Abstract
The migration of leukocytes in immune surveillance and inflammation is largely determined by their response to chemokines. While the chemokine specificities and expression patterns of chemokine receptors are well defined, it is still a matter of debate how leukocytes integrate the messages provided by different chemokines that are concomitantly produced in physiologic or pathologic situations in vivo. We present evidence for a novel regulatory mechanism of leukocyte trafficking. Our data are consistent with a mode of action where CC-chemokine receptor 7 (CCR7) agonists and unrelated, nonagonist chemokines first form a heteromeric complex, in the presence of which the triggering of CCR7 can occur at a much lower agonist concentration. The increase is synergistic and can be evoked by many but not all chemokines. Chemokine-induced synergism might provide an amplification system in “chemokine-rich” tissues, rendering leukocytes more competent to respond to migratory cues.
Introduction
Chemokines are the primary guidance cues used by leukocytes during their controlled trafficking to and from different compartments of the organism in physiology and in pathology. Similar to “classical” chemoattractants such as complement factor 5a (C5a) or N-formyl-methionyl-leucyl-phenylalanine (fMLP), all chemokines act by activating 7 transmembrane domain receptors that couple to heterotrimeric G proteins.1 An enormous body of work, accumulated during the past 15 years, has greatly clarified the rather large number as well as the diverse properties of chemokines and chemokine receptors.2,3 Numerous in vitro experiments have elucidated the agonist specificities of chemokines for their respective receptors, revealing a complex pattern of sometimes highly specific, but often surprisingly promiscuous interactions. A vast variety of in situ experiments have revealed a picture possibly even more complicated, since a variety of chemokines can be concomitantly produced at target sites of leukocyte trafficking and homing.4-7 Likewise, most leukocytes express more than one chemokine receptor of varying promiscuity, and are therefore potentially capable of responding to concomitant migratory signals.8-10 While we thus understand well the effects of different chemokines one by one, much less is known about the potential consequences of a rich chemokine “milieu” on leukocyte migration.
In inflammatory situations, the abundance of chemotactic factors and cellular detectors might provide the system as a whole with some degree of redundancy and hence robustness, as postulated previously.11 The recent discovery of human12-18 and viral19-25 chemokines that, apart from their known agonist functions, act as natural antagonists on certain receptors, provides an example of abundant chemokine expression serving a regulatory rather than redundant function. Proof of principle for the modulatory function of antagonists exists: viral MIP-II,26 and a truncated variant of monocyte chemoattractant protein-1 (MCP-1)27 were shown to be efficient inhibitors of leukocyte migration in animal models. In addition, in vivo experiments with human natural chemokine antagonists have recently confirmed the in vitro data observed in several laboratories.28
In this report, we present evidence for a novel, positive regulatory mechanism of leukocyte migration. We show that many chemokines, which were previously thought to have no effect on CC-chemokine receptor 7 (CCR7), synergistically augment chemotaxis induced by the selective agonists, namely secondary lymphoid tissue chemokine (SLC) and EBI1 ligand chemokine (ELC). In particular, we have investigated the effects of B-cell-attracting chemokine-1 (BCA-1), which augments not only chemotaxis but other cellular responses as well. The formation of heteromeric complexes consisting of agonistic and synergy-inducing chemokines correlates with the increased responses observed. Taken together, our findings suggest that sufficient tissue concentrations of many chemokines may trigger leukocytes to respond to agonist concentrations that per se would be inactive, thus lowering their “migratory threshold” capacity.
Materials and methods
Cells
Stable transfection of human CCR7 in murine pre-B 300.19 cells was performed as described before.29 Mature dendritic cells (DCs) were generated from peripheral blood leukocytes as previously described.30 Maturation was induced by addition of 1 μg/mL lipopolysaccharide (LPS; from Escherichia coli, 026:B6; Sigma-Aldrich Chemicals, St Louis, MO) for 43 hours of culture. CD19+ B lymphocytes were isolated by a positive immunoselection procedure (CD19 MicroBeads; Miltenyi Biotec, Bergisch Gladbach, Germany), and cultured for 24 hours before chemotaxis assays. CD4+ naive T lymphocytes were isolated by positive immunoselection (CD4 MicroBeads; Miltenyi Biotec), followed by sorting for CD45RA+ (Beckman Coulter, Marseille, France). The expression of CCR7 was assessed by flow cytometric analysis (FACScan; Becton Dickinson, San Jose, CA), using a rat anti-CCR7 antibody (3D12, kindly provided by Dr Martin Lipp, Berlin, Germany).
Chemokine synthesis
All chemokines were chemically synthesized using tBoc solid-phase chemistry.31
Chemotaxis assays
Chemotaxis was assayed in 48-well Boyden microchambers (Neuro Probe, Cabin John, MD) as previously described.32 CCR7-transfected cells (5 × 104), mature DCs, and naive T lymphocytes (10 × 104), respectively, were diluted in chemotaxis buffer, whereas B lymphocytes (10 × 104) were diluted in chemotaxis buffer containing 1 μM BCA-1. Cells were then added to the upper wells. After 60 minutes of incubation for mature DCs and 120 minutes for the other cell types, the membrane was removed, washed on the upper side with phosphate-buffered saline (PBS), fixed, and stained. Migrated cells were counted at 1000-fold magnification in 5 randomly selected fields. For inhibition studies, cells were pretreated with 1 μg/mL Bordetella pertussis toxin (Calbiochem, Bad Soden, Germany) at 37°C for 30 minutes. To investigate if glycosaminoglycans (GAGs) are involved in mediating the synergism, cells were pretreated before chemokine-induced migration with heparinase I (2 U/mL), heparinase III (0.05 U/mL), and chondroitinase ABC (0.012 U/mL; all obtained from Sigma-Aldrich Chemicals) at 37°C for 1 hour. For experiments indicated, 500 ng/mL low-molecular-weight heparin (Sigma-Aldrich Chemicals) was included in all buffers used.
Receptor internalization
CCR7-transfected cells were incubated at 37°C with 300 nM SLC or 100 nM ELC for 30 minutes. Controls were performed under the same conditions at 4°C. To assess the influence of BCA-1 in SLC- or ELC-induced internalization, cells were sequentially stimulated with 1 μM BCA-1 for 15 minutes and 300 nM SLC or 100 nM ELC for the time indicated. Receptor expression was evaluated by flow cytometry using the rat anti-CCR7 antibody. Statistical analysis of the differing CCR7 expression was performed using the analysis of variance (ANOVA) test.
Receptor binding
Competition binding assays were performed with CCR7-transfected cells using 0.15 nM 125I-ELC (Amersham Pharmacia Biotech, Uppsala, Sweden) as previously described,32 and increasing concentrations of unlabeled SLC, BCA-1, or macrophage-derived chemokine (MDC). The difference between total and nonspecific binding, as determined in the presence of 1000 nM unlabeled SLC, was taken as 100%. The data were analyzed with PRISM (GraphPad Software, San Diego, CA) using nonlinear regression in a one-site binding model. To investigate if the synergy-inducing chemokine can bind to CCR7 in the presence of CCR7 agonists, we performed binding studies using 0.15 nM 125I-MDC or 0.15 nM 125I-macrophage inflammatory protein-1α (MIP-1α) in the absence or presence of unlabeled ELC. Nonbound radioactivity was separated by centrifugation through 6% BSA in PBS, and cell-bound radioactivity was determined by gamma counting. A regression analysis was carried out with the radioactivity percentage variation as compared with the value without ELC as the dependent variable, and the concentration of unlabeled ELC and the labeled chemokines as the independent variables.
ERK phosphorylation
CCR7-transfected cells were starved for 5 hours in RPM-1640 20 mM Hepes (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid) pH 7.4. Cells (106) were incubated in the same medium for 10 minutes at 37°C, and stimulated with SLC, BCA-1, or both chemokines for the indicated time. Activated ERK (extracellular signal-regulated kinase) was detected as previously described.16 Equal loading was confirmed by reprobing with an antibody against total ERK-2 (C-14; Santa Cruz Biotechnology, Santa Cruz, CA). Densitometric analysis of the bands obtained by Western blotting was carried out with ImageQuant software (Amersham Biosciences, Uppsala, Sweden).
Coimmunoprecipitation of SLC and BCA-1
SLC, BCA-1, or SLC plus BCA-1 (60 ng) were incubated at 37°C for 15 minutes in chemotaxis buffer. Chemokine solutions were incubated at 4°C overnight with goat polyclonal antibodies (4 μg) to SLC or BCA-1 (AF366 and AF801, respectively; R&D Research Systems, Minneapolis, MN) in 20 mM Tris, 5 mM EDTA (ethylenediaminetetraacetic acid), 1% Nonidet P40 (NP40), and 0.1 mM dithiothreitol (DTT). After incubation, a biotinylated rabbit antigoat antibody (8 μg; E0466; DAKO, Glostrup, Germany) was added and the solutions were incubated for an additional 2 hours. Immune complexes were isolated with 25 μL streptavidin Dynabeads (M-280; Dynal, Oslo, Norway). Samples were analyzed by Western blotting. Briefly, immunoprecipitates were separated on 11% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gels and transferred to polyvinylidene difluoride (PVDF) membranes. To detect chemokines, the membranes were incubated overnight with the anti-BCA-1 or anti-SLC antibodies diluted 1:500. Enhanced chemiluminescence was used for detection of horseradish peroxidase-conjugated secondary antibodies (SC 2020, Santa Cruz; 170-6516, Bio-Rad, Hercules, CA) following the manufacturer's instructions (34 080 West Pico Chemiluminescent; Pierce, Rockford, IL). The same procedure was used to analyze a solution containing SLC and MIP-1α, using anti-SLC (AF366; R&D Research Systems) and anti-MIP-1α antibodies (2G6, kindly provided by Dr Charles MacKay).
Results
BCA-1 enhances agonist-induced migration in CCR7-transfected cells
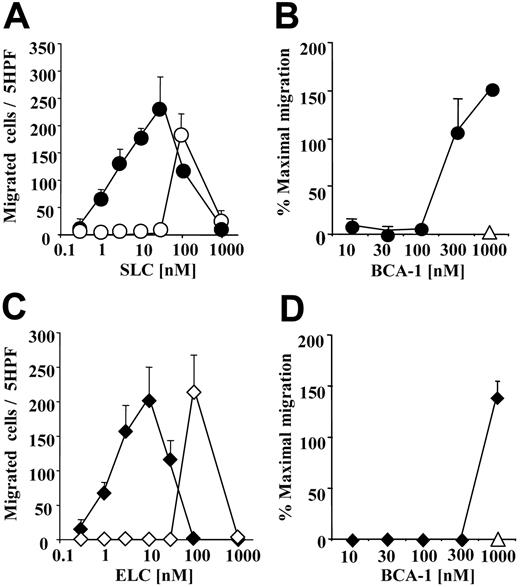
The selective CCR7 agonists, SLC and ELC, and the selective CXCR5 agonist, BCA-1, are concomitantly expressed in secondary lymphoid organs in discrete areas,29,33 and in inflammation in identical regions.34 We investigated if the presence of different concentrations of BCA-1 would influence the migration of CCR7+ cells to SLC or ELC, using murine pre-B cells transfected with CCR7. When 1 μM BCA-1 was applied to the lower wells of the chemotaxis chamber with increasing concentrations of SLC (Figure 1A) or ELC (Figure 1C), we observed an increase of 100-fold or greater in the potency of the chemotactic activity of the 2 agonists, as compared with the migration induced by the CCR7 agonists alone. This effect is synergistic because BCA-1, the agonist of CXCR5 and CXCR3,35,36 does not induce any cellular response via CCR7. A less pronounced effect was seen in SLC- and ELC-induced migration when 1 μM BCA-1 was added to the upper well together with the cells (migration to 10 nM SLC, mean ± standard deviation [SD], in the presence or absence of 1 μM BCA-1: 56 ± 22 and 6 ± 3 cells, respectively). In order to identify the minimal BCA-1 concentration required for the synergistic effect, we applied increasing concentrations of BCA-1 together with suboptimal chemotactic concentrations of SLC or ELC (10 nM), for which we did not detect migration per se. The BCA-1 dose necessary to enhance migration was 300 nM for SLC (Figure 1B), and 1 μM for ELC (Figure 1D).
BCA-1 enhances agonist-induced migration in CCR7-transfected cells. (A,C) Cell migration of CCR7-transfected cells was evaluated in Boyden microchambers. Chemotaxis was induced by SLC (circles) or ELC (diamonds) in the presence (filled symbols) or absence (open symbols) of 1 μM BCA-1. Mean ± SD of 3 independent experiments is shown. (B,D) Chemotaxis induced by 10 nM SLC (circles) or 10 nM ELC (diamonds) in the presence of increasing BCA-1 concentrations. In the absence of SLC or ELC, 1 μM BCA-1 alone does not induce migration of CCR7-transfected cells (▵). Mean ± SD of 3 independent experiments is shown. Cell count was performed in 5 high-power fields (5 hpf). Percentages of maximal migration were calculated taking as 100% the number of cells migrated to 100 nM SLC.
BCA-1 enhances agonist-induced migration in CCR7-transfected cells. (A,C) Cell migration of CCR7-transfected cells was evaluated in Boyden microchambers. Chemotaxis was induced by SLC (circles) or ELC (diamonds) in the presence (filled symbols) or absence (open symbols) of 1 μM BCA-1. Mean ± SD of 3 independent experiments is shown. (B,D) Chemotaxis induced by 10 nM SLC (circles) or 10 nM ELC (diamonds) in the presence of increasing BCA-1 concentrations. In the absence of SLC or ELC, 1 μM BCA-1 alone does not induce migration of CCR7-transfected cells (▵). Mean ± SD of 3 independent experiments is shown. Cell count was performed in 5 high-power fields (5 hpf). Percentages of maximal migration were calculated taking as 100% the number of cells migrated to 100 nM SLC.
This synergistic effect was completely unexpected and prompted further investigation. Hence, we performed the inverse experiment, using murine pre-B cells transfected with CXCR5. An optimal cell migration was obtained with 1000 nM BCA-1 (93 ± 2, mean ± SD), while we did not observed any migration at 100 nM. Surprisingly, 1 μM of either SLC or ELC induced the response of the CXCR5+ cells to 100 nM BCA-1 (117 ± 17 and 155 ± 6, respectively, mean ± SD). Taken together, these results hinted at the possibility that, in addition to the known chemokine receptor agonists and antagonists, the chemokine “milieu” of a tissue might modulate the responses of migrating cells as well.
BCA-1 enhances agonist-induced migration in CCR7+ human leukocytes
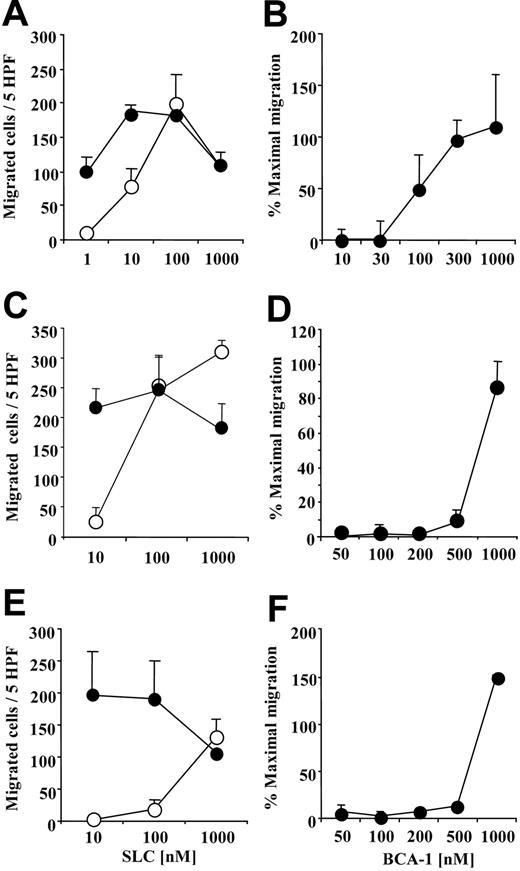
We investigated the synergistic effect of BCA-1 on cell migration for the 3 major CCR7+ leukocyte populations in order to determine if chemotaxis of primary human cells would be also enhanced. BCA-1 greatly augmented the migration to suboptimal concentrations of SLC on mature DCs (Figure 2A), B lymphocytes (Figure 2C), and naive T lymphocytes (Figure 2E). For B lymphocytes, which also express CXCR5, BCA-1 was applied together with the cells in order to desensitize CXCR5 receptor, as well as to the lower well. Under these conditions, no chemotaxis was observed in the absence of SLC. As for CCR7-transfected cells, dose-response studies were performed in order to assess the BCA-1 concentration required for the synergistic effect. The BCA-1 dose necessary to enhance migration was 100 nM for mature DCs (Figure 2B), 500 nM for B lymphocytes (Figure 2D), and 1 μM for naive T lymphocytes (Figure 2F).
BCA-1 enhances SLC-induced migration in human mature dendritic cells (DCs), B lymphocytes, and naive T lymphocytes. Chemotaxis of DCs (A), B lymphocytes (C), and T lymphocytes (E) induced by increasing concentrations of SLC in the presence (⬡) or absence (○) of 1 μM BCA-1. Chemotaxis of DCs (B), B lymphocytes (D), and T lymphocytes (F) induced by 10 nM SLC in the presence of increasing BCA-1 concentrations. For experiments with B lymphocytes, BCA-1 was applied to the upper well of the chemotaxis chamber together with the cells, and to the lower well. Mean ± SD of 3 independent experiments is shown. Percentages of maximal migration were calculated taking as 100% the number of cells migrated to 100 nM SLC for DCs, and to 1000 nM SLC for B and T lymphocytes.
BCA-1 enhances SLC-induced migration in human mature dendritic cells (DCs), B lymphocytes, and naive T lymphocytes. Chemotaxis of DCs (A), B lymphocytes (C), and T lymphocytes (E) induced by increasing concentrations of SLC in the presence (⬡) or absence (○) of 1 μM BCA-1. Chemotaxis of DCs (B), B lymphocytes (D), and T lymphocytes (F) induced by 10 nM SLC in the presence of increasing BCA-1 concentrations. For experiments with B lymphocytes, BCA-1 was applied to the upper well of the chemotaxis chamber together with the cells, and to the lower well. Mean ± SD of 3 independent experiments is shown. Percentages of maximal migration were calculated taking as 100% the number of cells migrated to 100 nM SLC for DCs, and to 1000 nM SLC for B and T lymphocytes.
BCA-1 enhances receptor internalization and ERK phosphorylation
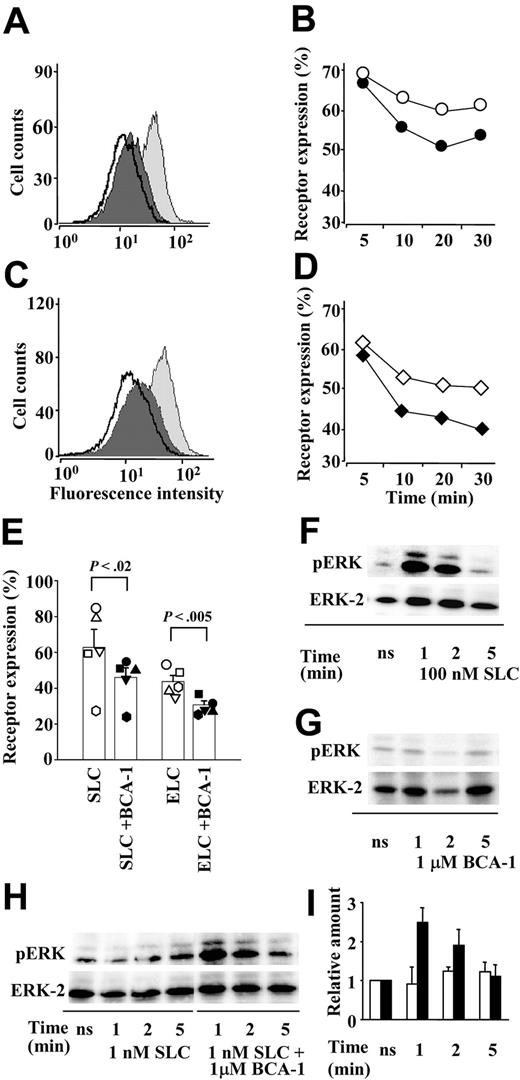
To characterize the synergistic effect of BCA-1 on SLC-induced responses more comprehensively, several cellular responses other than migration were investigated. Binding of agonist chemokines leads to receptor internalization, even though it has been shown previously37 that ELC is a significantly more potent inducer of CCR7 internalization than SLC. When CCR7 transfectants were sequentially stimulated with first 1 μM BCA-1 and then either 300 nM SLC (Figure 3A) or 100 nM ELC (Figure 3C), respectively, receptor internalization was augmented, compared with receptor internalization induced by the CCR7 agonists alone. In time course experiments with either 300 nM SLC (Figure 3B) or 100 nM ELC (Figure 3D), an accelerated internalization was observed in the presence of 1 μM BCA-1. This acceleration was minimal, but consistent. Therefore, we performed multiple experiments to assess CCR7 expression after a 30-minute stimulation with 300 nM SLC or 100 nM ELC in the presence or absence of 1 μM BCA-1. The results show that the difference between CCR7 expressions after stimulation with agonists alone or together with BCA-1 is statistically significant (Figure 3E). As for chemotaxis, BCA-1 activity on CCR7+ cells occurred only in the presence of the selective agonists, since 1 μM BCA-1 alone did not induce any CCR7 internalization (data not shown).
BCA-1 enhances receptor internalization and ERK phosphorylation in CCR7-transfected cells. BCA-1 enhances SLC- (A,B,E) and ELC- (C,D,E) induced CCR7 internalization. The histograms represent the fluorescence intensity of CCR7 in transfected cells unstimulated (light gray curves), stimulated with 300 nM SLC (A) or 100 nM ELC (C) (dark gray curves), or sequentially stimulated with 1 μM BCA-1 and SLC or ELC (bold line), as analyzed by flow cytometry. One representative experiment of 3 is shown. Time course of CCR7 internalization induced by 300 nM SLC (B) or 100 nM ELC (D) in the presence (closed symbols) or absence (open symbols) of 1 μM BCA-1. One of 2 independent experiments is shown. (E) CCR7 expression in cells stimulated for 30 minutes with 300 nM SLC or 100 nM ELC in the presence (filled symbols) or absence (open symbols) of 1 μM BCA-1. Columns represent mean ± SEM of 5 independent experiments. Differences between receptor expression after stimulation with the agonist or with the agonist plus BCA-1 are statistically significant. (F-G) ERK phosphorylation mediated by 100 nM SLC (F) or 1 μM BCA-1 (G) alone after times indicated. (H) ERK phosphorylation by a suboptimal dose (1 nM) of SLC and its synergistic increase in the presence of 1 μM BCA-1 after times indicated. pERK designates phosphorylated, and ERK-2 total ERK, respectively. One representative experiment of 3 is shown. (I) Densitometric analysis of the bands corresponding to phosphorylated ERK induced by SLC (1 nM; □) or SLC (1 nM) plus BCA-1 (1 μM; ▪). Values were normalized against their matching total ERK values, and the arbitrary value of 1 was given to the experiments obtained with unstimulated cells (ns). Mean ± SD of 3 independent experiments is shown.
BCA-1 enhances receptor internalization and ERK phosphorylation in CCR7-transfected cells. BCA-1 enhances SLC- (A,B,E) and ELC- (C,D,E) induced CCR7 internalization. The histograms represent the fluorescence intensity of CCR7 in transfected cells unstimulated (light gray curves), stimulated with 300 nM SLC (A) or 100 nM ELC (C) (dark gray curves), or sequentially stimulated with 1 μM BCA-1 and SLC or ELC (bold line), as analyzed by flow cytometry. One representative experiment of 3 is shown. Time course of CCR7 internalization induced by 300 nM SLC (B) or 100 nM ELC (D) in the presence (closed symbols) or absence (open symbols) of 1 μM BCA-1. One of 2 independent experiments is shown. (E) CCR7 expression in cells stimulated for 30 minutes with 300 nM SLC or 100 nM ELC in the presence (filled symbols) or absence (open symbols) of 1 μM BCA-1. Columns represent mean ± SEM of 5 independent experiments. Differences between receptor expression after stimulation with the agonist or with the agonist plus BCA-1 are statistically significant. (F-G) ERK phosphorylation mediated by 100 nM SLC (F) or 1 μM BCA-1 (G) alone after times indicated. (H) ERK phosphorylation by a suboptimal dose (1 nM) of SLC and its synergistic increase in the presence of 1 μM BCA-1 after times indicated. pERK designates phosphorylated, and ERK-2 total ERK, respectively. One representative experiment of 3 is shown. (I) Densitometric analysis of the bands corresponding to phosphorylated ERK induced by SLC (1 nM; □) or SLC (1 nM) plus BCA-1 (1 μM; ▪). Values were normalized against their matching total ERK values, and the arbitrary value of 1 was given to the experiments obtained with unstimulated cells (ns). Mean ± SD of 3 independent experiments is shown.
ERK belongs to a protein kinase family that is considered to play a crucial role in various signal transduction pathways upon activation by chemokines. In contrast to 100 nM SLC, which induced ERK phosphorylation (pERK; Figure 3F), 1 μM BCA-1 did not lead to ERK activation (Figure 3G) in CCR7-transfected cells. Using suboptimal concentrations of SLC (1 nM), which did not induce pERK per se, we could detect ERK activation when BCA-1 was applied together with the agonist (Figure 3H). Densitometric quantification revealed that 1 μM BCA-1 synergistically augmented pERK 2- to 3-fold (Figure 3I).
Several chemokines enhance migration of CCR7+ cells
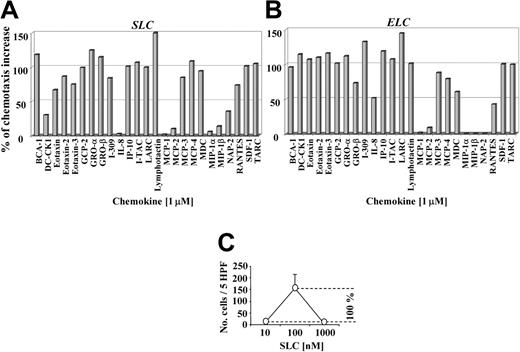
To assess if the synergistic effect on SLC- or ELC-induced migration is a unique property of BCA-1, we performed migration studies with 25 different homeostatic or inflammatory chemokines, applying them at 1 μM to the lower wells of the chemotaxis chamber together with a suboptimal chemotactic concentration of SLC or ELC (10 nM). Almost all chemokines tested elicited a synergistic effect on SLC- (Figure 4A) and ELC-induced migration (Figure 4B). It is important to underline that, when applied at 1 μM without SLC or ELC, they did not induce any migration of CCR7-transfected cells (data not shown).
Many chemokines enhance SLC- and ELC-induced migration in CCR7-transfected cells. CCR7-transfected cell migration induced by 10 nM SLC (A) or ELC (B) in the presence of 1 μM of different chemokines applied to the lower wells as indicated. Percentage values of the chemotactic increases were calculated as follows: the number of migrated cells at the suboptimal chemotactic concentration (10 nM SLC or ELC) was subtracted from the number of migrated cells at the optimal chemotactic concentration (100 nM SLC or ELC), with the difference representing 100% of the chemotactic increase (C). Synergistic effects are given as the percentage of the chemotactic increase. DC-CK1, dendritic cell-specific CC-chemokine; GCP, granulocyte chemotactic protein; GRO, growth-regulated oncogene; IP, interferon-inducible protein; NAP, neutrophil-activating peptide; RANTES, regulated on activation normal T cell expressed and secreted; SDF, stromal cell-derived factor; and TARC, thymus activated-regulated chemokine.
Many chemokines enhance SLC- and ELC-induced migration in CCR7-transfected cells. CCR7-transfected cell migration induced by 10 nM SLC (A) or ELC (B) in the presence of 1 μM of different chemokines applied to the lower wells as indicated. Percentage values of the chemotactic increases were calculated as follows: the number of migrated cells at the suboptimal chemotactic concentration (10 nM SLC or ELC) was subtracted from the number of migrated cells at the optimal chemotactic concentration (100 nM SLC or ELC), with the difference representing 100% of the chemotactic increase (C). Synergistic effects are given as the percentage of the chemotactic increase. DC-CK1, dendritic cell-specific CC-chemokine; GCP, granulocyte chemotactic protein; GRO, growth-regulated oncogene; IP, interferon-inducible protein; NAP, neutrophil-activating peptide; RANTES, regulated on activation normal T cell expressed and secreted; SDF, stromal cell-derived factor; and TARC, thymus activated-regulated chemokine.
In addition, we tested these 25 different chemokines on the B and T lymphocytes and the DCs. Interestingly, the synergistic effect was not only dependent on their concomitant presence, but also on the cell type. For SLC-induced migration on DCs (Figure 5A) and B lymphocytes (Figure 5B), we observed a synergistic effect with the same chemokines that had augmented the migration of CCR7-transfected cells. In contrast, only few chemokines revealed a synergistic activity on SLC-induced migration of T lymphocytes (Figure 5C). Again, none of the 25 chemokines we investigated showed chemotactic activity at 1 μM on any cell type per se (data not shown) with the exception of BCA-1, which, when applied to the lower wells, induced B lymphocyte migration, as expected. It is of note that some chemokines, such as MCP-1, MIP-1α, and MIP-1β, did not synergize with either SLC or ELC on any of the 4 cell types tested. Collectively, these results indicate that many chemokines are able to synergistically increase responses that are mediated by CCR7 and its agonists.
Many chemokines enhance SLC-induced migration in human leukocytes. Mature DC (A), B lymphocyte (B), and naive T lymphocyte (C) migration was induced by 10 nM SLC in the presence of 1 μM of different chemokines applied to the lower well as indicated. The percentage of the chemotactic increase was calculated as described in Figure 4.
Many chemokines enhance SLC-induced migration in human leukocytes. Mature DC (A), B lymphocyte (B), and naive T lymphocyte (C) migration was induced by 10 nM SLC in the presence of 1 μM of different chemokines applied to the lower well as indicated. The percentage of the chemotactic increase was calculated as described in Figure 4.
SLC forms heteromeric complexes with BCA-1 but not with MIP-1α
To determine if the synergistic effect of BCA-1 was due to an interaction with CCR7 or possibly some cellular component other than CCR7, we exposed parental 300.19 cells, which are devoid of CCR7, to the same combinations of BCA-1 and SLC used for CCR7-transfected cells, as described in Figure 1. We did not observe any migration under these conditions, indicating that CCR7 is a necessary component for migration as well as synergism (data not shown). Next, we preincubated CCR7 transfectants with 1 μM BCA-1 and, after washing, performed the same chemotaxis experiments as above. Under these conditions, cells pretreated with BCA-1 readily migrated toward SLC, but did not show an increase migration compared with cells pretreated with buffer only, arguing against a direct priming of CCR7 or of any other additional membrane component by BCA-1. To further characterize the mechanisms involved in chemokine-induced synergism, we performed chemotaxis with cells pretreated with Bordetella pertussis toxin, which prevents coupling of Gi proteins to G protein-coupled receptors. SLC-induced migration in the presence or absence of BCA-1 was significantly decreased (data not shown) suggesting that like agonist-induced migration, the synergistic effect of BCA-1 depends on functional Gi protein-coupled receptors.
Many chemokines are known to interact with GAGs, and this interaction is even required for full in vivo activity in certain cases.38 Shedding GAGs from the cell surface did not have any effect on chemotaxis induced by SLC alone, nor on the synergistic increase in the presence of 1 μM BCA-1 (migration of untreated or treated cells [mean ± SD] to 10 nM SLC in the presence of BCA-1: 72 ± 27 and 88 ± 29, respectively). Next, we tested chemotaxis in the presence of 500 ng/mL low-molecular-weight heparin. In accordance with a previous report,39 this treatment reduced the chemotactic potency of SLC (optimal cell migration at 1000 nM: 63 ± 10 [mean ± SD]), while the presence of 1 μM BCA-1 still led to a 10-fold-higher potency compared with SLC alone (migration to 100 nM SLC [mean ± SD] in the absence or presence of BCA-1: 10 ± 1 and 60 ± 7, respectively). These experiments clearly show that GAGs are not primarily involved in migratory synergism.
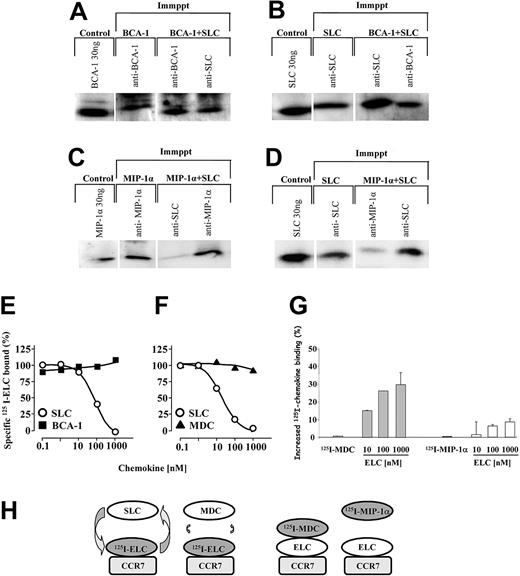
These findings led us to hypothesize that BCA-1 may bind to SLC in solution to form a complex, conferring to SLC a more potent chemotactic activity. In order to test the possible complex formation of SLC and BCA-1, the 2 chemokines (60 ng) were preincubated together in chemotaxis buffer for 15 minutes, and then immunoprecipitated with either anti-SLC or anti-BCA-1 antibodies. Immunoblot analysis revealed that both immunoprecipitates contained SLC and BCA-1 (Figure 6A-B), indicating that in solution, the chemokines bind to each other. The antibodies used for the immunoprecipitation and the detection were specific and did not cross-react with other chemokines in Western blotting or immunoprecipitation. We performed the same analysis using SLC and MIP-1α, a chemokine that did not display synergism in previous migration experiments. Under these conditions, the immunoprecipitation experiments failed to reveal the formation of heteromeric complexes between SLC and MIP-1α (Figure 6C-D).
Synergy-inducing chemokines form heteromeric complexes with SLC, do not interfere with the binding of CCR7 ligands, but can bind CCR7 in the presence of ELC. SLC, BCA-1, or SLC plus BCA-1 were subjected to immunoprecipitation (Immppt) with anti-BCA-1 or anti-SLC antibodies. The samples were then analyzed by Western blotting and the presence of chemokines was detected with anti-BCA-1 (A) or anti-SLC (B) antibodies. (C-D) MIP-1α or SLC plus MIP-1α were subjected to immunoprecipitation with anti-SLC or anti-MIP-1α antibodies. The samples were then analyzed by Western blotting, and the presence of MIP-1α or SLC detected with a specific antibody. (E-F) CCR7-transfected cells were incubated with 0.15 nM 125I-ELC and increasing concentrations of unlabeled SLC (○), BCA-1 (▪), or MDC (▴). The curves (means of duplicate determinations) are representative for 1 of 3 experiments performed. (G) CCR7-transfected cells were incubated with 0.15 nM 125I-MDC or 0.15 nM 125I-MIP-1α, as indicated, and increasing concentrations of unlabeled ELC. Data (mean ± SD) are shown as percentage of the increase of 125I-chemokine binding obtained in 3 independent experiments. The difference between the enhancements observed with 125I-MDC and 125I-MIP-1α in the presence of ELC is statistically significant (P < .005). (H) Schematic representation of the binding events corresponding to panels D-F. Please refer to text (“Synergy-inducing chemokine binds to CCR7 in the presence of ELC”) for details.
Synergy-inducing chemokines form heteromeric complexes with SLC, do not interfere with the binding of CCR7 ligands, but can bind CCR7 in the presence of ELC. SLC, BCA-1, or SLC plus BCA-1 were subjected to immunoprecipitation (Immppt) with anti-BCA-1 or anti-SLC antibodies. The samples were then analyzed by Western blotting and the presence of chemokines was detected with anti-BCA-1 (A) or anti-SLC (B) antibodies. (C-D) MIP-1α or SLC plus MIP-1α were subjected to immunoprecipitation with anti-SLC or anti-MIP-1α antibodies. The samples were then analyzed by Western blotting, and the presence of MIP-1α or SLC detected with a specific antibody. (E-F) CCR7-transfected cells were incubated with 0.15 nM 125I-ELC and increasing concentrations of unlabeled SLC (○), BCA-1 (▪), or MDC (▴). The curves (means of duplicate determinations) are representative for 1 of 3 experiments performed. (G) CCR7-transfected cells were incubated with 0.15 nM 125I-MDC or 0.15 nM 125I-MIP-1α, as indicated, and increasing concentrations of unlabeled ELC. Data (mean ± SD) are shown as percentage of the increase of 125I-chemokine binding obtained in 3 independent experiments. The difference between the enhancements observed with 125I-MDC and 125I-MIP-1α in the presence of ELC is statistically significant (P < .005). (H) Schematic representation of the binding events corresponding to panels D-F. Please refer to text (“Synergy-inducing chemokine binds to CCR7 in the presence of ELC”) for details.
Synergy-inducing chemokine binds to CCR7 in the presence of ELC
Collectively, the evidence suggests that a complex of an agonistic (ELC, SLC) and a synergistic chemokine binds to the receptor (CCR7), activating it more strongly than the agonistic chemokine by itself. To confirm this hypothesis, we first performed competition binding assays on CCR7-transfected cells to investigate if synergy-inducing chemokines interfere with the binding of CCR7 ligands. As shown in Figure 6E-F, SLC displaces 125I-ELC from the receptor, whereas neither BCA-1 (Figure 6E) nor MDC (Figure 6F), even at high concentrations, was able to compete for ELC binding. These results indicate that BCA-1 and MDC do not share their binding site with ELC either partially or completely, or do not bind CCR7 at all. More importantly, they suggest that ELC binding to CCR7 is not severely affected by the presence of BCA-1 or MDC. As shown in Figure 6G, 125I-MDC does not bind to CCR7 in the absence of ELC, but it does if ELC is added to the reaction, even at very low MDC concentrations. 125I-MIP-1α does not bind to CCR7, and in addition, the difference between the enhancements observed with 125I-MDC and 125I-MIP-1α in the presence of ELC is statistically significant (P < .005). These data support that heteromeric complexes formed by MDC and ELC can bind to CCR7.
A mixture of different synergy-inducing chemokines act equivalently to one alone
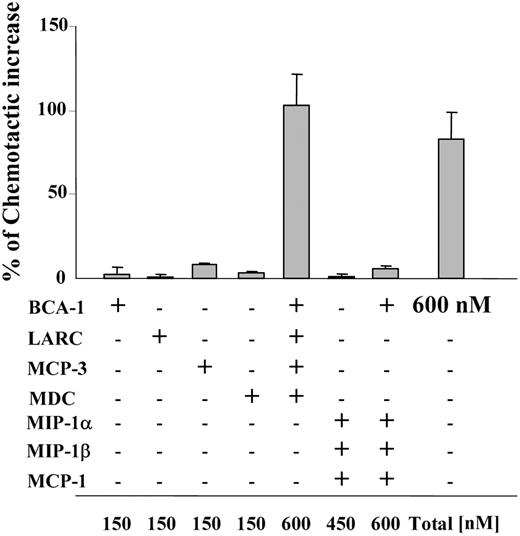
In order to synergistically increase chemotaxis, chemokine concentrations of 300 nM or greater were necessary for most cell types (Figures 1 and 2). While homeostatic receptors such as CCR7 or CXCR5 often require concentrations in this range for maximal migration, inflammatory chemokines are typically able to induce maximal responses at concentrations at least 10-fold lower, raising the question if migratory synergism might ever occur for these chemokines. However, concomitant expression of several chemokines is found under many homeostatic and inflammatory conditions, which led us to investigate if combinations of such chemokines could cooperate in enhancing SLC-induced migration. Hence, we applied 4 synergy-inducing chemokines, each at a concentration that did not enhance SLC-induced migration per se (150 nM), together with a suboptimal dose of SLC (10 nM). Of note, BCA-1, MCP-3, MDC, and LARC (liver and activation-regulated chemokine) induced the same effect as BCA-1 alone at 600 nM when applied together (Figure 7). In contrast, when we used a combination of BCA-1 with 3 of the chemokines that did not enhance SLC-induced migration (MCP-1, MIP-1α, and MIP-1β), the same final concentration of 600 nM was not capable of enhancing the agonist response. Synergy-inducing chemokines can thus act “in concert,” making it more likely that concentrations sufficient to elicit synergism may be reached in tissues.
At identical final concentrations, combinations of synergy-inducing chemokines are equivalent in their potency compared with a single synergy-inducing chemokine. CCR7-transfected cell migration to 10 nM SLC in the presence of different chemokines at 150 nM (if not indicated differently in the figure). Mean ± SD of the chemotactic increase of 3 independent experiments is shown. The percentage of the chemotactic increase was calculated as described in Figure 4.
At identical final concentrations, combinations of synergy-inducing chemokines are equivalent in their potency compared with a single synergy-inducing chemokine. CCR7-transfected cell migration to 10 nM SLC in the presence of different chemokines at 150 nM (if not indicated differently in the figure). Mean ± SD of the chemotactic increase of 3 independent experiments is shown. The percentage of the chemotactic increase was calculated as described in Figure 4.
Taken together, the present data indicate that (1) CCR7 agonists are more potent inducers of migration in the presence of synergy-inducing chemokines; (2) they can form heteromeric complexes with synergy-inducing chemokines, and (3) the complexes bind to CCR7.
Discussion
Our results clearly demonstrate that many chemokines, which per se have no effect on a particular chemokine receptor, can greatly augment the cellular response to specific receptor's agonist(s). The increase is dose dependent and requires concentrations more than 100 nM of the (per se inactive) synergy-inducing chemokines to occur. Hence, the enhancement is synergistic as, in the presence of synergy-inducing chemokines, cells migrate vigorously at agonist doses that do not induce any effect when applied alone. Notably, synergy-inducing chemokine “mixtures” proved as effective as single chemokines at corresponding concentrations at eliciting chemotactic synergism.
Our data are consistent with a mode of action where agonist and synergy-inducing chemokines first form a heteromeric complex, in the presence of which the triggering of the chemokine receptor can occur at much lower agonist concentration than the one needed in the presence of the agonist alone. The synergistic increase is transmitted by the chemokine receptor: the chemokine complex composed of ELC and MDC still bound to CCR7, and no synergism was observed with cells devoid of chemokine receptors.
The majority but not all of the chemokines analyzed featured synergistic activity on CCR7, and suggest the existence of complexes that are formed between the agonist and the synergistic chemokine as the cause of this effect. The advent of heteromeric complexes should not be too surprising: many chemokines are known to form homodimers,40 homomeric complexes of higher order,41,42 and heterodimers.43,44 The size, stochiometry, and shape of the synergy-inducing complexes remain to be elucidated for now. In the chemokine structure, the N-terminal domain is the most unordered part, as demonstrated for many chemokines by heteronuclear magnetic resonance spectroscopy.45-48 In the case of CCR7, the synergistic chemokine could, by forming a complex with the agonists, induce agonist structure changes that might fix its N-terminal domain, mask or exposing residues that are critical for conferring to the agonist an optimal capability for receptor triggering. Conversely, it has recently been proposed that the lower binding affinity of interleukin 8 (IL-8) dimer is not due to structural constraints but rather to its reduced conformational flexibility. Dimerization would therefore lock the protein into a low-affinity conformation.49 Of note, IL-8 is among the few chemokines that do not synergize with CCR7 ligands. Moreover, the responses induced by IL-8 on CXCR1 cannot be enhanced in the presence of synergy-inducing chemokines (B.O.G. and V.P., unpublished data, March 12, 2003). Conversely, in the case of CCR7 agonists, the heterocomplex formation between CCR7 agonists and synergy-inducing chemokines could lock SLC or ELC into a higher affinity conformation. Likewise, it is possible that the presence of a synergy-inducing chemokine might favor the homodimerization of chemokine receptors induced by a minimal agonist concentration.50
Further, our finding of a reciprocal synergistic effect of BCA-1 and SLC on agonist-induced responses of CCR7+ and CXCR5+ cells respectively, suggests that it may be a common feature of chemokine receptors to respond with enhanced activity when exposed to sufficient doses of nonagonist, synergy-inducing chemokines.
Chemotactic synergism, induced by a tissue environment rich in chemokines, would decrease the threshold and increase the dynamic range of leukocyte migration. While moving toward the agonist source and away from the source of the synergy-inducing chemokine(s), they would eventually be exposed to decreasing concentrations of the latter. As the synergistic effect itself is dose dependent, it would abate over time, slowly depriving the migrating cell of its increased sensitivity, and leave it finally with its normal capacity to respond to the agonist. Hence, the synergistic effect would act in concert with homologous receptor desensitization, rendering the cell less and less responsive and thus capable of detecting higher and higher agonist concentrations. Effectively, the migratory synergism would “flatten” the agonist gradient, and thus provide the cells with a wider dynamic range.
In vitro, substantial chemokine concentrations more than 100 nM were necessary to induce migratory synergism. Given the lack of any precise measurements about chemokine concentrations in vivo, it might be best to compare the threshold concentration for synergism to the concentrations required to induce chemotaxis in vitro. When using a standardized cellular background (300.19 murine pro-B cells), the receptors involved in homeostasis (CCR4, CCR6, CCR7, CXCR4, and CXCR5) require fairly high chemokine concentrations of 100 nM to 1000 nM to induce an optimal migration, whereas inflammatory receptors (CCR1-3, CCR5, and CXCR1-3) typically require lower agonist concentrations ranging between 10 nM and 100 nM. Therefore, it is reasonable to assume that homeostatic chemokines might be expressed at high enough concentrations to induce synergism, whereas inflammatory chemokines would be present at concentrations too low. However, inflammatory chemokines are just as likely to exert synergism: multiple chemokine expression in many inflamed tissues is well documented,4-7 and, as shown in Figure 7, a combination of chemokines reaching a threshold concentration was equal in its synergistic potential to the one induced by a single chemokine alone at the same concentration. Of note, synergistic activities of chemokines on leukocyte migration were shown recently by other groups. However, the authors ascribed the synergism observed to the concomitant expression of different chemokine receptors.8-10
The leukocytes that were analyzed in the present study express other chemokine receptors in addition to CCR7. Mature DCs express CCR7,51-53 CXCR4,54 and very low levels of CCR1 and CCR5.54 B lymphocytes express CCR7,55 CCR6,56,57 CXCR4,58,59 and CXCR5.35,60 Naive T lymphocytes express CCR761,62 and CXCR4.63 We cannot exclude that for a few of the chemokines tested, which bind and activate the above receptors, 2 mechanisms can concomitantly occur to induce the synergism observed: the presence of the chemokine heterocomplexes, and a possible but not-yet-characterized cross-talk.
It is tempting to make a few specific predictions about the potential consequences of migratory synergism for homeostatic and inflammatory trafficking of CCR7+ leukocytes such as mature DCs and lymphocytes. Upon antigen encounter and processing in inflamed tissues, DCs mature and change their chemokine receptor repertoire: inflammatory receptors such as CCR1, CCR2, and CCR5, as well as CCR6, are down-regulated, while CCR7 is newly expressed.51,54 This changing expression pattern provides a molecular basis for lymphoid tissue homing of mature DCs. Many inflammatory chemokines, either alone (Figures 4 and 5) or in concert (Figure 7), induce migratory synergism on CCR7+ cells. As a consequence, the inflamed tissue, “saturated” with such chemokines, would not only attract immature DCs in a first step, but subsequently increase the capacity of mature DCs to home back to secondary lymphoid organs as well. BCA-1, ordinarily considered a homeostatic chemokine, is expressed in pathologic conditions as well34,64,65 and might serve the same purpose under these conditions.
B lymphocytes express CXCR5 and home to BCA-1-expressing follicular zones of secondary lymphoid organs.35,66-68 After antigen binding and B-cell-receptor (BCR) triggering, they move to the boundary between B- and T-cell zones to interact with T-helper cells.69 Clearly, synergistic priming of antigen-presented CCR7+ cells by BCA-1 in follicular zones would serve to enhance this movement. A similar but retrograde effect can be imagined for follicular B-helper T cells that acquire CXCR5 after antigen presentation by lymphoid DCs70 : in accordance with our preliminary data on CXCR5, SLC and/or ELC would increase the responsiveness of CXCR5 to BCA-1.
T cells responded to a more restricted set of chemokines in a synergistic way, and the reason for this difference is unclear at present. Our results indicate that the chemokines have different potencies in inducing synergism. T lymphocytes might thus respond to the more potent ones only. Alternatively, an unidentified cellular component may be required for full synergism that is absent in T cells but present in the other cell types, or the lipid composition of the plasma membrane in T cells may differ sufficiently to allow for a more restricted repertoire of synergy-inducing chemokines only. Still, several chemokines such as BCA-1, eotaxin, eotaxin-2, eotaxin-3, interferon-inducible protein-10 (IP-10), interferon-inducible T-cell alpha chemoattractant (I-TAC), and lymphotactin were capable of inducing migratory synergism on T lymphocytes. Thus, synergy of BCA-1 on CCR7 might help in relocating follicular B-helper T cells to the T-cell zone after delivery of B-cell help. CCR7+ central memory T cells are known to coexpress other chemokine receptors in a subset-specific way: expression of CXCR3 and CCR3 is correlated with a Th1 or Th2 phenotype, respectively.71 Therefore, IP-10 and I-TAC, or eotaxin, eotaxin-2, and eotaxin-3, respectively, could synergize in repositioning central memory T lymphocytes to secondary lymphoid tissues after having recruited them into inflamed tissues first.
In short, we have presented evidence for a novel, positive regulatory mechanism in leukocyte trafficking. According to our data, various, nonagonist chemokines are capable of associating with known agonists of chemokine receptors, resulting in a stronger cellular response. As a consequence, inflamed and other “chemokine-rich” tissues would create an environment that renders many leukocyte types more competent to respond to migratory cues.
Prepublished online as Blood First Edition Paper, November 16, 2004; DOI 10.1182/blood-2004-04-1648.
Supported by the Helmut Horten Foundation, the Roche Research Foundation, the Swiss National Science Foundation (grant 3100A0 104 237/1 [M.U.]), and the EU FP5 and FP6 (BBW grants 02.0404 and 03.0441-1).
An Inside Blood analysis of this article appears in the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We dedicate this work to the memory of Ian Clark-Lewis. We thank Maria Pia Sormani for statistical analysis of the binding data, and Federica Sallusto and Alfonso Martin-Fontecha for critical reading of the manuscript.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal