Comment on Salomoni et al, page 3686
This report by Salomoni and colleagues demonstrates the pivotal role that PML plays in the molecular pathways regulating the induction of apoptosis upon distinct genotoxic stresses.
The promyelocytic leukemia protein (PML), as the name suggests, was first identified in acute promyelocytic leukemia (APL), which arises due to a reciprocal translocation between chromosomes 15 and 17. This unfortunate genetic event results in the expression of a fusion protein containing PML and retinoic acid receptor alpha (PML-RARα).1 A specific block in hematopoietic differentiation results from PML-RARα expression, manifesting itself as an accumulation of promyelocytes in APL patients. However, the clinical prognosis is good, as treatment using all trans retinoic acid and/or arsenic trioxide usually results in complete remission and an unblocking of the differentiation block. Nevertheless, the discovery of PML as an important tumor suppressor that is inactivated in APL led to numerous studies aimed at determining its normal cellular function, and we now know that PML has functional roles in transcriptional regulation, cellular senescence, apoptosis, and DNA repair. However, the molecular mechanisms by which PML mediates these activities, and in particular how PML mediates apoptosis after genotoxic stress, are only partly known. The paper by Salomoni and colleagues now provides a major advance in our understanding of this latter pathway, as they have identified a novel PML-dependent pathway for c-Jun transcriptional activation and induction of apoptosis in response to DNA damage.
Over the past 6 years or so, the Pandolfi laboratory and others have published a number of important papers showing that upon cellular stress such as gamma-irradiation, PML acts as a p53 transcriptional coactivator by recruitment and consequential acetylation by CBP (cyclic adenosine monophosphate [cAMP] response element-binding protein [CREB]-binding protein).2 In addition, they also showed that PML-/- mice and cells are protected from several p53-independent apoptotic stimuli.3 In this new study they now demonstrate that PML-/- fibroblasts are less sensitive to UV irradiation than PML+/+ cells and that PML is required for UV-induced apoptosis. Furthermore, they show that the mechanism of such protection is due to the functional interplay between PML and c-Jun upon UV exposure. The role of c-Jun in UV-induced apoptosis is not without controversy, but the demonstration that PML is able to regulate apoptosis in a p53-independent manner that involves the transcriptional activation of c-Jun was unexpected. Salomoni and colleagues show that overexpression of PML enhances UV-triggered c-Jun transcriptional activation and this coactivation of c-Jun by PML is only observed in UV-irradiated cells.
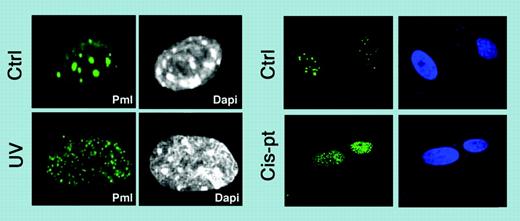
One of the most interesting aspects of PML function is the localization of PML to nuclear protein aggregates, termed nuclear bodies (NBs) or ND10, the proper formation of which requires PML.4 These NB structures are highly dynamic and their morphology changes in response to cellular stress.5 Remarkably in this new study, Salomoni and colleagues show that upon UV irradiation and cis-platinum treatment, PML relocalizes into microspeckle structures (termed UV-NBs) that dynamically accumulate c-Jun (see figure) and do not result from an increase in PML protein expression or changes in the pattern of PML small ubiquitin-like modifier-ylation (SUMOylation). Interestingly, upon UV treatment only phosphorylated c-Jun colocalizes with PML in UV-NBs, possibly via a direct physical interaction between PML and c-Jun. What about in APL? Intriguingly, the authors find that PML-RARα acts as a constitutive c-Jun transcriptional coactivator, which sets up the hypothesis that PML-RARα triggers the oncogenic potential of c-Jun, whereas normal PML regulates the UV-dependent proapoptotic function of c-Jun. What is more, in some aggressive cases of APL, point mutations of PML have been identified and it is tempting to speculate that these mutants might be defective in activating c-Jun upon DNA damage, thereby further protecting APL cells from DNA-damage-induced apoptosis. There is much to ponder with this new study, which will excite not only researchers interested in understanding the regulation of c-Jun but also those interested in PML NBs and how their reorganization leads to potentially different functional readouts. ▪
PML relocalizes into microspeckle structures. Relocalization upon UV irradiation and upon cisplatin treatment. See the complete figure in the article beginning on page 3686.
PML relocalizes into microspeckle structures. Relocalization upon UV irradiation and upon cisplatin treatment. See the complete figure in the article beginning on page 3686.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal