TPO-treated (day 7) wild-type and GATA-1low littermates, as indicated. Massive hyperplasia of Mks is observed in the spleen from both wild-type and GATA-1low mice 7 days after TPO treatment. Sections from marrow and spleen of the same animals were processed in parallel and the results are not shown. Original magnification: × 20 in the top panels and × 40 in all the others; numerical aperture of the objectives, 0.55 and 0.70, respectively. (B) Gomori-silver staining of spleen sections from untreated and TPO-treated (day 7) wild-type and GATA-1low littermates (alternate sections with respect to those presented in the top panels). Note the presence of extensive fiber deposition in TPO-treated wild-type animals while there is no appreciable change in the fibers of the GATA-1low mice after TPO. Similar results were observed on sections from bone marrow and liver (not shown). Original magnification × 200; numerical aperture, 0.55.
TPO treatment (100 μg/kg per day for 5 days) increased the frequency of Mks in hematopoietic tissues from both wild-type and GATA-1low littermates, but induced fibrosis only in those from wild-type animals. (A) Hematoxylin-eosin staining of spleen sections from untreated and TPO-treated (day 7) wild-type and GATA-1low littermates, as indicated. Massive hyperplasia of Mks is observed in the spleen from both wild-type and GATA-1low mice 7 days after TPO treatment. Sections from marrow and spleen of the same animals were processed in parallel and the results are not shown. Original magnification: × 20 in the top panels and × 40 in all the others; numerical aperture of the objectives, 0.55 and 0.70, respectively. (B) Gomori-silver staining of spleen sections from untreated and TPO-treated (day 7) wild-type and GATA-1low littermates (alternate sections with respect to those presented in the top panels). Note the presence of extensive fiber deposition in TPO-treated wild-type animals while there is no appreciable change in the fibers of the GATA-1low mice after TPO. Similar results were observed on sections from bone marrow and liver (not shown). Original magnification × 200; numerical aperture, 0.55.
TPO treatment (100 μg/kg per day for 5 days) increased the frequency of Mks and CFU-Mks in hematopoietic tissues from both wild-type and GATA-1low littermates. Frequency of Mks (A) and CFU-Mks (B) in marrow (▪), spleen (□), and liver (▦) from wild-type and GATA-1low mice at days 7, 10, 14, and 21 after receiving 100 μg TPO/kg of body weight/day for 5 consecutive days. The data are reported both in absolute number (top) and as percent change, with respect to values measured in each mouse 3 to 5 days before TPO treatment (bottom). Results represent the mean ± SD of data obtained on 4 mice at each time point. The numbers of Mks were determined in serial hematoxylineosin–stained sections similar to those presented in Figure 2. The numbers of CFU-Mks are expressed per 105 mononuclear cells (MNCs) for all the tissues. Values statistically higher than those observed in untreated animals: * indicates P < .05; §, P < .01.
TPO treatment (100 μg/kg per day for 5 days) increased the frequency of Mks and CFU-Mks in hematopoietic tissues from both wild-type and GATA-1low littermates. Frequency of Mks (A) and CFU-Mks (B) in marrow (▪), spleen (□), and liver (▦) from wild-type and GATA-1low mice at days 7, 10, 14, and 21 after receiving 100 μg TPO/kg of body weight/day for 5 consecutive days. The data are reported both in absolute number (top) and as percent change, with respect to values measured in each mouse 3 to 5 days before TPO treatment (bottom). Results represent the mean ± SD of data obtained on 4 mice at each time point. The numbers of Mks were determined in serial hematoxylineosin–stained sections similar to those presented in Figure 2. The numbers of CFU-Mks are expressed per 105 mononuclear cells (MNCs) for all the tissues. Values statistically higher than those observed in untreated animals: * indicates P < .05; §, P < .01.
TPO treatment had profound effects on the morphology of Mks both in wild-type and GATA-1low mice. In wild-type mice, the overall morphology of the Mks in the spleen became less mature, as revealed by reduced localization of P-selectin (Schmitt et al32 and results not shown) and von Willebrand factor (Table 2) on the α-granules. In addition, a significant portion (31 ± 2.6%) of these Mks had the heavy-electron dense para-apoptotic morphology originally described for GATA-1low Mks22 and contained neutrophils embedded in the cytoplasm, as confirmed by myeloperoxidase immunostaining (Figure 4). In contrast, in TPO-treated GATA-1low mice, the morphology of the Mks became more mature with clearly delineated platelet territories and significant increase, both in number and in localization to the α-granules, of von Willebrand factor–related gold particles (Figure 5; Table 2). These stage III Mks, never detected in untreated mutants,22 represented 28.5% of the Mks in spleen from TPO-treated GATA-1low Mks.
Numbers of immunogold particles for von Willebrand factor in Mks from the spleen of untreated wild-type and GATA-1low littermates and from mice after 7 days of TPO treatment, as indicated
. | von Willebrand factor, no. particles . | . | |
|---|---|---|---|
. | Granules . | Cytoplasm/DMS . | |
| Wild-type mice | |||
| Untreated | |||
| Light Mk | 103 ± 8 | 91 ± 8 | |
| Heavy Mk | - | - | |
| TPO treated | |||
| Light Mk | 62 ± 10* | 135 ± 4* | |
| Heavy Mk | 33 ± 7* | 138 ± 2* | |
| GATA-1low mice | |||
| Untreated | |||
| Light Mk | 21 ± 2 | 86 ± 2 | |
| Heavy Mk | 8.0 ± 4.5 | 81 ± 5 | |
| TPO treated | |||
| Light Mk | 70 ± 10* | 142 ± 11* | |
| Heavy Mk | 63 ± 9* | 183 ± 6* | |
. | von Willebrand factor, no. particles . | . | |
|---|---|---|---|
. | Granules . | Cytoplasm/DMS . | |
| Wild-type mice | |||
| Untreated | |||
| Light Mk | 103 ± 8 | 91 ± 8 | |
| Heavy Mk | - | - | |
| TPO treated | |||
| Light Mk | 62 ± 10* | 135 ± 4* | |
| Heavy Mk | 33 ± 7* | 138 ± 2* | |
| GATA-1low mice | |||
| Untreated | |||
| Light Mk | 21 ± 2 | 86 ± 2 | |
| Heavy Mk | 8.0 ± 4.5 | 81 ± 5 | |
| TPO treated | |||
| Light Mk | 70 ± 10* | 142 ± 11* | |
| Heavy Mk | 63 ± 9* | 183 ± 6* | |
At least 15 to 20 wild-type and more than 50 GATA-1low Mks were analyzed for the presence of von Willebrand factor-related gold particles. The numbers of gold particles present in 14 μm2 of Mks are presented as mean (± SD) of determinations obtained in 5 randomly selected cells for experimental point. The number of gold granules present in 5 randomly selected 14-μm2 surfaces outside the cells as control are as follows: for untreated wild-type mice, 2.3 ± 0.5; TPO-treated wild-type mice, 1.5 ± 0.5; GATA-1low untreated mice, 1.7 ± 0.4; and GATA-1low TPO-untreated mice, 1.8 ± 0.5.
Data significantly different (P < .05) from those observed in Mks from the corresponding untreated controls
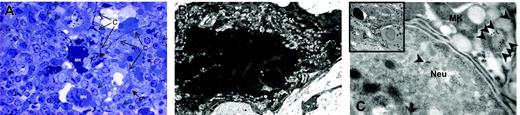
The Mks in the spleen from TPO-treated wild-type mice expressed several morphologic abnormalities characteristic of GATA-1low Mks. Semithin (A) and ultrathin (B-C) sections of the spleen from TPO-treated wild-type mice. The semithin section (A) shows a cluster of light- and heavy-electron dense Mks (Mk) surrounded by numerous neutrophils (arrowheads), fibroblasts (F plus arrows), and connectival fibers (CF). Upon ultrastructural examination (B), heavy-electron dense Mks present a morphology of cytoplasm and nucleus compatible with that of cells undergoing para-apoptosis.22 Furthermore, heavy-electron dense Mks contain neutrophils (Neu) embedded in their cytoplasm (insert in C), as proved by immunogold staining for myeloperoxidase (C) that shows gold particles (arrowheads) in the cytoplasm both of the neutrophil and of the Mks. Similar results were observed in the spleen of at least 3 separate TPO-treated wild-type mice. Magnification: × 100 (A), × 3000 (B, insert in C), and × 30 000 (C). Numerical aperture of the objective lenses: 0.1.
The Mks in the spleen from TPO-treated wild-type mice expressed several morphologic abnormalities characteristic of GATA-1low Mks. Semithin (A) and ultrathin (B-C) sections of the spleen from TPO-treated wild-type mice. The semithin section (A) shows a cluster of light- and heavy-electron dense Mks (Mk) surrounded by numerous neutrophils (arrowheads), fibroblasts (F plus arrows), and connectival fibers (CF). Upon ultrastructural examination (B), heavy-electron dense Mks present a morphology of cytoplasm and nucleus compatible with that of cells undergoing para-apoptosis.22 Furthermore, heavy-electron dense Mks contain neutrophils (Neu) embedded in their cytoplasm (insert in C), as proved by immunogold staining for myeloperoxidase (C) that shows gold particles (arrowheads) in the cytoplasm both of the neutrophil and of the Mks. Similar results were observed in the spleen of at least 3 separate TPO-treated wild-type mice. Magnification: × 100 (A), × 3000 (B, insert in C), and × 30 000 (C). Numerical aperture of the objective lenses: 0.1.
TPO treatment restored Mk maturation in GATA-1low mice. Immunoelectron microscopy for von Willebrand factor of Mks from the spleen of untreated GATA-1low mouse (A) and from a mutant mouse after 7 days of TPO treatment (B). The cytoplasm of the Mks from TPO-treated animals contains clearly recognizable platelet territories with numerous electron-dense granules, which, when analyzed at higher magnification, contain several von Willebrand factor–related gold particles (Bii). The rectangle in panels A and B indicates the region of the Mks shown at higher magnification in Aii and Bii, respectively. The arrows in Aii and Bii indicate von Willebrand factor–related gold particles. Magnification, × 4400 (Ai,Bi) and × 30 000 (Aii,Bii). Numerical aperture of the objective lenses: 0.1.
TPO treatment restored Mk maturation in GATA-1low mice. Immunoelectron microscopy for von Willebrand factor of Mks from the spleen of untreated GATA-1low mouse (A) and from a mutant mouse after 7 days of TPO treatment (B). The cytoplasm of the Mks from TPO-treated animals contains clearly recognizable platelet territories with numerous electron-dense granules, which, when analyzed at higher magnification, contain several von Willebrand factor–related gold particles (Bii). The rectangle in panels A and B indicates the region of the Mks shown at higher magnification in Aii and Bii, respectively. The arrows in Aii and Bii indicate von Willebrand factor–related gold particles. Magnification, × 4400 (Ai,Bi) and × 30 000 (Aii,Bii). Numerical aperture of the objective lenses: 0.1.
Therefore, TPO treatment raised, with similar kinetics, but in different anatomic sites, the number of Mks and CFU-Mks in both wild-type and GATA-1low mice. The treatment, however, had opposite effects on the morphology of Mks generated by the 2 animals.
TPO treatment induced fibrosis in wild-type animals but not in GATA-1low mice
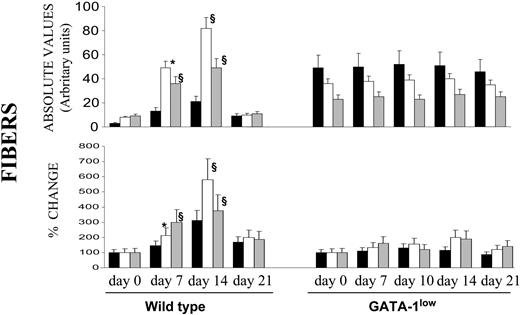
As expected,17 TPO-treated wild-type mice expressed a transient fibrosis, with a peak at day 14, in all of the organs examined, but especially in the spleen (Figures 2, 6), where maximal increases in Mk frequency had also been detected (Figure 3A). In contrast, in GATA-1low mice, TPO treatment had no significant effect on fibrosis in any of the organs examined, not even in the liver (Figure 6), in spite of the massive Mk increase induced by TPO in this organ (Figure 3B).
Quantification of fibrosis in wild-type and GATA-1low animals killed at different time points after TPO treatment. Fibers were measured in arbitrary units as described in “Materials and methods” from stained sections similar to those presented in Figure 2, and the data are presented either in absolute values (top) or as mean (SD) percent change, compared with values recorded from untreated controls (bottom). The mean number of fiber intersections (SD/cm) was calculated from values obtained at least on 3 different sections per organ per mouse. At least 3 to 4 mice were analyzed per each experimental point. Values statistically significant from those of untreated animals are indicated: * indicates P < .05; §, P < .01.
Quantification of fibrosis in wild-type and GATA-1low animals killed at different time points after TPO treatment. Fibers were measured in arbitrary units as described in “Materials and methods” from stained sections similar to those presented in Figure 2, and the data are presented either in absolute values (top) or as mean (SD) percent change, compared with values recorded from untreated controls (bottom). The mean number of fiber intersections (SD/cm) was calculated from values obtained at least on 3 different sections per organ per mouse. At least 3 to 4 mice were analyzed per each experimental point. Values statistically significant from those of untreated animals are indicated: * indicates P < .05; §, P < .01.
TPO treatment restored GATA-1 content in Mks from GATA-1low mice
Although Mks from GATA-1low mice did not express detectable levels of GATA1 mRNA,14,18 it is possible that the mutation allows leakage expression at the single Mk level. Therefore, to further clarify the nature of the different response to TPO by wild-type and GATA-1low mice, the GATA-1 content of Mks from TPO-treated mice was investigated by immunohistochemistry and quantitative RT-PCR.
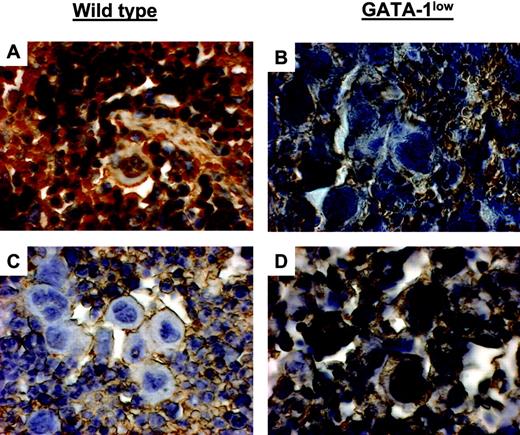
GATA-1 protein was clearly detected by immunohistochemistry in Mks from wild-type mice but not in those from their mutant littermates (Figure 7). Variegation in intensity of GATA-1 staining was observed in Mks from both wild-type and GATA-1low mice. The majority (83%) of Mks in the spleen from wild-type animals was strongly stained by the GATA-1–specific antibody. Similarly, although none of the GATA-1low Mks reacted with the antibody as strongly as those from wild-type mice (Figure 7), 30% of them were faintly stained. Such a variegation was enhanced by TPO treatment. In wild-type mice, TPO treatment decreased to 5% the number of Mks containing detectable GATA-1 protein in the spleen at day 7, and as many as 40% of normal Mks still reacted poorly with the GATA-1 antibody by days 14 to 21 (Figure 7 and results not shown). In contrast, in GATA-1low mice, TPO treatment sharply increased to 40% to 87% the number of mutant Mks expressing high levels of GATA-1 in spleen (and in liver, not shown) from day 7 to day 21 (Figure 7 and results not shown).
TPO treatment increased GATA-1 content in Mks from GATA-1low mice while decreasing GATA-1 content in Mks from wild-type littermates. GATA-1 immunostaining of spleen sections from untreated (A-B) and TPO-treated (day 7 after 100μg TPO/kg per day for 5 days) (C-D) wild-type and GATA-1low mice, as indicated. Mks in spleen sections from untreated wild-type and TPO-treated GATA-1low mice strongly reacted with the GATA-1 antibody, while those from untreated GATA-1low and TPO-treated wild-type animals did not. Similar results were observed in sections from marrow and liver from these same animals (not shown). Original magnification × 400. Numerical aperture of the objective lenses: 1.30.
TPO treatment increased GATA-1 content in Mks from GATA-1low mice while decreasing GATA-1 content in Mks from wild-type littermates. GATA-1 immunostaining of spleen sections from untreated (A-B) and TPO-treated (day 7 after 100μg TPO/kg per day for 5 days) (C-D) wild-type and GATA-1low mice, as indicated. Mks in spleen sections from untreated wild-type and TPO-treated GATA-1low mice strongly reacted with the GATA-1 antibody, while those from untreated GATA-1low and TPO-treated wild-type animals did not. Similar results were observed in sections from marrow and liver from these same animals (not shown). Original magnification × 400. Numerical aperture of the objective lenses: 1.30.
The changes in GATA-1 content in Mks observed at 7 days after TPO treatment by immunomicroscopy were confirmed at the mRNA level by quantitative RT-PCR analysis. The ΔCT for GATA1 mRNA in Mks purified from untreated wild-type and GATA-1low mice was 4.1 ± 0.16 and 9.7 ± 0.48, respectively, which corresponds to a significantly (P < .01) lower (by 2-log) expression of GATA1 in Mks from GATA-1low mice. After TPO treatment, the ΔCT of GATA1 mRNA in wild-type Mks was 4.6 ± 0.30 (a significant, P < .05, 30% reduction in mRNA content with respect to untreated wild-type Mks), while that from GATA-1low mice became 7.7 ± 0.39 (a significant, P < .01, 1-log increase over the values in the same cells before treatment).
These results indicate that after TPO treatment, GATA-1 (both as protein and mRNA) decreased in normal Mks while increasing in GATA-1low Mks.
TPO treatment increased the levels of TGF-β1 in plasma and extracellular fluids of wild-type mice, but not in those from GATA-1low mice
TGF-β1 has been implicated in the pathobiology of myelofibrosis33,34 by the observation that Mks from TPOhigh rats17 and mice29,32 express high levels of TGF-β1 that are reflected by high concentrations of this factor in marrow extracellular fluids and plasma. In TPOhigh mice, the pathobiologic role of TGF-β1 in the development of the disease was formally proved by the finding that mice that received transplants of normal stem cells transfected with a TPO-containing retrovirus, but not those that received transplants of TGF-β1null stem cells transfected with the same retrovirus, develop myelofibrosis.29 To clarify the role of TGF-β1 in the development of myelofibrosis in the GATA-1low model, we compared the level of TGF-β1 expression in femur and spleen and its concentration in extracellular fluids and plasma from untreated and TPO-treated wild-type and GATA-1low mice.
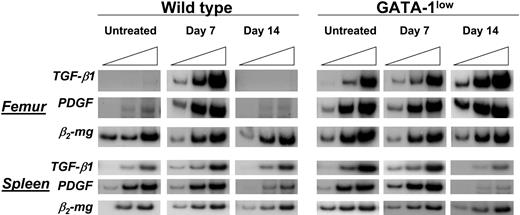
TGFB1 mRNAwas barely expressed by femur and spleen cells from wild-type mice (Figure 8). In these animals, expression of TGFB1 significantly increased in both organs at day 7 after TPO treatment and returned to basal levels by day 14 (Figure 8). In contrast, femur and spleen cells from GATA-1low mice expressed TGFB1 at levels nearly as high as those found in wild-type animals at day 7 after TPO treatment (Figure 8). TPO treatment of GATA-1low mice did not further raise the levels of TGFB1 expressed by these organs and even decreased expression in the spleen at day 14 (Figure 8). Of note, expression of PDGF, another growth factor produced by Mks, was found to be up-regulated in a fashion similar to TGFB1 both in untreated GATA-1low and in TPOhigh mice.
TPO treatment increased expression of TGFB1 mRNA in cells from femur and spleen of wild-type mice but not in those from their GATA-1low littermates. Semiquantitative RT-PCR analysis for the expression of TGFB1, PDGF, and β2-microglobulin (β2-mg, as control), in femur and spleen from untreated or TPO-treated (7 and 14 days after being treated with 100 μg TPO/kg per day for 5 days) wild-type and GATA-1low littermates. Each product was amplified for increasing number of cycles,25,30,35 as indicated by the triangle on the top of the panels. Similar results were obtained in 2 additional experiments.
TPO treatment increased expression of TGFB1 mRNA in cells from femur and spleen of wild-type mice but not in those from their GATA-1low littermates. Semiquantitative RT-PCR analysis for the expression of TGFB1, PDGF, and β2-microglobulin (β2-mg, as control), in femur and spleen from untreated or TPO-treated (7 and 14 days after being treated with 100 μg TPO/kg per day for 5 days) wild-type and GATA-1low littermates. Each product was amplified for increasing number of cycles,25,30,35 as indicated by the triangle on the top of the panels. Similar results were obtained in 2 additional experiments.
High levels of TGFB1 mRNA in cells from marrow and spleen from GATA-1low and TPOhigh mice were associated with high levels of TGF-β1 protein in extracellular fluids from these organs (Table 1). The extracellular fluids of GATA-1low mice contained significantly more TGF-β1 than those from the corresponding organs of untreated wild-type animals (1.9-32 vs 0.9-19 ng/mL, P < .005). On the other hand, TPO treatment increased significantly the levels of TGF-β1 detected in extracellular fluids from wild-type mice after 14 days, but did not affect the concentration of TGF-β1 in tissues from GATA-1low animals (Table 1). These results were complemented by analysis of the TGF-β1 present in plasma: no statistical difference was observed between the TGF-β1 present in plasma from wild-type and GATA-1low mice (∼ 2 ng/mL in both cases), while increases of TGFB1 mRNA and protein in marrow and spleen in TPO-treated wild-type mice were mirrored by increased levels of the growth factor in plasma (Table 1).
The observation that the levels of TGF-β1 in extracellular fluids from both TPOhigh and GATA-1low mice, but only those in plasma from TPOhigh animals were higher than normal indicates that the overall levels of TGF-β1 were higher in TPOhigh than in GATA-1low littermates.
Discussion
The interactions between TPO and GATA-1 in the control of thrombocytopoiesis and in the pathobiology of myelofibrosis are here clarified by comparing the effects of TPO treatment in wild-type and GATA-1low littermates.
Preliminary experiments determined that, in spite of their thrombocytopenia, GATA-1low mice have a normal TPO metabolism. In fact, the steady-state TPO levels in the plasma of these mice was normal (∼ 0.75 ng/mL) and transiently rose after TPO treatment up to values observed in TPO-treated wild-type animals (∼ 5.7-6.0 ng/mL by day 7) (Table 1). These results are expected since the bulk of TPO receptors (ie, the number of Mks plus platelets that is inversely correlated with the circulating TPO levels expressed by an animal10 ) is roughly normal in GATA-1low mice as a result of the counterbalance between high number of Mks and reduced (15% of normal) platelet numbers14 and levels of mpl expression.18 Of note, the TPO treatment used in this study raised the plasma levels of the factor to values comparable with those (∼ 3-100 ng/mL) observed in TPOhigh animals.15,29
GATA-1low mice responded to TPO as wild-type animals by increasing the number of platelets in the blood (Figure 1) and the frequency of Mks and CFU-Mks in the hematopoietic tissues (Figures 2, 3). However, significant qualitative and quantitative differences were observed in the response to TPO by the 2 animal groups.
At the level of Mk progenitor and precursor cells, the most evident differences were represented by the amplitude of the response (higher in GATA-1low mice) and by the hematopoietic sites involved (spleen and spleen plus liver in wild-type and GATA-1low animals, respectively). Both results were not unexpected. GATA-1low progenitor cells, probably because of their reduced GATA-1 content,35 have more proliferative potential than their normal counterparts.26 Moreover, a similarly higher than normal progenitor cell amplification had been observed in these animals in response to EPO.20 On the other hand, hematopoiesis could not increase further in the spleen from 12-month-old GATA-1low mice, which were already heavily recruited to compensate for the effects of the mutation20 and marrow fibrosis.21
At the level of platelets, the major difference in response to TPO shown by the 2 animal groups was represented by the kinetics. In wild-type animals, circulating platelet counts peaked at days 7 to 10 (Figure 1), slightly before the increases in Mks and CFU-Mks observed at days 7 to 14 (Figures 2, 3). In contrast, in GATA-1low mice, the peak number of circulating platelets occurred at days 14 to 16 and followed the maximal increase of Mks and CFU-Mks observed, as in normal mice, at days 7 to 10 (Figures 1, 2). Therefore, in wild-type animals, TPO treatment increased platelet counts, at least partially, by stimulating platelet formation from a preexisting Mk pool, while in GATA-1low mice, the platelets released in response to TPO derived mostly from newly generated Mks. Since the majority of newly generated Mks detected at day 7 in TPO-treated GATA-1low mice contained GATA-1 (Figure 7) and expressed normal platelet territories (Figure 5), it is not surprising that many of the platelets detected in the blood from these mice at the peak of their TPO response were apparently normal as well (Figure 1B).
The GATA-1– and the TPO-dependent control of megakaryocytopoiesis had been indirectly linked by the observation that Mks from GATA-1low mice express reduced levels of mpl,18 suggesting that GATA-1 is required for proper expression of mpl in these cells.Afurther link between the 2 control pathways is provided here by the observation that TPO treatment had opposite effects on GATA-1 content in Mks from normal and GATA-1low mice (Figure 7). These opposite effects were, at least partially, mirrored by changes in the levels of GATA1 mRNA expressed by purified Mk populations. These data suggest that the regulatory sequences of the GATA1 gene contain both TPO-enhancer and -suppressor elements. The suppressor elements might be specifically located within the region deleted by the GATA-1low mutation and exert a negative feedback on GATA1 expression in normal cells. Since these sequences are also implicated in the up-regulation of GATA1 expression necessary for erythroid differentiation, the presence of a TPO-suppressor element within this region might favor Mk over erythroid differentiation36 at high TPO levels. However, further studies are necessary to clarify whether TPO regulates the GATA-1 content in Mks by directly suppressing the transcription of the gene, or by other indirect mechanisms.
A corollary to the finding that both Mks from GATA-1low and those from TPOhigh mice contain similar low levels of GATA-1 protein is that they should present similar maturation abnormalities. On the other hand, Mks from TPO-treated GATA-1low mice, containing detectable levels of GATA-1, should be morphologically normal. The results obtained confirm both hypotheses. Mks from TPOhigh and GATA-1low mice, in fact, share several morphologic alterations, including abnormal localization of P-selectin on the demarcation membrane system (DMS)22,32 and increased neutrophil emperipolesis with consequently death by paraapoptosis (Figure 4; Centurione et al22 ; and Schmitt et al32 ). In contrast, at day 7 of TPO treatment, the cytoplasm of a significant proportion of GATA-1low Mks presented normal platelet territories with appropriate localization of von Willebrand factor to the α-granules (Figure 5; Table 2). Of note, while TPO treatment induced transient myelofibrosis in wild-type mice, it halted the manifestation of the disease in GATA-1low animals (Figures 2, 4). TPO treatment actually increased the frequency of CFU-Mks, and presumably of other progenitors, in marrow from GATA-1low mice, but not in that of wild-type littermates (Figure 3B). Since a raise in progenitor frequency presumes availability of appropriate niches,37 it is possible that TPO treatment had made possible the regress of marrow fibrosis in these animals, although at levels not appreciated by the insensitive method used for its quantification. Further experiments in which younger (6- to 8-months old) GATA-1low mice will be exposed, by retroviral technology, to high levels of TPO until an age at which they would normally develop the disease, are necessary to clarify whether TPO represents a cure for myelofibrosis in GATA-1low mice.
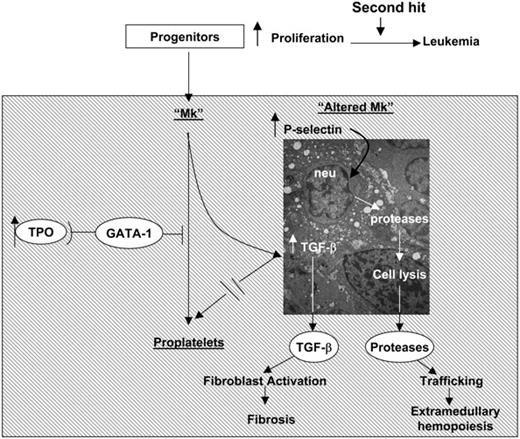
Recently it has been shown that Mks from patients with IM, either generated in vitro or obtained from marrow biopsy, express normal levels of GATA1 mRNA but contain significantly lower levels of GATA-1 protein than their counterparts from healthy donors (Vannucchi et al38 and A.M.V., A.P., L.B., Paolo Gugliemelli, S. Di Lollo, C. Bogdani, G. Baroni, A. R. Migliaccio, A. Bosi, and F. Paoletti, manuscript submitted November 2004). Therefore, since the process of Mk maturation, and its regulation, is well conserved between mice and humans, it is not surprising that Mks from IM patients express abnormalities similar to those expressed by Mks from TPOhigh and GATA-1low mice (ie, increased neutrophil emperipolesis through abnormal P-selectin localization on the DMS32 leading to para-apoptosis39 ). On the basis of these considerations, we propose the unifying pathobiologic pathway for the development of myelofibrosis described in Figure 9. In this pathway, TPO, GATA-1, and TGF-β1 are linked in an upstream-downstream relationship: reduced GATA-1 content in Mks, either as result of TPO treatment32 and of hypomorphic GATA-1low mutations22 in mice or of a still-unidentified posttranscriptional mechanism(s)38 in humans, blocks Mk maturation and results in P-selectin localization on the DMS. Abnormal P-selectin localization increases; then, neutrophils undergo emperipolesis within the Mks, whose specific pathologic consequences are the results of the neutrophil enzymes released in the Mk cytoplasm. These enzymes would, in fact, dissolve the rudimental α-granules and release TGF-β1 in the microenvironment through the canaliculi of the DMS. According to this model, myelofibrosis does not have a single leading cause, but may be a consequence of any mutation along this pathobiologic pathway that results in increased neutrophil emperipolesis within the Mk through the DMS. It also predicts that different mutations may induce diseases manifesting themselves with different morbidity and mortality, depending on the levels of TGF-β1 and neutrophil proteases they release in the microenvironment. Indeed, the TPOhigh alterations, which are associated with significantly more TGF-β1 in extracellular fluids and plasma than the GATA-1low mutation (Table 1), induce a more severe myelofibrosis than that observed in GATA-1low mice.4,5,16,21 A corollary of this model is that any pharmacologic manipulation that would restore the function of the elements in the pathway represents a potential candidate for targeted therapy of IM. The GATA-1low mice, because of their long half-life, represent invaluable tools to identify which treatment holds the highest promise for a cure.
A pathobiologic pathway linking TPO, GATA-1, and TGF-β1 in the development of myelofibrosis in mice and, possibly, of IM in humans. Both TPOhigh and GATA-1low mutations decrease the GATA-1 content of Mks (Figure 7) and induce similar Mk abnormalities, including abnormal P-selectin localization on the DMS, increased emperipolesis of neutrophils, and high levels of para-apoptosis (Figure 4).22,32 In both cases, the animals express high levels of TGF-β1 in extracellular fluids of marrow and spleen (Table 1), while neutrophil proteases are also detected in the microenvironment surrounding the Mks in association with the fibers (Figure 4). According to these data, we propose that marrow fibrosis is caused by TGF-β1 activation of the fibroblasts. On the other hand, extramedullary hematopoiesis is the consequence not only of reductions in stem/progenitor cell niches in the marrow because of fibrosis, but also of the proteases released by the neutrophils as part of the emperipolesis process that would dislodge stem/progenitor cells by cleaving from their surface the adhesion proteins necessary for attachment to the niches.37 Dislodged stem/progenitor cells would, then, have no other choice than to home and colonize lower affinity niches in other tissues.
A pathobiologic pathway linking TPO, GATA-1, and TGF-β1 in the development of myelofibrosis in mice and, possibly, of IM in humans. Both TPOhigh and GATA-1low mutations decrease the GATA-1 content of Mks (Figure 7) and induce similar Mk abnormalities, including abnormal P-selectin localization on the DMS, increased emperipolesis of neutrophils, and high levels of para-apoptosis (Figure 4).22,32 In both cases, the animals express high levels of TGF-β1 in extracellular fluids of marrow and spleen (Table 1), while neutrophil proteases are also detected in the microenvironment surrounding the Mks in association with the fibers (Figure 4). According to these data, we propose that marrow fibrosis is caused by TGF-β1 activation of the fibroblasts. On the other hand, extramedullary hematopoiesis is the consequence not only of reductions in stem/progenitor cell niches in the marrow because of fibrosis, but also of the proteases released by the neutrophils as part of the emperipolesis process that would dislodge stem/progenitor cells by cleaving from their surface the adhesion proteins necessary for attachment to the niches.37 Dislodged stem/progenitor cells would, then, have no other choice than to home and colonize lower affinity niches in other tissues.
Prepublished online as Blood First Edition Paper, January 21, 2005; DOI 10.1182/blood-2004-04-1320.
Supported by Progetto Strategico Oncologia CNR MIUR (Centro Nazionale Ricerche e Ministero Istruzione Universitá Ricerca) legge 449/99; Progetti di Ricerca di Interesse Nazionale (PRIN) 2000, 2001, and 2002 from the Ministry of Health; PRIN no. 2003064888-3, MIUR 60% grant 2002; grant no. E1172 from the Telethon Foundation; and a grant from Associazione Italiana per le Leucemie (AIL) “30 ore,” Florence. L.B. is a fellow of Fondazione Italiana Ricerca sul Cancro, Milan, and A.P. is a fellow of AIL, Florence.
M.N. is employed by Brewery Pharmaceutical Research Laboratory, whose potential product was studied in the present work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors gratefully acknowledge Prof Francesco Antonio Manzoli and Dr William Vainchenker for advice and discussion, Dr Lucia Centurione for performing the GATA-1 immunostaining, and Dr Antonio Di Virgilio for managing the animal colony. Murine TPO was provided by Kirin (transfer of material agreement of March 29, 2002).


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal