Abstract
Latent membrane protein 1 (LMP-1) of Epstein-Barr virus (EBV) promotes tumorigenesis by inhibiting apoptosis. We show that an important antiapoptotic activity of LMP-1 is the inhibition of Bcl2-associated protein X (Bax), a potent proapoptotic protein. BAX expression was regulated by LMP-1 activation of nuclear factor κB (NF-κB) via the C-terminal activation region 1 (CTAR-1) and CTAR-2. Interestingly, p65/p50 inhibited, whereas p50/p50 increased, BAX promoter activity as demonstrated by overexpression and selective inhibition of these NF-κB isoforms. Electrophoretic mobility shift analysis revealed that LMP-1 activates 2 of the 3 NF-κB binding sites (κB1-κB3) in the BAX promoter. LMP-1 induced binding of the NF-κB heterodimer p65/p50 to the κB2 site and of the p50/p50 homodimer to the κB3 site. Promoter mutation analysis revealed that the κB2 site is necessary for inhibition of BAX promoter activity and the κB3 site, for its activation. However, the activation of the BAX promoter by LMP-1 was observed only in the presence of specific inhibitors of p65/p50. In all other cases, LMP-1 inhibited BAX promoter activity. Most importantly, the antiapoptotic activity of LMP-1 was considerably decreased in cells deficient for BAX. These results indicate that the inhibition of Bax may be an important antiapoptotic activity of LMP-1. (Blood. 2005;105:3263-3269)
Introduction
Epstein-Barr virus (EBV) latent infection is associated with malignancy.1 The latent membrane protein 1 (LMP-1) encoded by EBV plays a potential key role in the pathogenesis of EBV-associated malignancies such as Hodgkin lymphoma2 and nasopharyngeal carcinoma.3 The mechanisms by which LMP-1 affects tumorigenesis are not fully understood.
LMP-1 consists of 386 amino acids. Its carboxyterminal cytoplasmic domain contains 2 C-terminal activation regions (CTARs), CTAR-1 and CTAR-2. CTAR-1 binds to tumor necrosis factor (TNF) receptor-associated factors (TRAFs) through a PxQxT motif 4,5 ; whereas, CTAR-2 binds to the TNF receptor-associated death domain (TRADD) through the tyrosine residue at amino acid position 384 (Tyr384).6 Binding of LMP-1 to TRAF and TRADD initiates the formation of a signaling complex6-8 that leads to activation of the nuclear factor κB (NF-κB) and p38/mitogen-activated protein kinase (MAPK) signaling pathways.9-11 In addition, LMP-1 activation of the Janus kinase (JNK) signaling pathway is mediated through CTAR-1 and CTAR-28,12,13 (Figure 2A).
Mutations in CTAR-1 and CTAR-2 abrogate the inhibitory effect of LMP-1 on BAX promoter activity. (A) Schematic presentation of LMP-1 and LMP-1-activated signaling pathways. Alterations in mutated LMP-1 proteins are indicated (PQT > AAA, Y384G, ΔCOOH). (B) HEK 293-T cells were cotransfected with plasmids encoding wild-type LMP-1 or various mutants of LMP-1 (see Figure 2A) and with pBAX-CAT. The expression of wild-type LMP-1 and the respective mutants was analyzed by Western blot. Detection of GAPDH was included as loading control. (C) Quantitative evaluation of CAT assay of HEK 293-T cells transfected with pBAX-CAT (left side) or pSV2-CAT (right side) and wild-type or mutant pLMP-1. Mean values and standard deviations of 3 independent experiments are shown; *P < .05.
Mutations in CTAR-1 and CTAR-2 abrogate the inhibitory effect of LMP-1 on BAX promoter activity. (A) Schematic presentation of LMP-1 and LMP-1-activated signaling pathways. Alterations in mutated LMP-1 proteins are indicated (PQT > AAA, Y384G, ΔCOOH). (B) HEK 293-T cells were cotransfected with plasmids encoding wild-type LMP-1 or various mutants of LMP-1 (see Figure 2A) and with pBAX-CAT. The expression of wild-type LMP-1 and the respective mutants was analyzed by Western blot. Detection of GAPDH was included as loading control. (C) Quantitative evaluation of CAT assay of HEK 293-T cells transfected with pBAX-CAT (left side) or pSV2-CAT (right side) and wild-type or mutant pLMP-1. Mean values and standard deviations of 3 independent experiments are shown; *P < .05.
Inhibition of apoptosis is a key feature of EBV tumorigenicity. LMP-1 inhibits apoptosis in latently EBV-infected cells.14,15 Inhibition of apoptosis in EBV-infected cells correlates with the induction of antiapoptotic factors such as Bcl-2, A20, and Bfl-1 by LMP-1.14-18 Since apoptosis is regulated through the balance of both proapoptotic and antiapoptotic genes,19 the mechanism by which LMP-1 inhibits apoptosis in virally infected cells may involve the regulation of both pro- and antiapoptotic genes. Accordingly, Bcl-2 and Bfl-1, which are up-regulated by LMP-1, inhibit apoptosis by sequestration of the proapoptotic factor Bax (Bcl2-associated protein X).20,21 Additionally, decreased Bax levels have been observed in various tumors, including EBV-associated Hodgkin disease,22 and associated with poor prognosis in lung23 and stomach carcinomas.24 Inactivating mutations of BAX have been detected in tumors of the colon25 and in EBV-negative Burkitt lymphomas.26,27 Collectively, these findings suggest that functional deactivation of BAX and/or inhibition of BAX expression may be involved in EBV-associated lymphomas.
Here, we show that LMP-1 inhibits the expression of BAX via NF-κB p65/p50 signaling, and we provide evidence that BAX may be an important target of the antiapoptotic activity of LMP-1.
Materials and methods
Cells, plasmids, and inhibitory drugs
The cell lines DG-75,26 EBV-negative Akata (an Akata subclone that has lost the virus and has been shown to be EBV negative28 ), HeLa, and CHO were kept in RPMI 1640/10% fetal calf serum (FCS; Gibco-BRL, Karlsruhe, Germany); BC-1,29 in RPMI 1640/20% FCS; and HEK 293-T, in DMEM/10% FCS. 1852.4 human B cells30 were grown in RPMI 1640/10% tetracycline-free FCS (Clontech, Heidelberg, Germany) with 2 μg/mL tetracycline.
The plasmids pSV2-CAT,31 pHIV-1-LTR-CAT,32 pro237-GBP-1,33 pHEBo,34 pHEBo-LMP-1 (pLMP-1) and its derivates pLMP-1(PQT>AAA), pLMP-1(Y384G), pLMP-1(PQT>AAA/Y384G), and pLMP-1(ΔCOOH),12 and pIκBα(S32/36A)35 were previously described. pBAX-CAT contains nucleotide (nt) -1416 to nt -317 relative to the translation start of the BAX gene including 3 κB consensus sites at nts -702 to -693 (κB1), -687 to -678 (κB2), and -502 to -493 (κB3).36 pBAX-CATm-κB2 and pBAX-CATm-κB3 are mutants of pBAX-CAT, with the nucleotide exchanges described in “Electrophoretic mobility shift assay (EMSA).” pK15 contains a cDNA of human herpesvirus 8 (HHV-8) open reading frame (ORF) K15 encoding a 23-kDa product37 in the expression vector pcDNA-3. Expression plasmids for p65, p50, and IRF-1 in pCMV-T38 were provided by Dr John Hiscott (McGill University, Montreal, QC).
siRNA to p65, p50, and a sequence-scrambled control with previously published sequences39,40 were purchased from MWG Biotech (Ebersberg, Germany). The plasmid psi-BAX encoding siRNA to Bax was constructed by cloning the published siRNA sequence41 in the vector pSuppressorRetro (Biocarta, Hamburg, Germany). Accordingly, a sequence-scrambled control siRNA was cloned.
Caffeic acid phenetyl ester (CAPE; Calbiochem, Schwalbach, Germany), SN50 (Biomol, Hamburg, Germany), and Bay11-7082 (Calbiochem) were added daily at 5 μg/mL, 100 μg/mL, and 1 μM, respectively.
Transfection
Adherent cells were transfected with Effectene (Qiagen, Hilden, Germany). Nonadherent cells were electroporated in 4-mm cuvettes in an Easyject (EquiBio, Maidstone, United Kingdom). Consistent transfection efficiency was monitored by cotransfection of pFRED encoding the green fluorescent protein (gift from Dr Severine Démart, GSF, Neuherberg, Germany).
CAT assays
At 3 days after transfection with the reporter plasmid containing the chloramphenicol-acetyltransferase (CAT) gene and the effector plasmid or the plain vector as a reference, cells were ruptured by freeze-thawing in 0.25 M Tris (tris(hydroxymethyl)aminomethane) HCl, pH 7.8. Equal amounts of protein were used in the standard CAT assay.42 The separated reaction products were quantitatively evaluated using a Storm 86 phosphoimager (Amersham-Pharmacia, Freiburg, Germany).
In each experiment, the fraction of acetylated products was expressed in percent of the acetylated products present in reference samples. Means and standard deviations were calculated from 3 independent experiments. The reference value was set to 100% and thus has no standard deviation.
Immunoblotting
Western blot analysis was carried out as previously described.43,44 Primary antibodies used were mouse anti-LMP-1 (1: 400, CS1-4; Dakopatts, Hamburg, Germany), rabbit anti-Bax (1:1000; New England Biolabs, Frankfurt, Germany), anti-p50 and anti-p65 (both 1:1000; Santa Cruz, Heidelberg, Germany), and mouse anti-glyceraldehyde phosphate dehydrogenase (GAPDH, 1: 20 000; Chemicon, Hofheim, Germany).
Electrophoretic mobility shift assay (EMSA)
EMSA was carried out as described.33 Oligonucleotides had the sequences 5′-GCTGAGGTG(C)G(T)G(C)AGGCTTCAGCCCGGG-3′ (w-κB1), 5′-TTCAGCCCG(C)G(T)G(C)AATTCCAGACTGCAG-3′ (w-κB2), and 5′-GCTGAGACG(T)G(C)GG(T)TTATCTCTTGGGCT-3′ (w-κB3). Nucleotide exchanges in m-κB1, m-κB2, and m-κB3 are given in parentheses. Mutant sequences were obtained from Bentires-Alj et al36 ;w-κBc (sc-2505) and m-κBc (sc-2511) were purchased from Santa Cruz. Experiments were repeated 3 times.
Flow cytometric apoptosis assay
Akata and DG-75 were treated for 24 hours with streptonigrin, fixed and permeabilized using the Cytofix/Cytoperm kit (BD Pharmingen), and incubated for 30 minutes on ice with 5% fluorescein isothiocyanate (FITC)-anti-poly(ADP-ribose) polymerase (PARP) and 50% phycoerythrin-anti-LMP-1 antibody (both BD Pharmingen). Subsequently, cells were washed and fixed in 1% paraformaldehyde/phosphate-buffered saline (PBS). HEK 293-T cells were stained with the LIVE/DEAD Reduced Biohazard Cell Viability Kit no. 1 (Molecular Probes, Leiden, The Netherlands), which detects an apoptosis-related increase in cell membrane permeability, and were subsequently fixed. Fluorescence was determined using a FACScalibur (BD Pharmingen). Experiments were repeated 3 times.
Statistical analysis
Frequency of apoptosis was described by a generalized linear mixed model. We considered the classification parameters (1) streptonigrin (added or not), (2) cell type (Akata or DG-75; control-si or Bax-si), and type of LMP-1 (wild type or mutant) and assumed that the apoptosis event was binomially distributed. As variance components, the influences on individual samples were considered. Based on this model, adjusted frequencies as well as differences between cell type/LMP-1 type settings were calculated and statistically tested. Differences between the samples were transformed into odds ratios with 95% confidence intervals. P < .05 was considered statistically significant. All analyses were performed using the module GLIMMIX of the statistical software package SAS Version 8.2 (SAS, Heidelberg, Germany).
Results
LMP-1 inhibits BAX promoter activity
To determine whether LMP-1 regulates BAX promoter activity, transient expression assays using the reporter plasmid pBAX-CAT and a mammalian expression vector for LMP-1 were performed in various human cell lines (Akata [EBV-negative Burkitt lymphoma], BC-1 [HHV-8/EBV-infected peripheral effusion lymphoma], HEK 293-T [kidney]) and in Chinese hamster ovary (CHO) cells. In untransfected BC-1 cells, LMP-1 was not detectable by fluorescence-activated cell sorter (FACS) analysis (data not shown).
LMP-1 reduced relative levels of BAX promoter-directed CAT expression in all of the cells tested, suggesting that LMP-1 functionally inhibits BAX promoter activity (Figure 1A, lane 2; 1B, upper panels, black bars). Also, Western blot analysis confirmed that LMP-1 repressed the expression of Bax protein in transiently transfected HEK 293-T cells (data not shown). In contrast, a cDNA of the HHV-8 ORF K15 encoding a 23-kDa protein that has similarities to EBV LMP-1, but that does not activate the NF-κB pathway,37 did not affect BAX promoter-directed CAT expression in these cells (Figure 1A, lane 4; 1B, upper panels, gray bars).
LMP-1 inhibits BAX promoter activity and protein expression. (A) CAT assay of HEK 293-T cells cotransfected with pBAX-CAT (lanes 1-4) or pHIV-1-LTR-CAT (lanes 5-8) in combination with pHEBo (lanes 1,5), pLMP-1 (lanes 2,6), pcDNA-3 (lanes 3,7), or pK15 (lanes 4,8), or transfected with pHEBo only (lane 9). (B) Different cell lines were cotransfected with pBAX-CAT (top row) or pSV2-CAT (bottom row) and pLMP-1 (▪), pK15 (▦), or the control vectors (pHEBo for pLMP-1; pcDNA-3 for pK15; □). CAT activity in transfections with the control vector was set to 100% and compared with CAT activity in the presence of pLMP-1 and pK15. (C) Western blot analysis of LMP-1 (top), Bax (middle), and GAPDH (bottom) protein in 1852.4 cells. Cells were maintained in the absence of tetracycline for 7 days and subsequently 2 μg/mL tetracycline was added for 6 days. DG75 cells that express neither LMP-1 nor Bax were used as a control (right lane). (D) 1852.4 cells were grown 6 days in the absence (-Tet, left) or presence (+et, right) of 2 μg/mL tetracycline and transfected with pBAX-CAT (□) or pSV2-CAT (▪). CAT activity was measured as described for panel B. (B,D) The means and standard deviations of 3 independent experiments are shown.
LMP-1 inhibits BAX promoter activity and protein expression. (A) CAT assay of HEK 293-T cells cotransfected with pBAX-CAT (lanes 1-4) or pHIV-1-LTR-CAT (lanes 5-8) in combination with pHEBo (lanes 1,5), pLMP-1 (lanes 2,6), pcDNA-3 (lanes 3,7), or pK15 (lanes 4,8), or transfected with pHEBo only (lane 9). (B) Different cell lines were cotransfected with pBAX-CAT (top row) or pSV2-CAT (bottom row) and pLMP-1 (▪), pK15 (▦), or the control vectors (pHEBo for pLMP-1; pcDNA-3 for pK15; □). CAT activity in transfections with the control vector was set to 100% and compared with CAT activity in the presence of pLMP-1 and pK15. (C) Western blot analysis of LMP-1 (top), Bax (middle), and GAPDH (bottom) protein in 1852.4 cells. Cells were maintained in the absence of tetracycline for 7 days and subsequently 2 μg/mL tetracycline was added for 6 days. DG75 cells that express neither LMP-1 nor Bax were used as a control (right lane). (D) 1852.4 cells were grown 6 days in the absence (-Tet, left) or presence (+et, right) of 2 μg/mL tetracycline and transfected with pBAX-CAT (□) or pSV2-CAT (▪). CAT activity was measured as described for panel B. (B,D) The means and standard deviations of 3 independent experiments are shown.
Although LMP-1 inhibited BAX promoter activity, control experiments showed that LMP-1 activated the HIV-1-LTR (Figure 1A, lane 6) and the human GBP-1 promoter (6.6-fold compared with basal activity; data not shown), which are both activated by NF-κB.9,33 The activity of the sv40 promoter (pSV2-CAT) was not affected by LMP-1 (Figure 1B, lower panels). These data indicated that LMP-1 selectively inhibits BAX promoter activity. In all cases, HHV-8 K15 failed to demonstrate a significant effect (Figure 1A lanes 4,8; 1B, lower panel, gray bars).
LMP-1 inhibits the expression of BAX in EBV-infected cells
To determine whether LMP-1 affects endogenous BAX expression, the B-cell line 1852.4 that is infected with a mini EBV and in which LMP-1 expression can be induced by tetracycline was used.30 Cultivation of 1852.4 cells for 7 days in the absence of tetracycline caused a complete shut-off of LMP-1 protein expression (Figure 1C, day 0). After addition of tetracycline, LMP-1 expression increased constantly and reached plateau levels after 4 days as determined by Western blot analysis (Figure 1C, upper panel). Maximal LMP-1 expression in tetracycline-treated 1852.4 cells was comparable with that found in EBV-infected lymphoblastoid cell lines (data not shown; Kilger et al30 ). Inversely to the increase of LMP-1, Bax protein expression decreased gradually and was almost undetectable at day 6 (Figure 1C, middle panel). As a control, DG-75 B cells that do not express LMP-1 and Bax were included (Figure 1C, last lane). Transient expression assays demonstrated that BAX promoter activity was less than 50% in LMP-1-expressing 1852.4 cells compared with 1852.4 cells in which LMP-1 expression was reduced by tetracycline withdrawal for 6 days (Figure 1D, white bars). The sv40 promoter activity in 1852.4 cells was unaffected by tetracycline-induced expression of LMP-1 (Figure 1D, black bars).
CTAR-1 and CTAR-2 of LMP-1 cooperate in the inhibition of BAX promoter activity
To map the region in the LMP-1 protein that mediates inhibition of BAX promoter activity, LMP-1 mutants were expressed in HEK 293-T cells and their effect on BAX promoter activity was investigated. The LMP-1 mutants used included LMP-1(PQT>AAA), which has an inactivated CTAR-1; LMP-1(Y384G), which has an inactivated CTAR-2; and LMP-1(PQT>AAA/Y384G), which is mutated in both CTARs and LMP-1(ΔCOOH) in which the entire carboxyterminal cytoplasmic region is deleted (Figure 2A). Western blot analysis confirmed the expression of wild-type and mutant LMP-1 proteins (Figure 2B). pLMP-1(ΔCOOH) expression was not detected because the anti-LMP-1 antibody used is directed against the cytoplasmic moiety of LMP-1. BAX promoter activity was reduced to 50% of its normal level in wild-type LMP-1-expressing cells (P = .0004) and to 58% (P = .0012) and 72% (P = .0185) in LMP-1(PQT>AAA)- and LMP-1(Y384G)-expressing cells, respectively (Figure 2C, left). In cells that expressed LMP-1(PQT>AAA/Y384G) or LMP-1(ΔCOOH), BAX promoter activity was not significantly reduced (Figure 2C, left). Neither LMP-1 nor any of its mutants had significant effects on the sv40 promoter (Figure 2C, right). These results suggested that LMP-1 repressed BAX expression via the cooperative activity of CTAR-1 and CTAR-2 signaling motifs.
LMP-1 inhibits BAX promoter activity via the NF-κB signaling pathway
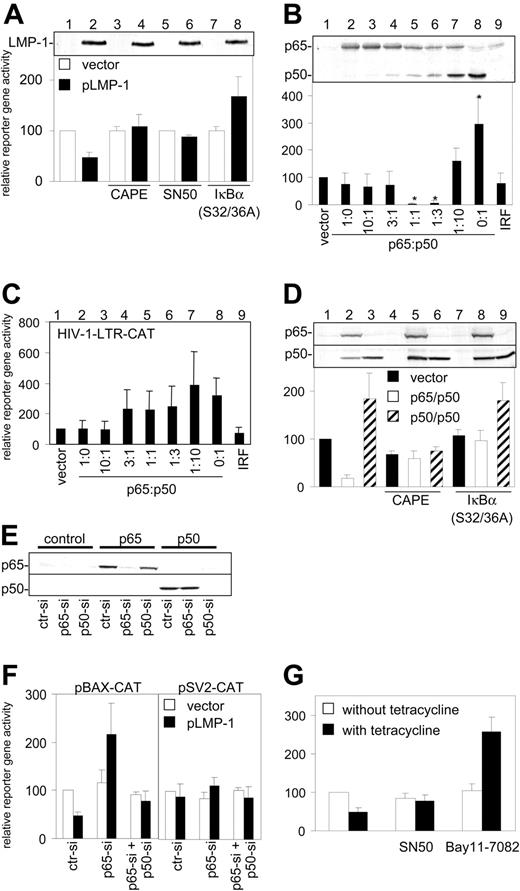
Since CTAR-1 and CTAR-2 are known to activate the NF-κB signaling pathway,4 various inhibitors were used to determine whether LMP-1 inhibition of BAX promoter activity is mediated through NF-κB. The NF-κB inhibitors used include CAPE, which inhibits various forms of NF-κB45 ; SN50, which inhibits homodimers and heterodimers containing p50 (p65/p50, p50/p50)46 ; and IκBα(S32/36A), which selectively inhibits p65/p50,35 but not p50/p50, activity.47 Western blot analysis showed that LMP-1 expression in transfected HEK 293-T cells was not affected by these inhibitors (Figure 3A, upper panel, lanes 2,4,6,8). Interestingly, CAPE and SN50 abolished the inhibitory effect of LMP-1 on the BAX promoter, whereas, IκBα(S32/36A) stimulated BAX promoter activity 1.7-fold in the presence of LMP-1 (Figure 3A, lower panel, black bars). This suggested that the different NF-κB forms targeted by these inhibitors may exert opposite effects on Bax expression.
Inhibition of BAX promoter activity by LMP-1 is mediated by the NF-κB pathway. (A) Western blot for LMP-1 (top) and CAT assay (bottom) of HEK 293-T cells cotransfected with pBAX-CAT and with pHEBo (□) or pLMP-1 (▪) and with pcDNA-3 (columns 1-6) or pIκBα(S32/36A) (columns 7-8) and grown in the presence of 5 μg/mL CAPE (columns 3-4) or 100 μg/mL SN50 (columns 5-6). (B) Detection of p65 and p50 protein expression by Western blot (top) and CAT assay (bottom) of HEK 293-T cells cotransfected with pBAX-CAT and the vector pCMV-T (lane 1) or pCMV-T-p65 and pCMV-T-p50 in gradually changing ratios but at constant total amount (0.3 μg) (lanes 2-8) or with an IRF-1 expression plasmid (lane 9). *Significantly different from vector control (P < .05). (C) CAT assay as in panel B with pBAX-CAT replaced by pHIV-1-LTR-CAT. (D) Western blot for p65 and p50 expression (top) and CAT assay (bottom) of HEK 293-T cells cotransfected with pBAX-CAT and with vector pCMV-T (▪), equal amounts of pCMV-T-p65 and pCMV-T-p50 (□), or pCMV-T-p50 alone (▨) and with pcDNA-3 (columns 1-6) or pIκBα(S32/36A) (columns 7-9) and grown in the absence (columns 1-3, 7-9) or presence (columns 4-6) of 5 μg/mL CAPE. (E) Western blot for p65 (top) and p50 (bottom) in HEK 293-T cells cotransfected with pBAX-CAT and pCMV-T (left), pCMV-T-p65 (middle), or pCMV-T-p50 (right) expression plasmids and control siRNA (ctr-si), p65-siRNA (p65-si), or p50-siRNA (p50-si). (F) CAT assay of HEK 293-T cotransfected with pBAX-CAT (left) or pSV2-CAT (right) together with pHEBo (□) or pLMP-1 (▪) and with ctr-si, p65-si, or p65-si and p50-si. (G) CAT assay of 1852.4 cells grown 6 days in the absence (□) or presence (▪) of 2 μg/mL tetracycline, then transfected with pBAX-CAT and grown 3 days in the absence (left) or presence of 100 μg/mL SN50 (middle) or 1 μM Bay11-7082 (right). (A-D,F-G) CAT activity in control-transfected cells (first bar in each panel) was set to 100%. All experiments were performed in triplicate, and the mean and standard deviations are shown.
Inhibition of BAX promoter activity by LMP-1 is mediated by the NF-κB pathway. (A) Western blot for LMP-1 (top) and CAT assay (bottom) of HEK 293-T cells cotransfected with pBAX-CAT and with pHEBo (□) or pLMP-1 (▪) and with pcDNA-3 (columns 1-6) or pIκBα(S32/36A) (columns 7-8) and grown in the presence of 5 μg/mL CAPE (columns 3-4) or 100 μg/mL SN50 (columns 5-6). (B) Detection of p65 and p50 protein expression by Western blot (top) and CAT assay (bottom) of HEK 293-T cells cotransfected with pBAX-CAT and the vector pCMV-T (lane 1) or pCMV-T-p65 and pCMV-T-p50 in gradually changing ratios but at constant total amount (0.3 μg) (lanes 2-8) or with an IRF-1 expression plasmid (lane 9). *Significantly different from vector control (P < .05). (C) CAT assay as in panel B with pBAX-CAT replaced by pHIV-1-LTR-CAT. (D) Western blot for p65 and p50 expression (top) and CAT assay (bottom) of HEK 293-T cells cotransfected with pBAX-CAT and with vector pCMV-T (▪), equal amounts of pCMV-T-p65 and pCMV-T-p50 (□), or pCMV-T-p50 alone (▨) and with pcDNA-3 (columns 1-6) or pIκBα(S32/36A) (columns 7-9) and grown in the absence (columns 1-3, 7-9) or presence (columns 4-6) of 5 μg/mL CAPE. (E) Western blot for p65 (top) and p50 (bottom) in HEK 293-T cells cotransfected with pBAX-CAT and pCMV-T (left), pCMV-T-p65 (middle), or pCMV-T-p50 (right) expression plasmids and control siRNA (ctr-si), p65-siRNA (p65-si), or p50-siRNA (p50-si). (F) CAT assay of HEK 293-T cotransfected with pBAX-CAT (left) or pSV2-CAT (right) together with pHEBo (□) or pLMP-1 (▪) and with ctr-si, p65-si, or p65-si and p50-si. (G) CAT assay of 1852.4 cells grown 6 days in the absence (□) or presence (▪) of 2 μg/mL tetracycline, then transfected with pBAX-CAT and grown 3 days in the absence (left) or presence of 100 μg/mL SN50 (middle) or 1 μM Bay11-7082 (right). (A-D,F-G) CAT activity in control-transfected cells (first bar in each panel) was set to 100%. All experiments were performed in triplicate, and the mean and standard deviations are shown.
In order to investigate this, p65 and p50 were expressed in HEK 293-T cells in gradually changing ratios ranging from exclusive expression of p65 (Figure 3B, lane 2) toward exclusive expression of p50 (Figure 3B, lane 8). BAX promoter activity was significantly inhibited (P < .001) in cells that expressed predominantly p65/p50 after transfection with p65- and p50-encoding plasmids in 1:1 or 1:3 ratios (Figure 3B, lower panel, lanes 5-6); whereas, transfection of p50 plasmid alone or in a large excess to the p65 plasmid increased BAX promoter activity 2.9- and 1.6-fold (P = .025 and P = .0968), respectively (Figure 3B, lower panel, lanes 7-8). Interferon regulatory factor-1 (IRF-1) did not affect BAX promoter activity (Figure 3B, lower panel, lane 9). The HIV-1-LTR was activated by both p65/p50 and p50/p50 (Figure 3C). CAPE abrogated both the inhibition and the activation of BAX promoter activity in HEK 293-T cells that expressed ectopically p65/p50 and p50/p50 (Figure 3D, lanes 5-6). In contrast, pIκBα(S32/36A) abolished only the inhibition of BAX promoter activity by p65/p50 (Figure 3D, lane 8) but not its activation by p50/p50 (Figure 3D, lane 9). Western blot analysis showed that CAPE and IκBα(S32/36A) did not affect p65 and p50 protein expression (Figure 3D, upper panel).
Finally, siRNA was used to specifically inhibit p65 and p50 expression in HEK 293-T cells. Western blot revealed that overexpression of p65 (Figure 3E, middle) and p50 (Figure 3E, right) was specifically and efficiently inhibited by the respective p65 (p65-si) and p50 (p50-si) siRNAs, whereas a control siRNA (ctr-si) had no effect (Figure 3E). Analysis of BAX promoter activity in these cells showed that p65-si and p50-si applied together abolished the inhibitory effect of LMP-1 (Figure 3F, left panel, right columns) and p65-si increased BAX promoter activity to more than 200% (Figure 3F, left panel, middle columns). sv40 promoter activity was unaffected by LMP-1 or by the various siRNAs (Figure 3F, right panel).
These results were confirmed in EBV-infected 1852.4 cells. Tetracycline-induced LMP-1 decreased BAX promoter activity to 50% (Figure 3G, left, black bar). This effect was abrogated by SN50 (Figure 3G, middle); whereas, in the presence of Bay11-7082, a specific inhibitor of p65/p50 that does not affect p50/p50,48 BAX promoter activity was increased in these cells 2.5-fold (Figure 3G, right, black bar).
These results showed that LMP-1 activates both BAX inhibiting (p65/p50) and BAX activating (p50/p50) pathways of NF-κB. In all cells tested, the BAX inhibiting pathway was predominant (compare Figure 1B, D). BAX activation via p50/p50 was detected only in the presence of specific repressors of p65/p50 (Figure 3A,F-G).
LMP-1 inhibits BAX promoter activity via the κB2 motif
The BAX promoter contains 3 κB motifs (κB1: -702 to -693; κB2: -687 to -678; and κB3: -502 to -493 relative to the translation start).36 LMP-1-induced specific complexes at the κB2 and κB3 sites were detected by EMSA with nuclear extracts of tetracycline-treated 1852.4 cells (Figure 4A, black arrows), whereas a complex at κB1 appeared independently of LMP-1 (Figure 4A, white arrow). No complexes were detected with the mutated oligonucleotides (oligos) m-κB1, m-κB2, and m-κB3 (Figure 4A). In addition, formation of these complexes was competed with an excess of unlabeled w-κB1, w-κB2, and w-κB3 oligos, respectively (Figure 4B). The κB2 and κB3 complexes, but not the κB1 complex, could also be competed with a consensus κB oligo (κBc; Figure 4B). Supershift analyses demonstrated that the κB2 complex contained both p65 (Figure 4C, middle, white arrow) and p50 (Figure 4C, middle, black arrow), whereas the κB3 complex contained only p50 (Figure 4C, right, arrow). None of these proteins were detected in the κB1 complex (Figure 4C, left).
κB2 of the BAX promoter is necessary for the inhibition of BAX promoter activity by LMP-1. (A) EMSA with κB1, κB2, and κB3 wild-type and mutant oligonucleotides as probes without (-) or with nuclear extracts from 1852.4 cells grown 6 days in the absence (Tet-) or presence (Tet+) of 2 mg/mL tetracycline. LMP-1-dependent complexes are indicated by black arrows. An LMP-1-independent complex is indicated by a white arrow. (B) EMSA competition experiment with nuclear extracts of 1852.4 grown in the presence of tetracycline and radiolabeled wild-type κB1, κB2, and κB3 oligos and an excess unlabeled κB1, κB2, κB3, or κB consensus region (κBc) wild-type (w) or mutant (m) oligonucleotides. (C) EMSA supershift analysis with κB1, κB2, and κB3 oligonucleotides as probes and nuclear extracts of 1852.4 cells grown in the presence of tetracycline. Antibodies to p65, p50, and p52 were added to the binding reactions before incubation with the probe. Supershifted complexes are indicated by white (p65) and black (p50) arrows. (D) CAT assay of HEK 293-T cells cotransfected with reporter plasmids with wild-type (pBAX-CAT) or mutant BAX promoters (pBAX-CATm-κB2 or pBAX-CATm-κB3) and with pHEBo (□) or pLMP-1 (▪). (E) CAT assay of HEK 293-T cells transfected with either pBAX-CAT, pBAX-CATm-κB2, or pBAX-CATm-κB3 in combination with either pCMV-T (□), pCMV-T-p50 (▨), or pCMV-T-p65 together with pCMV-T-p50 (▪). (A-C) Representative example of 3 independent experiments. (D-E) CAT activity in the presence of the control vector was set to 100%. Mean and standard deviation of 3 independent experiments. *P < .01.
κB2 of the BAX promoter is necessary for the inhibition of BAX promoter activity by LMP-1. (A) EMSA with κB1, κB2, and κB3 wild-type and mutant oligonucleotides as probes without (-) or with nuclear extracts from 1852.4 cells grown 6 days in the absence (Tet-) or presence (Tet+) of 2 mg/mL tetracycline. LMP-1-dependent complexes are indicated by black arrows. An LMP-1-independent complex is indicated by a white arrow. (B) EMSA competition experiment with nuclear extracts of 1852.4 grown in the presence of tetracycline and radiolabeled wild-type κB1, κB2, and κB3 oligos and an excess unlabeled κB1, κB2, κB3, or κB consensus region (κBc) wild-type (w) or mutant (m) oligonucleotides. (C) EMSA supershift analysis with κB1, κB2, and κB3 oligonucleotides as probes and nuclear extracts of 1852.4 cells grown in the presence of tetracycline. Antibodies to p65, p50, and p52 were added to the binding reactions before incubation with the probe. Supershifted complexes are indicated by white (p65) and black (p50) arrows. (D) CAT assay of HEK 293-T cells cotransfected with reporter plasmids with wild-type (pBAX-CAT) or mutant BAX promoters (pBAX-CATm-κB2 or pBAX-CATm-κB3) and with pHEBo (□) or pLMP-1 (▪). (E) CAT assay of HEK 293-T cells transfected with either pBAX-CAT, pBAX-CATm-κB2, or pBAX-CATm-κB3 in combination with either pCMV-T (□), pCMV-T-p50 (▨), or pCMV-T-p65 together with pCMV-T-p50 (▪). (A-C) Representative example of 3 independent experiments. (D-E) CAT activity in the presence of the control vector was set to 100%. Mean and standard deviation of 3 independent experiments. *P < .01.
To determine whether κB2 and κB3 are involved in inhibition of BAX gene expression by LMP-1, we investigated the activity of BAX promoters with mutations in κB2 and κB3 sites. While LMP-1 inhibited the wild-type promoter pBAX-CAT activity by 71% (Figure 4D, left, black bar), a promoter with a mutated κB2 site (pBAX-CATm-κB2) was not affected by LMP-1 (Figure 4D, middle). A promoter with a mutated κB3 site (pBAX-CATm-κB3) was inhibited by LMP-1 with almost the same efficiency as was wild-type pBAX-CAT (53%; Figure 4D, right, black bar). The predominant role of κB2 in the inhibition of BAX expression was further supported by the finding that a promoter with a mutated κB2 site failed to respond to overexpressed p65/p50 or p50/p50 (Figure 4E, middle). In contrast, a promoter with a mutated κB3 was still inhibited by p65/p50 but was not activated by p50/p50 (Figure 4E, right). Altogether, these data suggested that binding of p65/p50 to κB2 is sufficient for the inhibition of BAX expression, whereas the up-regulation of BAX by p50/p50 requires both the κB2 and κB3 sites of the BAX promoter.
Inhibition of BAX is an important antiapoptotic function of LMP-1
Finally, to determine how the regulation of BAX expression affects the antiapoptotic activity of LMP-1, HEK 293-T cells that express BAX, BCL-2, and BFL-1 (data not shown) were transfected with plasmids encoding Bax-siRNA (Figure 5B) or scrambled control-siRNA (Figure 5A). By Western blot analysis, we demonstrated that Bax protein expression is strongly decreased after transfection with Bax-siRNA in these cells (data not shown). In addition, the cells were transfected with pLMP-1 or the nonfunctional mutant pLMP-1(PQT>AAA/Y384G) and with pGFP (green fluorescence) as a transfection marker. At 3 days after transfection, the cells were treated with 1 μM of the DNA-damaging agent streptonigrin for 24 hours. Apoptosis-related changes in cell membrane permeability were detected with a specific dye (ethidium homodimer-2, red fluorescence). The relative numbers of apoptotic cells (red fluorescence) within the population of transfected cells (green fluorescence) were determined by FACS. In the absence of streptonigrin, the fraction of apoptotic cells was consistently less than 3% (Figure 5A-B, left; representative experiments are shown; mean values of 3 independent experiments are given in parentheses and are displayed with confidence intervals in Figure 5C). In contrast, a high fraction (72.2%) of cells transfected with control-siRNA and mutant LMP-1 was apoptotic after streptonigrin treatment (Figure 5A, upper right panel). Of note, cells transfected with control-siRNA and wild-type LMP-1 were significantly (P < .05; odds ratio [OR] = 3.73; Figure 5C) less susceptible to apoptosis (41.0%; Figure 5A, lower right panel). The same decrease of apoptotic cells was observed in streptonigrin-treated HEK 293-T cells transfected with Bax-siRNA independent of the expression of mutant or wild-type LMP-1 (42.0% each; Figure 5B, right). These results were significantly different from those obtained with cells transfected with control-siRNA and expressing mutant LMP-1 (P < .01 and P < .05, respectively; OR = 3.58 for both; Figure 5C). These results demonstrated that the antiapoptotic activity of LMP-1 is absent in HEK 293-T cells with impaired BAX expression.
Bax is required for the antiapoptotic activity of LMP-1. (A-B) HEK 293-T cells were cotransfected with pGFP, with control-siRNA (A) or Bax-siRNA (B), and with mutant (top row) or wild-type (bottom row) LMP-1 and subsequently incubated with (right column) or without (left column) 1 μM streptonigrin. After staining with Dead Red, cells were gated for pGFP expression by FACS analysis (horizontal axis), and the fraction of Dead Red-stained cells (vertical axis) within the transfected cells was determined. (C) Mean values and 95% confidence intervals (CI95) of 3 experiments as in panels A-B; *P < .05. □ indicates control-si; ▦, Bax-si. (D-E) Akata (D) and DG-75 (E) cells were transfected with mutant (top row) or wild-type (bottom row) LMP-1 and subsequently incubated with (right column) or without (left column) 0.25 μM (Akata) or 1 μM (DG-75) streptonigrin. After staining with FITC/anti-PARP and PE/anti-LMP-1, the expression of PARP (horizontal axis) within the fraction of LMP-1-positive cells (vertical axis) was assayed. (F) Mean values and CI95 intervals of 3 experiments as in panels D-E. *P < .05; **P < .001; n. s. indicates not significant. □ indicates Akata; ▦, DG-75. (A-B, D-E) Numbers show the fractions of apoptotic cells within the gate of transfected cells in the experiment shown; numbers in parentheses refer to the mean of 3 experiments.
Bax is required for the antiapoptotic activity of LMP-1. (A-B) HEK 293-T cells were cotransfected with pGFP, with control-siRNA (A) or Bax-siRNA (B), and with mutant (top row) or wild-type (bottom row) LMP-1 and subsequently incubated with (right column) or without (left column) 1 μM streptonigrin. After staining with Dead Red, cells were gated for pGFP expression by FACS analysis (horizontal axis), and the fraction of Dead Red-stained cells (vertical axis) within the transfected cells was determined. (C) Mean values and 95% confidence intervals (CI95) of 3 experiments as in panels A-B; *P < .05. □ indicates control-si; ▦, Bax-si. (D-E) Akata (D) and DG-75 (E) cells were transfected with mutant (top row) or wild-type (bottom row) LMP-1 and subsequently incubated with (right column) or without (left column) 0.25 μM (Akata) or 1 μM (DG-75) streptonigrin. After staining with FITC/anti-PARP and PE/anti-LMP-1, the expression of PARP (horizontal axis) within the fraction of LMP-1-positive cells (vertical axis) was assayed. (F) Mean values and CI95 intervals of 3 experiments as in panels D-E. *P < .05; **P < .001; n. s. indicates not significant. □ indicates Akata; ▦, DG-75. (A-B, D-E) Numbers show the fractions of apoptotic cells within the gate of transfected cells in the experiment shown; numbers in parentheses refer to the mean of 3 experiments.
To confirm these results in B cells, LMP-1 antiapoptotic activity was analyzed in Akata cells that have a functional BAX gene (Figure 5D) and in DG-75 cells that have inactivating mutations in both BAX alleles26 (Figure 5E). Western blot analysis confirmed that DG-75 cells did not express BAX (Figure 1C) but did express BCL-2 and BFL-1, whereas, Akata cells expressed BAX, BCL-2, and BFL-1 (data not shown). Akata and DG-75 cells were transfected with expression plasmids encoding either wild-type or mutant LMP-1. Subsequently, these cells were treated with streptonigrin in concentrations that induce similar rates of apoptosis (about 25%) in mutant LMP-1 transfectants (Akata: 0.25 μM; DG-75: 1 μM). To exclude assay-specific artifacts, a proteolytic fragment of PARP was used as an alternative indicator of apoptosis in these experiments. The fraction of apoptotic cells within the gate of LMP-1-positive cells was quantitatively determined by FACS analysis (Figure 5D-F).
In Akata and DG-75 cells expressing mutant and wild-type LMP-1, the fractions of apoptotic cells were below 6.6% (Figure 5D-E, left panels). After treatment with streptonigrin, a large fraction of the Akata cells with mutant LMP-1 were apoptotic (22.8%), whereas in cells with wild-type LMP-1 the fraction of apoptotic cells was only slightly increased (9.3%; Figure 5D, right panels). These values differed significantly (P < .001; OR = 2.86; Figure 5F). In contrast, the apoptotic fractions in mutant and wild-type LMP-1-transfected DG-75 cells differed only slightly (25.0% versus 18.0%; not significant; Figure 5F). These results demonstrated that, in B cells, Bax is also an important target of the antiapoptotic activity of LMP-1.
Discussion
Here, we showed that EBV LMP-1 inhibits BAX promoter activity in all 5 of the different cell lines used. The inhibition of BAX promoter activity was mediated cooperatively by CTAR-1 and -2 of LMP-1 as demonstrated by ectopic expression of LMP-1 mutants. Interestingly, previous studies have demonstrated that CTAR-1 and -2 cooperatively activate the NF-κB signaling pathway,4 that the BAX promoter harbors 3 κB consensus sites (κB1-κB3),36 and that BAX expression is regulated by NF-κB.36,49-51 Collectively, these findings suggested that LMP-1 may regulate BAX expression via NF-κB signaling.
This was confirmed by analysis of LMP-1 effects on BAX promoter activity in the presence of inhibitors of different NF-κB isoforms. CAPE or SN50 treatment of cells, or simultaneous transfection of p65 and p50-siRNAthat targeted both p65/p50 and p50/p50, abrogated the effect of LMP-1 on BAX promoter activity. However, IκBα(S32/36A) and p65-si RNA that selectively inhibited p65/p50, but not p50/p50, activated BAX promoter activity in the presence of LMP-1. These results suggested that LMP-1 activates both p65/p50 and p50/p50, but p65/p50 inhibits and p50/p50 increases BAX promoter activity. Consistently, overexpression of p65/p50 inhibited BAX promoter activity, while the BAX promoter was activated by overexpressed p50/p50 (Figure 3B). This was in agreement with previous reports suggesting that an IκBα-sensitive NF-κB pathway may inhibit Bax expression36,49 and an IκBα-insensitive NF-κB pathway may activate Bax expression.50,51
We showed by 2 approaches that the κB2 and κB3 consensus sites of the BAX promoter regulated the differential responses to the different NF-κB isoforms. First, EMSA demonstrated that in LMP-1-expressing cells the κB2 element forms a complex with p65/p50, whereas the κB3 element binds to p50/p50 (Figure 4A-C). Binding of p65/p50 to κB2 has also been shown by others.36 Second, promoter mutation analyses clarified the functional role of the 2 κB sites in the BAX promoter response to LMP-1. The κB2 site was found to be key for the inhibitory effect of LMP-1 on BAX promoter activity, whereas the κB3 element regulated the activation of the BAX promoter by p50/p50. However, in LMP-1-expressing cells the latter effect was observed only when an excess of p50/p50 was experimentally induced by specific inhibitors of p65/p50 (Figure 3D,F-G). Otherwise, in all of the different cell types investigated in this study, the predominant effect of LMP-1 was the inhibition of the BAX promoter by p65/p50.
As yet, it was known that LMP-1 can exert antiapoptotic activity by up-regulation of the expression of BCL-2, BFL-1, and A20.14-18 We showed that LMP-1, in addition, inhibits BAX promoter activity and that in cells with impaired BAX function or expression the antiapoptotic activity of LMP-1 is significantly reduced. The latter showed that Bax is an important target of the antiapoptotic activity of LMP-1. This is consistent with the fact that both Bcl-2 and Bfl-1 also inhibit apoptosis by sequestration and inactivation of Bax.20,21
Altogether our results indicate that LMP-1 in latently EBV-infected cells activates 2 antiapoptotic defense mechanisms converging on Bax. First, LMP-1 inhibits BAX promoter activity. Second, LMP-1 activates the expression of BCL-215 and BFL-1,18 which are potent inhibitors of Bax. The latter may neutralize residual Bax activity when inhibition of the BAX promoter is not sufficiently achieved. The relative contributions of both mechanisms may vary in different cell types.
Most importantly, our data suggest that the opposite effects of LMP-1 on BAX promoter activity mediated by p65/p50 and p50/p50 may be of clinical relevance. Specific inhibition of p65/p50 may result in activation of the BAX promoter via p50/p50 in the presence of LMP-1. This may render LMP-1-expressing cells more sensitive to apoptosis-inducing drugs in the therapy of EBV-related tumors and in other tumors in which NF-κB is constitutively activated. On the other hand, the selective inhibition of p50/p50 may increase the antiapoptotic effect of LMP-1 on BAX expression and may exacerbate tumorigenesis.
Prepublished online as Blood First Edition Paper, December 21, 2004; DOI 10.1182/blood-2004-07-2752.
Supported by grants of the Deutsche Forschungsgemeinschaft (DFG-SPP 1130), the Bavarian State Ministry of Sciences, Research and the Arts (Bavaria-Quebec Research Cooperation), the German Cancer Aid (Deutsche Krebshilfe, Apoptose-Schwerpunktprogramm), and the Interdisciplinary Center for Clinical Research (IZKF, projects A1 and B11) of the University of Erlangen to M.S.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Dr Graham Packham, University of Southampton, Southampton, United Kingdom, for providing DG-75 cells; Dr John Hiscott, Mc Gill University, Montreal, QC, for p65, p50, and IRF-1 expression plasmids; Dr Michael Nüsse, Flow Cytometry Unit, GSF, Neuherberg, Germany, for help with the flow cytometric assays; and Dr Wolfgang Hammerschmidt, Institute of Clinical Molecular Biology and Tumor Genetics, GSF, Munich, Germany, for helpful discussions.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal