Comment on Arruda et al, page 3458
The authors have shown sustained correction of bleeding in hemophiliac dogs using a new method of viral vector–mediated gene transfer to skeletal muscle.
The blight of hemophilia is well documented, dating back to ancient times. Two distinct forms of the disease (A and B) were recognized over 50 years ago. Hemophilia A (factor VIII deficiency; “classic” hemophilia) affects 1 in 10 000 live male births, whereas hemophilia B (factor IX deficiency; Christmas disease) is less common, affecting about 1 in 35 000 male births. Even though modern approaches using recombinant DNA technologies to produce clotting factors have greatly enhanced the quality and length of life, such therapies remain expensive and cumbersome. Given that both hemophilias are single-gene defects, the lure of gene transfer as a permanent cure has been like the Siren's song.
Early results in large animal models had suggested that hemophilia B might be easily treated using gene transfer,1 but these preclinical results could not be reproduced in human subjects in early phase 1 trials.2,3 The issue appeared to be that an effective dose of vector could not be practically delivered to skeletal muscle in humans. In this issue of Blood, Arruda and colleagues report a new technique that ostensibly overcomes the hurdle of dose delivery. Using the canine model of hemophilia B, the authors showed that extensive regions of skeletal muscle could be transduced using recombinant adeno-associated virus (rAAV) vectors carrying the canine factor IX gene. They borrowed a technique previously tested in rodents wherein the limb vasculature is isolated, clamped, and infused with papaverine followed by a mixture of vector and histamine.4 The hemophiliac dogs so treated were found to have a rise in circulating factor IX levels from less than 1% of normal to 4% to 14%. Importantly, these levels have remained undiminished for over 3 years. Clinically, the treated dogs have had one tenth the bleeding episodes of untreated animals over the same period, thereby shifting the disease phenotype from severe to mild. At the cellular level, factor IX synthesized in the muscle was biologically active as demonstrated by the high correlation between antigen and activity. Inhibitor formation, a potential show-stopping side effect, was blocked by transient immunosuppression with cyclophosphamide at the time of vector delivery.
These data have implications beyond the clotting world. Any disorder that requires large-scale transduction of skeletal muscle is fair game. For example, gene transfer for certain muscular dystrophies could be carried out in this same fashion. Also, delivery of therapeutic molecules for a variety of disease states is worthy of consideration.
There are at least 2 potential drawbacks to this approach. First, the technique is highly invasive and requires a certain level of expertise. Second, even with isolation of the limb vasculature, vasoactive drugs must be given during the procedure to achieve efficient vector delivery. Less invasive alternatives can and should be sought and tested in the same dog model. However, this general approach has great merit and is likely to be successful when transferred to human subjects. Indeed, this dog might hunt. ▪
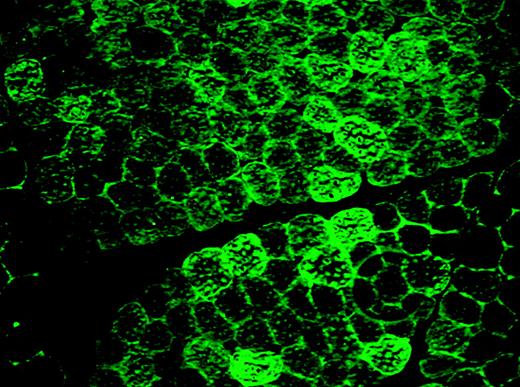
Histology of normal dog muscle 8 weeks after intravascular vector delivery by isolated limb perfusion. Immunofluorescence staining for canine F.IX, showing extensive positive staining. See the related figure in the article beginning on page 3458.
Histology of normal dog muscle 8 weeks after intravascular vector delivery by isolated limb perfusion. Immunofluorescence staining for canine F.IX, showing extensive positive staining. See the related figure in the article beginning on page 3458.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal