Abstract
The refractoriness of T cells to the interferon-γ (IFN-γ)/signal transducer and activator of transcription 1 (STAT1) pathway, which shields them from the antiproliferative effect of IFN-γ, is attributed mainly to down-regulation of the IFN-γR2 signaling chain. However, the mechanisms responsible for this down-regulation are unclear. Here we show that iron uptake mediated by the transferrin receptor (TfR) delivers a signal that leads to IFN-γR2 internalization and thus plays an essential role in attenuating activation of the IFN-γ/STAT1 pathway in human T lymphocytes. The effect of iron on IFN-γR2 internalization was specific as it did not affect expression of the IFN-γR1 binding chain. Deferoxamine (DFO), an iron-chelating agent, up-regulated IFN-γR2 surface expression and reinstated IFN-γ/STAT1 activation in proliferating T lymphocytes. Resistance of malignant T cells to the antiproliferative effect of IFN-γ in vitro was abrogated by addition of DFO. Conversely, iron inhibited IFN-γ–induced apoptosis in malignant T cells in serum-free conditions. In combination but not individually, DFO and IFN-γ strongly inhibited growth of human malignant T cells in an in vivo severe combined immunodeficient (SCID) mouse model. These data provide valuable insights for novel therapeutic approaches aimed at reinstating the IFN-γ/STAT1 apoptotic signaling pathway in autoreactive or neoplastic T cells by means of iron chelation.
Introduction
Interferon-γ (IFN-γ), produced by T and natural killer (NK) cells, is considered the principal effector cytokine of cell-mediated immunity and exerts its effects on target cells through a high-affinity receptor complex linked to a specific Janus kinase (Jak)/signal transducer and activator of transcription (STAT) signaling cascade.1,2 The IFN-γ receptor (IFN-γR) complex consists of 2 chains: an IFN-γR1 binding chain and an IFN-γR2 signaling chain.1 The intracellular portions of the 2 chains provide the Jak1 and Jak2 docking sites. Upon phosphorylation, Jak1 and Jak2 activate STAT1: Following phosphorylation and dimerization, STAT1 is translocated into the nucleus where it activates transcription of numerous sets of IFN-γ–inducible genes.2
The IFN-γ/STAT1 pathway plays an essential role in controlling the expansion of normal and neoplastic cell types of different origin. Activation of this pathway switches on many proapoptotic and antiproliferative genes such as interferon regulatory factor 1 (IRF1), p21waf/cip, Fas and FasL, and activates caspases.3-10 However, the signals transduced by IFN-γ do not always induce apoptosis or block proliferation, and lymphoid cells become resistant to the antiproliferative effects of IFN-γ. Resting, malignant, or normal T cells that develop toward the T helper 1 (Th1) pathway become resistant to the antiproliferative effects of the IFN-γ/STAT1 pathway9,11,12 or rather, IFN-γ favors their proliferation and differentiation.13-15
The refractoriness of T cells to the IFN-γ/STAT1 pathway has been attributed mainly to down-regulation of the IFN-γR chains, especially IFN-γR2, which protects these cells from the antiproliferative/proapoptotic effects of IFN-γ.11,16-19 Both IFN-γ–dependent and –independent mechanisms have been reported to down-regulate IFN-γR2 expression in T lymphocytes. During murine Th cell differentiation, IFN-γ itself induces IFN-γ resistance by down-regulating IFN-γR2,16 whereas in human T lymphocytes, IFN-γR2 internalization occurs mostly in clathrin-coated pits independently from IFN-γ17 and is selectively induced by insulin-like growth factor 1 (IGF1).20 Since the IFN-γ/STAT1 pathway is usually down-regulated in T lymphocytes, information on the mechanisms that maintain low IFN-γR2 expression in these cells might prove useful for devising therapeutic approaches centered on selectively reinstating the IFN-γ/STAT1 apoptotic signaling pathway in autoreactive or neoplastic T cells.
Besides T-cell receptor (TCR) engagement,9,12 up-regulation of surface IFN-γR2 in T cells may also be induced by serum deprivation,5 exposure to nitric oxide (NO),21 or low extracellular pH,22 all of which increase expression of inducible nitric oxide synthase (iNOS) and NO production in macrophages.23 This suggests that serum factors capable of blocking NO production play a critical role in maintaining low IFN-γR2 expression in T lymphocytes.
Among the plethora of factors present in serum, iron has profound effects on numerous critical cell functions, such as electron and oxygen transport, mitochondrial energy metabolism, and detoxification, thus requiring tight homeostatic regulation.24 Iron binds to cytoplasmic iron regulatory protein 1 (IRP1) and IRP2 which in turn regulate expression of proteins such as ferritin, erythroid 5-aminolaevulinic acid synthase (e-ALAS), and the transferrin receptor (TfR) by binding to the iron-responsive elements (IREs) present in their respective mRNA untranslated regions.24,25 In addition, there is a regulatory loop connecting NO and iron: On the one hand, NO modulates IRP activity26,27 and, on the other, iron impairs iNOS transcription.28 These data prompted us to evaluate the effects of iron on IFN-γR2 expression in human T lymphocytes.
In this paper, we investigated the effects of iron and its chelating agent deferoxamine (DFO) on IFN-γR2 surface expression, STAT1 activation, and apoptosis, and demonstrate that iron delivers a signal that causes IFN-γR2 internalization and induces refractoriness to IFN-γ/STAT1 signaling in human T lymphocytes. Furthermore, pretreatment with iron chelators reinstated IFN-γ–mediated growth inhibition in human malignant ST4 cells injected into severe combined immunodeficient (SCID) mice. All these data suggest the possibility of using iron deprivation to restore IFN-γ responsiveness in human T lymphocytes.
Materials and methods
Media
The culture media were RPMI 1640 (BioWhittaker, Walkersville, MD) containing gentamycin (Schering-Plough, Milan, Italy), with or without 10% fetal calf serum (FCS; Invitrogen, Carlsbad, CA), designated complete medium and serum-free medium, respectively. All in vitro cultures were maintained at 37°C in a 5% CO2 humidified atmosphere.
PBMCs and malignant cells
Human peripheral blood mononuclear cells (PBMCs) were isolated from heparinized venous blood obtained from 5 healthy donors by Ficoll-Type 400 (Pharmacia, Uppsala, Sweden) gradient centrifugation and stimulated (1 × 106 cells/mL) with 2.5 μg/mL phytohemagglutinin (PHA; Sigma-Aldrich, St Louis, MO). Blood was obtained after informed consent, according to the Declaration of Helsinki. This study was approved from the Department of Medicine and Experimental Oncology Review Board, University of Turin, Italy. After 3 days, PHA-activated T lymphocytes (T lymphoblasts) were cultured in complete medium containing recombinant human (r-h) interleukin 2 (IL-2; 20 U/mL; EuroCetus, Milan, Italy). ST4 T cells display irregular nuclei with deep indentation typical of childhood, convoluted-type T-cell lymphoma; PF382 is a human T-acute lymphoblastic leukemia stabilized both in vitro and in nu/nu mice starting from biopsy material.13 Jurkat (ATCC, CRL8161) are human T cells from an acute lymphoblastic leukemia.
Cell proliferation assay
Malignant T cells (0.25 × 106/mL) were cultured in triplicate in round-bottom 96-well plates in complete medium with or without 1000 U/mL IFN-γ (kindly provided by Dr M. Brunda, Hoffman-La Roche, Nutley, NJ) in the presence of 0 to 10 μM DFO (Sigma-Aldrich). After 42 hours, cultures were pulsed with 1 μCi (0.037 MBq) [3H]thymidine (TdR) (Amersham, Milan, Italy). After 6 hours, cells were directly collected using the FilterMate Universal Harvester (PerkinElmer Life Sciences, Zaventem, Belgium) onto UniFilter plates (PerkinElmer Life Sciences) and [3H]TdR uptake was quantitated using a TopCount NXT Microplates Scintillation and Luminescence Counter (PerkinElmer Life Sciences).
Flow cytometry
To evaluate surface expression of the IFN-γR chains, ST4 cells (1 × 106/mL) were cultured in complete or serum-free medium. After 24 hours, 10 μM FeSO4 (Sigma-Aldrich) or control buffer was added and the culture was allowed to proceed for another 24 hours. In some wells, cells were further cultured for 48 hours in complete medium with DFO (10 μM) after which cells were recovered, washed, and stained for surface expression of IFN-γR chains with unconjugated γR99 mAb or anti–IFN-γR2 mAb (PBL Biomedical Laboratories, New Brunswick, NJ), followed by fluorescein isothiocyanate (FITC)–conjugated rabbit anti–mouse immunoglobulin G (IgG; DakoCytomation, Glostrup, Denmark) as previously described.20 In parallel experiments, ST4 cells were cultured in complete or serum-free medium. After 24 hours, cells were further cultured with or without FeSO4 for 4 hours. Every hour, cells were recovered and stained for surface IFN-γR2 expression. Alternatively, 24-hour-serum-starved cells were incubated with or without FeSO4 for 1 hour and IFN-γR2 expression was assessed. After this, cells were washed 3 times in serum-free medium, further cultured for 1 hour with or without 10 μM DFO and IFN-γR2 expression was evaluated again. To assess the role of TfR on iron-dependent IFN-γR2 internalization, ST4 cells (1 × 106/mL) were cultured for 24 hours in serum-free medium, then incubated for 30 minutes at 4°C in the presence of 10 μg/mL OKT-9, an IgG1 isotype-matched anti-TfR monoclonal antibody (mAb) used as control since it does not block internalization, or anti-TfR Ap3c10 mAb which instead does block internalization (both kindly provided by Dr F. Malavasi, University of Turin, Italy), and cultured with or without 10 μM FeSO4 at 37°C. After 1 hour, surface expression of IFN-γR2 was evaluated by staining cells with biotin-conjugated anti–IFN-γR2 mAb for 30 minutes followed by phycoerythrin (PE)–conjugated streptavidin (DakoCytomation). In another series of experiments, after a 30-minute incubation with OKT-9 or Ap3c10 mAb, cells were further cultured for 1 hour with or without 50 μg/mL iron-loaded transferrin (Holo-transferrin [Holo-Tf]) or unloaded transferrin (Apo-transferrin [Apo-Tf]) (Sigma-Aldrich), followed by evaluation of IFN-γR2 expression. To measure expression of IFN-γR2 in CD3+ resting PBMCs, cells were simultaneously stained with an FITC-conjugated anti-CD3 mAb (DakoCytomation) and biotinylated anti–IFN-γR2 mAb for 30 minutes followed by PE-conjugated streptavidin. To evaluate IFN-γ–induced apoptosis, ST4 cells were cultured in complete and serum-free medium with or without FeSO4. After 24 hours, cells were further cultured for 24 hours with or without IFN-γ (1000 U/mL). In parallel wells, ST4 cells were cultured in the presence of 10 μM DFO with or without 1000 U/mL IFN-γ. Then, cells were stained for DNA strand breaks (Tunel) with In Situ Cell Death Detection Kit, Fluorescein (Roche Diagnostics, Milan, Italy), according to the manufacturer's instructions. All experiments were performed using a FACSCalibur flow cytometer (Becton Dickinson, Franklin Lakes, NJ). Each plot represents the results from 10 000 events.
Endocytosis experiments
ST4 cells were cultured for 24 hours with or without serum, collected, and then incubated with anti–IFN-γR2 mAb or isotype-matched mouse IgG1 control mAb for 1 hour at 37°C or at 4°C in the absence or presence of FeSO4 (10 μM). Cell surface–associated mAb was removed by treating twice with acid pH (2 minutes at pH 3.0) as described.17 Cells were then fixed and permeabilized as described elsewhere17 and incubated for 30 minutes at 4°C with PE-conjugated anti–mouse Ig. IFN-γR2 endocytosis was measured as cell-associated specific fluorescence by flow cytometry.
RT-PCR
Total RNA was extracted from malignant T cells, cultured for 24 hours in the same conditions as described in “Flow cytometry” for IFN-γR1 and IFN-γR2 evaluation, using the Trizol (Invitrogen). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) primers were obtained from Clontech (Palo Alto, CA). The cDNA synthesis, reverse transcriptase–polymerase chain reaction (RT-PCR), and gel analysis were carried out as described previously.20
Western blot analysis
Phosphorylation of STAT1 nuclear protein in ST4 cells, cultured with or without IFN-γ (1000 U/mL) for 15 minutes, was evaluated as previously described.20 Briefly, nuclear proteins (from 25 μg–30 μg of protein) extracted from 5 × 106 ST4 cells were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE), electroblotted onto nitrocellulose membranes (Bio-Rad, Hercules, CA), and incubated overnight with a 1:1000 dilution of anti–phospho-Tyr701-STAT1, or anti-STAT1 rabbit polyclonal Abs (Cell Signaling, Beverly, MA). Membranes were incubated with 1:2000 horseradish peroxidase (HRP)–conjugated goat anti–rabbit IgG Ab (Cell Signaling). Antibody binding was visualized by ECL Plus (Amersham) according to the manufacturer's instructions. Nonphosphorylated STAT1 was used as a control for equal protein loading.
Mice
Five-week-old female immunodeficient SCID (CB17 scid/scid) mice (Charles River Laboratories, Calco, Italy) were fed and maintained under specific pathogen-free conditions in the animal facility of the Department of Clinical and Biological Sciences, University of Turin, and were treated in accordance with European guidelines.
Injection of ST4 cells into mice
ST4 cells (5 × 106 per mouse) were washed 3 times with phosphate-buffered saline (PBS), resuspended in 0.2 mL PBS containing 0.01% murine serum albumin (MSA; Sigma-Aldrich) and then injected subcutaneously in the inguinal region of SCID mice.
Treatment of mice inoculated with ST4 cells
At 24 hours before tumor challenge, and then at weekly intervals, all mice received intraperitoneal injections with 0.2 mL of a 1/20 dilution of anti–asialo GM1 rabbit antiserum (Wako Chemicals GmbH, Dusseldorf, Germany) in PBS. Two hours after inoculation of ST4 cells, SCID mice received a subcutaneous injection of either 10 μM DFO in 0.4 mL PBS/MSA or PBS/MSA alone for 5 days in the site of tumor challenge. At 7 days after the tumor challenge, SCID mice with or without DFO were further inoculated with 0.4 mL PBS/MSA with or without 1000 U IFN-γ for 5 days. Tumor incidence and growth were evaluated twice a week by a blinded observer. Neoplastic masses were measured with calipers along the 2 perpendicular diameters for 90 days. At the end of this period, tumor-free mice were classified as survivors. Latency time was considered as the period (in days) between challenge and the growth of neoplastic masses to a mean diameter of 3 mm. Mice were killed for humane reasons when the tumor exceeded 12 mm mean diameter.
Statistical analysis
Pearson t test (GraphPad Prism 3; GraphPad Software, San Diego, CA) was used to analyze the effect of IFN-γ on the in vivo growth of ST4 cells and the results of IFN-γR2 expression in normal T lymphoblasts. P values of less than .05 were considered significant.
Results
Effect of iron on expression of the IFN-γR chains in human malignant T cells
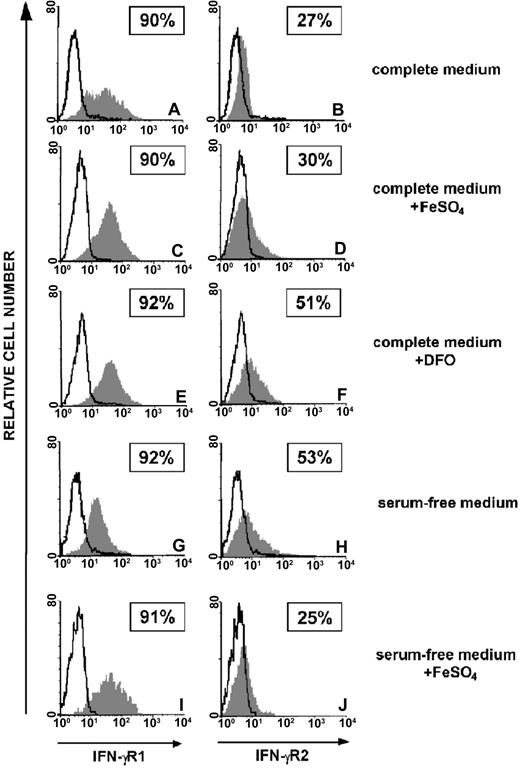
We analyzed the effects of iron, provided as FeSO4, and the iron chelator DFO on IFN-γR chains expression in human malignant T cells. Serum deprivation represents a simple and convenient way to deplete iron from cells in culture. ST4 cells were cultured in complete or serum-free medium for 24 hours and then further cultured for 24 hours with or without 10 μM FeSO4. In parallel, cells were cultured for 48 hours in complete medium supplemented with 10 μM DFO. Flow cytometric analysis showed high surface expression of IFN-γR1 which was not affected by serum, FeSO4,or DFO (Figure 1A,C,E,G,I), whereas expression of IFN-γR2 in ST4 cultured in complete medium was low regardless of FeSO4 administration (Figure 1B,D). In contrast, IFN-γR2 surface expression was increased in cells cultured in complete medium supplemented with DFO (Figure 1F) or in serum-free medium (Figure 1H). In the latter condition, addition of FeSO4 led to a return to low expression of IFN-γR2 (Figure 1J). Similar results were obtained with PF382 and Jurkat T-cell lines (data not shown). Increased surface expression of IFN-γR2 was also observed in T lymphoblasts from healthy donors cultured for 48 hours in the presence of DFO. In complete medium only few cells were positive (IFN-γR2+ % ± SEM: 4.4 ± 1.2), whereas the expression significantly augmented in the presence of DFO (13.8 ± 2.8, P < .05). On the contrary, DFO had no effect on resting CD3+ PBMCs (medium, 2.6 ± 0.7; DFO, 2.5 ± 0.2) indicating that the IFN-γR2 internalization signal delivered by iron is restricted to T lymphocytes that have entered the cell cycle, independent of their normal or malignant status (data not shown).
Effect of iron on expression of the IFN-γR chains in human malignant T cells. Flow cytometric analysis of IFN-γR1 and IFN-γR2 surface expression in ST4 malignant T cells cultured in complete medium (A-B), in complete medium with 10 μM FeSO4 (C-D), in complete medium with DFO (E-F), in serum-free medium (G-H), and in serum-free medium with FeSO4 (I-J). The histogram represents the expression of IFN-γR1 (left panels, gray histograms), IFN-γR2 (right panels, gray histograms), or background of mouse IgG1 negative control (open histograms). Shown are the results of 1 representative experiment of 3 independently performed. Boxed results show percentage of positive cells calculated by subtracting the positivity of nonspecific fluorescence detected with isotype-matched control Ig from that obtained with specific fluorescence.
Effect of iron on expression of the IFN-γR chains in human malignant T cells. Flow cytometric analysis of IFN-γR1 and IFN-γR2 surface expression in ST4 malignant T cells cultured in complete medium (A-B), in complete medium with 10 μM FeSO4 (C-D), in complete medium with DFO (E-F), in serum-free medium (G-H), and in serum-free medium with FeSO4 (I-J). The histogram represents the expression of IFN-γR1 (left panels, gray histograms), IFN-γR2 (right panels, gray histograms), or background of mouse IgG1 negative control (open histograms). Shown are the results of 1 representative experiment of 3 independently performed. Boxed results show percentage of positive cells calculated by subtracting the positivity of nonspecific fluorescence detected with isotype-matched control Ig from that obtained with specific fluorescence.
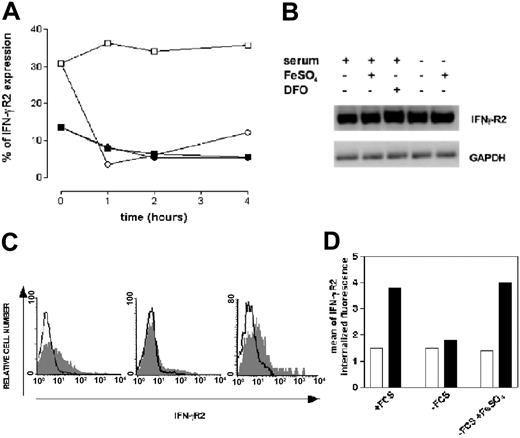
To better define the mechanisms of iron-dependent down-regulation of IFN-γR2, we used ST4 cells to measure the short-term kinetics of surface IFN-γR2 expression under the effects of iron. Cells were cultured in complete or serum-free medium for 24 hours, then further cultured for 4 hours with or without FeSO4 in which IFN-γR2 expression was evaluated every hour by FACS analysis. In complete medium, only a low percentage of ST4 cells were IFN-γR2+. This low percentage slightly decreased throughout the 4-hour experiment and was unaffected by addition of FeSO4 (Figure 2A). Compared with cells cultured in complete medium, serum-free medium–cultured cells displayed a 2.5-fold higher percentage of IFN-γR2 positivity which endured throughout the 4 hours of culture (Figure 2A). Conversely, IFN-γR2 expression was strongly down-regulated already after 1 hour of treatment with FeSO4. The percentage of IFN-γR2+ cells fell to the levels observed in complete medium and was maintained throughout the following 3 hours of culture (Figure 2A). Similar results were obtained in PF382 and Jurkat cell lines (data not shown). RT-PCR analysis of ST4 cells cultured in complete medium revealed that IFN-γR2 mRNA was constitutively expressed (Figure 2B).20 This was not modified by 24 hours of culture in complete medium supplemented with FeSO4, in complete medium plus DFO, in serum-free medium, and in serum-free medium plus FeSO4 (Figure 2B). Neither iron nor DFO induced variations in the level of IFN-γR2 detected by Western blot in total protein extracts from ST4 cells (data not shown). These results indicate that modulation of IFN-γR2 surface expression by iron or iron-chelating agents do not affect IFN-γR2 transcription.
Effect of iron on IFN-γR2 chain internalization. (A) ST4 cells were cultured at 37°C in complete medium (▪ and ▪) or in serum-free medium (□ and ○). After 24 hours of culture, 10 μM FeSO4 was added (▪ and ○); aliquots were recovered at different time points and IFN-γR2 surface expression was evaluated by flow cytometric analysis. Results are expressed as percentage of positive cells calculated by subtracting nonspecific fluorescence detected with isotype-matched control IgG1 from specific fluorescence. One of 3 independently performed experiments is shown. (B) Total RNA was extracted from ST4 T cells cultured for 24 hours in complete or serum-free medium, with or without 10 μM DFO or 10 μM FeSO4, and IFN-γR2 and GAPDH (housekeeping gene) mRNAs were analyzed. (C) IFN-γR2 surface expression was evaluated in ST4 cells cultured in serum-free medium (left), serum-free medium plus 10 μM FeSO4 for 1 hour (middle), serum-free medium plus 10 μM FeSO4 for 1 hour, washed 3 times, and then treated for 1 hour with 10 μM DFO (right). Open histograms represent background of mouse IgG. Negative control and gray histograms represent the expression of IFN-γR2. (D) ST4 T cells cultured for 24 hours with or without serum, or without serum plus 10 μM FeSO4, were recovered and incubated with anti–IFN-γR2 mAb at 4°C (□) or 37°C (▪). After 1 hour, cells were permeabilized and stained with PE-conjugated anti–mouse Ig. Mean of IFN-γR2 internalized fluorescence was calculated by subtracting the mean of internalized fluorescence obtained with isotype-matched mAb from that obtained with specific anti–IFN-γR2 mAb.
Effect of iron on IFN-γR2 chain internalization. (A) ST4 cells were cultured at 37°C in complete medium (▪ and ▪) or in serum-free medium (□ and ○). After 24 hours of culture, 10 μM FeSO4 was added (▪ and ○); aliquots were recovered at different time points and IFN-γR2 surface expression was evaluated by flow cytometric analysis. Results are expressed as percentage of positive cells calculated by subtracting nonspecific fluorescence detected with isotype-matched control IgG1 from specific fluorescence. One of 3 independently performed experiments is shown. (B) Total RNA was extracted from ST4 T cells cultured for 24 hours in complete or serum-free medium, with or without 10 μM DFO or 10 μM FeSO4, and IFN-γR2 and GAPDH (housekeeping gene) mRNAs were analyzed. (C) IFN-γR2 surface expression was evaluated in ST4 cells cultured in serum-free medium (left), serum-free medium plus 10 μM FeSO4 for 1 hour (middle), serum-free medium plus 10 μM FeSO4 for 1 hour, washed 3 times, and then treated for 1 hour with 10 μM DFO (right). Open histograms represent background of mouse IgG. Negative control and gray histograms represent the expression of IFN-γR2. (D) ST4 T cells cultured for 24 hours with or without serum, or without serum plus 10 μM FeSO4, were recovered and incubated with anti–IFN-γR2 mAb at 4°C (□) or 37°C (▪). After 1 hour, cells were permeabilized and stained with PE-conjugated anti–mouse Ig. Mean of IFN-γR2 internalized fluorescence was calculated by subtracting the mean of internalized fluorescence obtained with isotype-matched mAb from that obtained with specific anti–IFN-γR2 mAb.
To investigate if iron induces IFN-γR2 recirculation between the cell surface and the cytoplasm, we assessed the ability of DFO to rapidly counteract the effect of iron in inducing IFN-γR2 down-regulation. Exposure of 24-hour-serum-deprived ST4 cells to iron for 1 hour led to the disappearance of cell surface IFN-γR2 (Figure 2C, left and middle panels). When these cells were recovered, washed, and exposed to DFO for 1 hour, IFN-γR2 reaccumulated at the surface (Figure 2C, right panel). Next, we evaluated the effects of iron on accumulation of anti–IFN-γR2 mAb, at 37°C or at 4°C, in ST4 cells cultured without serum. In the presence of serum, an approximately 2.5-fold increase of cell-associated specific fluorescence of anti–IFN-γR2 mAb was observed after a 1-hour incubation at 37°C (Figure 2D). In contrast, no anti–IFN-γR2 mAb uptake was observed in the absence of serum (Figure 2D), indicating that IFN-γR2 internalization was inhibited. In the absence of serum, iron completely restored the uptake of anti–IFN-γR2 mAb (Figure 2D).
Thus, it seems that in T lymphocytes iron delivers a signal that induces IFN-γR2 internalization but does not affect IFN-γR1.
Effect of TfR blockade in iron-dependent internalization of the IFN-γR2 chain
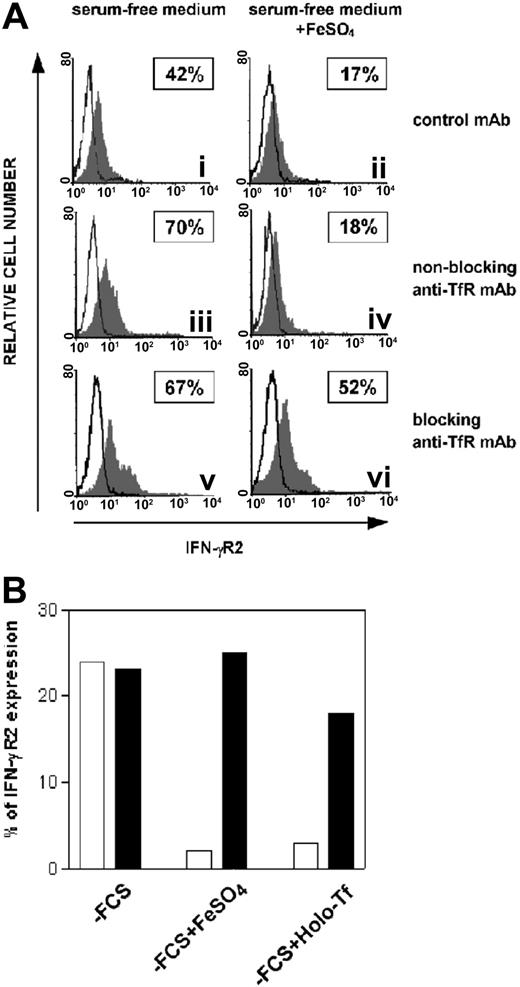
Since normal and malignant T cells (1) require iron to proliferate, and (2) express high levels of TfR29-31 (data not shown), the role of TfR on iron-dependent internalization of IFN-γR2 was evaluated. ST4 cells were cultured for 24 hours in serum-free medium to induce overexpression of IFN-γR2. Cells were then incubated for 30 minutes at 4°C with one of the following mAbs: (1) OKT9, which binds TfR without inhibiting internalization (referred to as `nonblocking anti-TfR mAb'), (2) Ap3c10, which binds TfR and blocks internalization (`blocking anti-TfR mAb'), and (3) isotype-matched control mAb. Cultures were then incubated for 1 hour at 37°C with or without 10 μM FeSO4. In the absence of FeSO4, none of the mAbs affected surface expression of IFN-γR2, which remained high (Figure 3Ai,iii,v). Instead, in the presence of FeSO4, internalization of IFN-γR2 was observed with the nonblocking anti-TfR and control mAbs (Figure 3Aii,iv) but prevented by the presence of the anti-TfR blocking mAb (Figure 3Avi). Holo-Tf induced internalization of IFN-γR2 in a manner similar to that observed with treatment with iron salt and, similarly, the blocking anti-TfR mAb inhibited this internalization (Figure 3B). By contrast, Apo-Tf did not induce down-regulation of IFN-γR2 (data not shown).
Effect of TfR internalization blockade in iron-induced internalization of IFN-γR2. (A) ST4 cells were cultured for 24 hours without serum, then incubated for 30 minutes at 4°C with isotype-negative control mouse IgG1 (i-ii), nonblocking anti-TfR mAb (iii-iv), or blocking anti-TfR mAb (v-vi). Cells were further cultured in the absence (i,iii,v) or presence (ii,iv,vi) of 10 μM FeSO4 at 37°C. The histogram represents the expression of IFN-γR2 (gray histogram) or background of mouse IgG1 negative control (open histogram) in the ST4 T-cell line in 1 representative experiment of 3 independently performed. Boxed results show the percentage of positive cells calculated by subtracting nonspecific fluorescence detected with isotype-matched control IgG1 from that obtained with specific fluorescence. (B) ST4 cells were cultured as described in legend of Figure 3A with nonblocking anti-TfR mAb (□) or blocking anti-TfR mAb (▪). Cells were further cultured in the absence or presence of 10 μM FeSO4 or 50 μg/mL Holo-Tf. After 1 hour, IFN-γR2 cell-surface expression was evaluated by flow cytometry. Percentage of IFN-γR2 expression was calculated as described in legend of Figure 3A. The percentage of IFN-γR2 expression was similar in cells cultured in isotype control mAb (data not shown) and nonblocking anti-TfR mAb.
Effect of TfR internalization blockade in iron-induced internalization of IFN-γR2. (A) ST4 cells were cultured for 24 hours without serum, then incubated for 30 minutes at 4°C with isotype-negative control mouse IgG1 (i-ii), nonblocking anti-TfR mAb (iii-iv), or blocking anti-TfR mAb (v-vi). Cells were further cultured in the absence (i,iii,v) or presence (ii,iv,vi) of 10 μM FeSO4 at 37°C. The histogram represents the expression of IFN-γR2 (gray histogram) or background of mouse IgG1 negative control (open histogram) in the ST4 T-cell line in 1 representative experiment of 3 independently performed. Boxed results show the percentage of positive cells calculated by subtracting nonspecific fluorescence detected with isotype-matched control IgG1 from that obtained with specific fluorescence. (B) ST4 cells were cultured as described in legend of Figure 3A with nonblocking anti-TfR mAb (□) or blocking anti-TfR mAb (▪). Cells were further cultured in the absence or presence of 10 μM FeSO4 or 50 μg/mL Holo-Tf. After 1 hour, IFN-γR2 cell-surface expression was evaluated by flow cytometry. Percentage of IFN-γR2 expression was calculated as described in legend of Figure 3A. The percentage of IFN-γR2 expression was similar in cells cultured in isotype control mAb (data not shown) and nonblocking anti-TfR mAb.
These data indicate that iron-induced internalization of IFN-γR2 is dependent largely on TfR.
Effect of iron on the activation of the IFN-γ/STAT1 pathway in human T cells
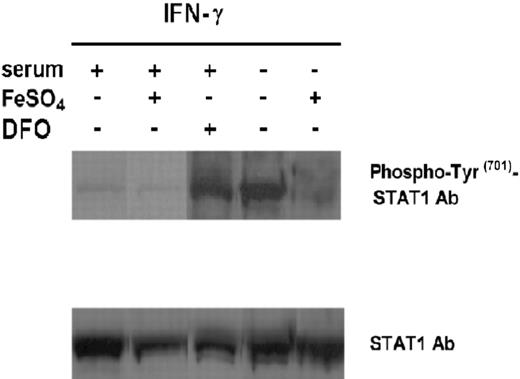
The interaction of IFN-γ with its receptor leads to the phosphorylation of Jak1 and Jak2, which in turn activate STAT1.2 The amount of STAT1 induced by IFN-γ is controlled by the level of IFN-γR2 surface expression.5 To evaluate the effect of iron on IFN-γ–mediated STAT1 activation in malignant T cells, we cultured ST4 cells in serum-free medium with or without FeSO4, and in complete medium with or without FeSO4, or with or without DFO for 24 hours. Cells were then harvested and treated with IFN-γ (1000 U/mL) for 15 minutes. Nuclear proteins were extracted and analyzed by Western blotting with a specific anti–phospho-Tyr701-STAT1 polyclonal Ab (Figure 4). No phosphorylation of STAT1 was observed in cells cultured in complete or serum-free medium, with or without FeSO4 or with or without DFO, without IFN-γ (data not shown), whereas addition of IFN-γ to cells cultured in complete medium weakly induced STAT1 phosphorylation and this was further decreased by FeSO4. In contrast, STAT1 phosphorylation was enhanced by addition of DFO (Figure 4). Strong STAT1 activation was also detected in cells cultured in serum-free medium plus IFN-γ but this effect was almost completely abrogated by FeSO4 (Figure 4). These data together with the results on IFN-γR2 expression indicate that iron regulates activation of the IFN-γ/STAT1 pathway in human T cells by inducing internalization of IFN-γR2.
Effect of iron on IFN-γ–induced activation of STAT1 in ST4 T cells. ST4 cells were cultured in complete medium, with or without 10 μM FeSO4 or DFO, and in serum-free medium with or without FeSO4. After 24 hours cells were recovered and treated with 1000 U/mL IFN-γ for 15 minutes. STAT1 activation was evaluated by Western blot analysis of nuclear cell extracts with anti–phospho-Tyr701-STAT1 polyclonal Ab. Membranes were subsequently probed with an anti-STAT1 antibody to confirm equal protein loading in each lane of the gel. The experiments were performed independently at least 3 times.
Effect of iron on IFN-γ–induced activation of STAT1 in ST4 T cells. ST4 cells were cultured in complete medium, with or without 10 μM FeSO4 or DFO, and in serum-free medium with or without FeSO4. After 24 hours cells were recovered and treated with 1000 U/mL IFN-γ for 15 minutes. STAT1 activation was evaluated by Western blot analysis of nuclear cell extracts with anti–phospho-Tyr701-STAT1 polyclonal Ab. Membranes were subsequently probed with an anti-STAT1 antibody to confirm equal protein loading in each lane of the gel. The experiments were performed independently at least 3 times.
Iron prevents IFN-γ–dependent apoptosis in malignant T cells
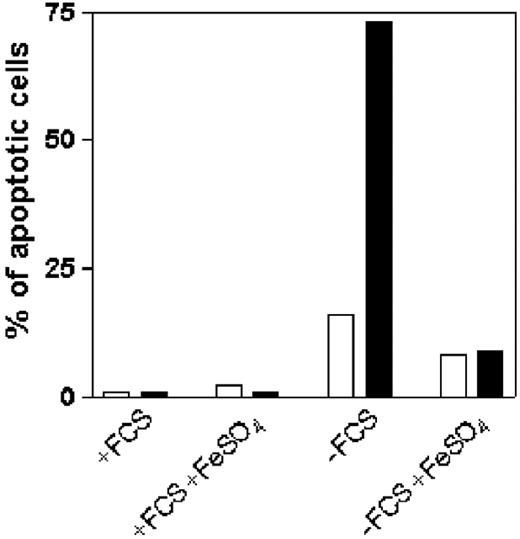
IFN-γ triggers STAT1-dependent apoptosis in T cells that express high levels of IFN-γR2.5,21 Since iron can block the activation of STAT1 in malignant T cells in serum-free medium, we investigated if iron could rescue these cells from IFN-γ–dependent apoptosis. ST4 cells were cultured in complete or serum-free medium with or without FeSO4. After 24 hours, cells were treated with or without 1000 U/mL IFN-γ and culture continued for 24 hours, after which cells were stained simultaneously using the Tunel assay to evaluate apoptosis (Figure 5). Very few apoptotic cells were observed when ST4 cells were cultured in complete medium with or without FeSO4, irrespective of the presence of IFN-γ (Figure 5). On the other hand, apoptosis was increased in cells cultured in serum-free medium (Figure 5) and was further enhanced by IFN-γ (Figure 5). However, the apoptosis-enhancing effect of IFN-γ was completely prevented when cells were grown in serum-free medium plus FeSO4 (Figure 5). Similar results were obtained by staining cells with annexin V and propidium iodide (data not shown). Thus, iron limits IFN-γ–induced apoptosis in human T cells by inducing internalization of IFN-γR2.
Effects of iron on IFN-γ–induced apoptosis in serum-deprived T cells. ST4 cells were cultured for 24 hours in complete or serum-free medium, with or without 10 μM FeSO4, and then further cultured in the absence (□) or presence (▪)of 1000 U/mL IFN-γ. After 24 hours, cells were recovered and stained with Tunel kit to evaluate IFN-γ–induced apoptosis. The number of apoptotic cells was determined by flow cytometric analysis. Percentage of DNA fragmentation is indicated. Results of 1 of 3 independently performed experiments are shown.
Effects of iron on IFN-γ–induced apoptosis in serum-deprived T cells. ST4 cells were cultured for 24 hours in complete or serum-free medium, with or without 10 μM FeSO4, and then further cultured in the absence (□) or presence (▪)of 1000 U/mL IFN-γ. After 24 hours, cells were recovered and stained with Tunel kit to evaluate IFN-γ–induced apoptosis. The number of apoptotic cells was determined by flow cytometric analysis. Percentage of DNA fragmentation is indicated. Results of 1 of 3 independently performed experiments are shown.
Iron chelation restores IFN-γ–dependent growth inhibition in human malignant T cells
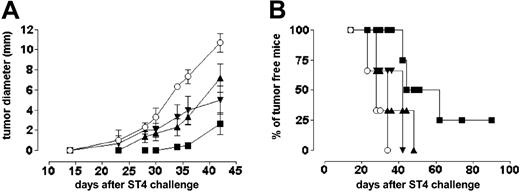
The in vitro proliferation of ST4 cells was not affected by addition of 1000 U/mL IFN-γ or by serially increased doses of DFO (from 0 to 10 μM; Figure 6A). However, when administered in combination, DFO restored the antiproliferative effect of IFN-γ in a dose-dependent manner (Figure 6A). Tunel experiments showed that neither 10 μM DFO nor 1000 U/mL IFN-γ (data not shown) induced apoptosis in ST4 cells which was instead significantly induced by their combined administration (Figure 6B). We then tested the ability of the DFO/IFN-γ combination to inhibit ST4 cell growth in a SCID mouse model: Prior to tumor cell inoculation, mice were further immunosuppressed with anti–asialo GM1 rabbit antiserum to eliminate residual NK activity.32 Growth of ST4 cells (evaluated as mean tumor diameter) was only slightly inhibited by the administration of either DFO or IFN-γ alone, whereas the combination of IFN-γ and DFO significantly decreased ST4 tumor cells growth (Figure 7A). Compared with mice treated with vehicle solution, DFO, or IFN-γ alone, 25% of mice treated with DFO/IFN-γ were still tumor free 90 days after ST4 challenge (Figure 7B).
Effect of iron chelation on IFN-γ-dependent growth inhibition of ST4 cells in vitro. (A) ST4 cells (0.25 × 106/mL) were cultured in triplicate in round-bottom 96-well plates in complete medium alone (▪) or in complete medium plus 1000 U/mL IFN-γ (□) in the presence of serially increasing doses of DFO (from 0 to 10 μM). After 48 hours, [3H]TdR uptake was measured. The results are expressed as the arithmetic mean of cpm plus or minus the standard deviation (SD). A representative experiment of 3 independently performed is shown. (B) ST4 cells were cultured in complete medium in the presence of 10 μM DFO with (gray histogram) or without (open histogram) 1000 U/mL IFN-γ for 24 hours. Apoptosis was measured using Tunel assay. Tunel staining profile of ST4 cells cultured in complete medium was used to set the marker of nonapoptotic cells (M). A representative experiment of 3 independently performed is shown.
Effect of iron chelation on IFN-γ-dependent growth inhibition of ST4 cells in vitro. (A) ST4 cells (0.25 × 106/mL) were cultured in triplicate in round-bottom 96-well plates in complete medium alone (▪) or in complete medium plus 1000 U/mL IFN-γ (□) in the presence of serially increasing doses of DFO (from 0 to 10 μM). After 48 hours, [3H]TdR uptake was measured. The results are expressed as the arithmetic mean of cpm plus or minus the standard deviation (SD). A representative experiment of 3 independently performed is shown. (B) ST4 cells were cultured in complete medium in the presence of 10 μM DFO with (gray histogram) or without (open histogram) 1000 U/mL IFN-γ for 24 hours. Apoptosis was measured using Tunel assay. Tunel staining profile of ST4 cells cultured in complete medium was used to set the marker of nonapoptotic cells (M). A representative experiment of 3 independently performed is shown.
Effect of IFN-γ and DFO on ST4 cell growth in SCID mice. Anti–asialo GM1–treated SCID mice were challenged subcutaneously with 5 × 106 ST4 cells. After 2 hours, mice were injected at the site of tumor challenge with 0.4 mL PBS/MSA alone (○ and ▾) or 10 μM DFO (▴ and ▪). This treatment was repeated daily for 5 days. At day 7 after inoculum, ST4 cell–treated mice received 0.4 mL PBS/MSA alone (○ and ▴) or 1000 U of IFN-γ (▾ and ▪) daily, for 5 days. Results are indicated (A) as the kinetics of the mean plus or minus SD of the tumor diameter and (B) as percentage of tumor-free mice. Each group consisted of 6 mice except the DFO plus IFN-γ–treated group, which instead consisted of 8 mice.
Effect of IFN-γ and DFO on ST4 cell growth in SCID mice. Anti–asialo GM1–treated SCID mice were challenged subcutaneously with 5 × 106 ST4 cells. After 2 hours, mice were injected at the site of tumor challenge with 0.4 mL PBS/MSA alone (○ and ▾) or 10 μM DFO (▴ and ▪). This treatment was repeated daily for 5 days. At day 7 after inoculum, ST4 cell–treated mice received 0.4 mL PBS/MSA alone (○ and ▴) or 1000 U of IFN-γ (▾ and ▪) daily, for 5 days. Results are indicated (A) as the kinetics of the mean plus or minus SD of the tumor diameter and (B) as percentage of tumor-free mice. Each group consisted of 6 mice except the DFO plus IFN-γ–treated group, which instead consisted of 8 mice.
Discussion
The critical role played by iron in numerous physiologic and pathologic conditions has placed this element at the focus of numerous studies aimed at better understanding its role in the workings of the immune system. For example, iron has been found to play an important regulatory role in monocyte/macrophage function, mostly mediated by the interplay between iron and the IFN-γ signaling pathway.33,34 Several studies have demonstrated a role for iron in T-cell regulation35,36 but the role it plays in adaptive immunity requires further investigation. Better knowledge of the connection between iron and the immune system would be beneficial in understanding the pathogenesis of conditions characterized by altered T-cell development and function. In particular, the reported interaction between iron and IFN-γ in macrophages34,37-40 together with the existence of a regulatory loop between iron and NO (whose production is induced by IFN-γ26-28 ) strongly suggest an iron/IFN-γ interplay in lymphocytes. T lymphocytes lack sensitivity to the antiproliferative/proapoptotic effect of IFN-γ/STAT1, which has been attributed mainly to the down-regulation of the IFN-γR2 signaling chain.5,11,16,17
This study shows that iron is a critical environmental factor that determines IFN-γR2 internalization and prevents activation of the IFN-γ/STAT1 pathway in human T cells. By using malignant T-cell lines characterized by lack of IFN-γ production and by prevalently cytoplasmic expression of IFN-γR2,5 we have demonstrated that iron depletion, obtained by means of either serum deprivation or addition of iron-chelating agents, induced up-regulation of IFN-γR2 expression on the cell surface, whereas addition of iron to serum-deprived T cells restored low surface expression of the receptor. Up-regulation of IFN-γR2 induced by DFO was also observed in Jurkat cell transfectants expressing hemagglutinin (HA)–tagged IFN-γR2. When these cells were cultured for 48 hours in the presence of DFO, a clear increase in surface IFN-γR2 could be detected with anti-HA mAb (data not shown).
The down-modulation of IFN-γR2 from the cell surface is due to internalization because (1) iron rapidly induced the disappearance of IFN-γR2 at the T-cell surface, unlike DFO, which rapidly induced its reappearance; (2) neither iron nor DFO had any effect on IFN-γR2 mRNA and protein expression; (3) iron induced the uptake of anti-IFNγR2 mAb. The effect of iron on IFN-γR2 expression requires the TfR since IFN-γR2 down-regulation could be prevented by a TfR-blocking mAb that hampers its internalization. The possibility that other pathways of iron uptake could be involved was ruled out by the observation that the internalization of IFN-γR2 was equally induced by iron salt and iron-loaded Tf. In human T cells, TfR and IFN-γR2 are colocalized, probably within clathrin-coated pits17 (data not shown), implying that iron-induced internalization of TfR has broader consequences on the regulation of IFN-γR2 expression. We have previously reported that IGF1 induces internalization of IFN-γR2 in human T cells.20 Interestingly, IGF1 might regulate iron uptake by inducing rapid redistribution of TfR at the cell surface.41 Therefore, it is likely that a functional interplay exists between IGF1 and iron bound to TfR, which critically governs internalization of IFN-γR2 in human T cells.
The possibility that TfR, via direct interaction with IFN-γR2, regulates IFN-γ signaling in antigen-activated T cells, could be taken into account. TfR physically associates with the zeta chain of the TCR42 and is recruited to the immunologic synapse in response to TCR engagement.43 Engagement of the TCR on T-helper precursor cells causes rapid copolarization of IFN-γR with the TCR within the developing immunologic synapse.44 The interplay between TfR and IFN-γR2 during T-cell activation is currently under investigation in our laboratory.
Negative regulation of IFN-γR2 also occurs in normal activated T cells. We observed that in the presence of iron chelators, surface expression of IFN-γR2 is enhanced in T lymphoblasts but not in resting CD3+ PBMCs. Thus iron regulates internalization only in activated T cells that have entered the cell cycle. This is consistent with the observation that the iron-dependent internalization of IFN-γR2 is dependent on TfR, which is highly expressed in activated but not resting T cells.30 In human T lymphocytes, iron induces internalization of IFN-γR2 but not of IFN-γR1. This provides further evidence of the independent regulation of these 2 chains. In effect, IFN-γR1 expression is down-regulated by IFN-γ9 and, in IFN-γ–stimulated Jurkat T cells, IFN-γR1 is endocytosed in plasma membrane lipid microdomains or “rafts”45 which constitute a clathrin-independent endocytic pathway.46 Thus it would seem that distinct pathways of endocytosis are involved in ligand-dependent IFN-γR1 and iron-dependent IFN-γR2 internalization in human T cells.
Our data clearly show that iron desensitizes IFN-γ/STAT1 signaling in T cells since IFN-γ–dependent phosphorylation of STAT1 was inhibited by iron. Thus, by limiting the availability of IFN-γR2 at the T-cell surface, iron is one of the factors that can directly prevent IFN-γ from triggering a sufficient number of heterodimeric receptors.1 On the other hand, iron depletion perturbs IFN-γR2 recycling, allowing this receptor chain to accumulate at the cell surface. This greatly enhances the ability of IFN-γ to activate STAT1 and also restores the antiproliferative effect of IFN-γ. The down-regulation of IFN-γR2 observed in the presence of serum led to weak STAT1 activation in response to IFN-γ. By contrast, iron deprivation obtained by culturing T cells in serum-free medium or in the presence of chelating agents, resulted in strong IFN-γ–dependent STAT1 activation, which led to increased T-cell apoptosis. The specificity of this effect was also demonstrated by the fact that DFO alone did not affect the in vitro proliferation of malignant T lymphocytes, whereas this was greatly inhibited by the combination of iron chelating agents and IFN-γ.
The observation that iron and its chelation may determine the fate of T lymphocytes exposed to IFN-γ could be of critical importance in understanding and controlling several pathologic situations such as autoimmune diseases and malignancies in which IFN-γ plays a major role.47 Many studies have explored the antitumor effect of iron-chelating agents, albeit with contradictory results. In some cases, there was no evidence of a clinical benefit; in others, a transient inhibition of tumor proliferation was observed.48,49 Our results indicate that DFO may restore the STAT1 pathway in cells, such as T lymphocytes, that have become resistant to the antiproliferative/proapoptotic effects of IFN-γ. This suggested to us that administration of IFN-γ following treatment with iron-chelating agents might restore the ability of IFN-γ to inhibit the growth of malignant T lymphocytes in vivo. Our results clearly indicate that the combined administration of DFO and IFN-γ was able to inhibit the growth of human malignant ST4 cells in immunodeficient mice. In vivo, but not in vitro, a certain degree of ST4 growth inhibition was observed after administration of either DFO or IFN-γ alone. The ability of DFO to slightly inhibit the in vivo growth of ST4 cells may result from accumulation of DFO at the inoculation site, since the treatment was repeated for 5 days after tumor cell challenge. In effect, higher doses of DFO were shown to inhibit growth of human hepatocarcinoma cells injected into immunosuppressed mice.50 IFN-γ alone slightly inhibited in vivo growth of ST4 cells. Since human IFN-γ is species specific, it seems likely that IFN-γR2 surface expression on ST4 cells growing in vivo had increased. Indeed, we have shown that in an acidic pH, which is a typical feature of tumor growth in vivo,51 IFN-γR2 expression is up-regulated,22 resulting in partial restoration of ST4 sensitivity to IFN-γ.
The interaction between iron and IFN-γ may help explain the immune system malfunctions observed in patients with abnormal iron concentrations. Iron overload, such as occurs in polytransfused thalassemic patients, leads to excessive iron storage in macrophages and inhibits the immune system.52 But iron deprivation could be also detrimental for the immune response, for example, the anemia that accompanies infection, inflammation, or malignancy, commonly termed anemia of chronic diseases (ACDs), may induce an up-regulation of IFN-γR2 on T cells and increase their sensitivity to the apoptotic effect of IFN-γ, thus contributing to the elimination of effector T cells. In conclusion, comprehension of the mechanisms underlying the interaction between iron and IFN-γ could be helpful in answering some as-yet-unsolved questions about human lymphocyte regulation. The resistance of T lymphocytes to IFN-γ–induced apoptosis may be a consequence of IFN-γR2 down-regulation. Our data demonstrate that by restoring IFN-γ–induced STAT1 activation and growth inhibition, iron chelation offers new prospects for overcoming the resistance to the antiproliferative effect of IFN-γ in malignant and autoimmune T cells.
Supported by grants from Fondazione Piemontese Studi e Ricerche sulle Ustioni (FPSRU), Associazione Italiana per la Ricerca sul Cancro (AIRC), Compagnia di San Paolo (special project Oncology) and Ministero dell'Istruzione, dell'Università e della Ricerca (MIUR), ex 40% and Fondo per l'Innovazione e la Ricerca di Base (FIRB). S.D.A. and P.B. were supported by a fellowship from Fondazione Internazionale di Ricerca in Medicina Sperimentale (FIRMS).
G.R. and M.B. contributed equally to this study.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
Prepublished online as Blood First Edition Paper, December 30, 2004; DOI 10.1182/blood-2004-07-2686.
We thank Dr Enza Ferrero for critically reading the manuscript.

![Figure 6. Effect of iron chelation on IFN-γ-dependent growth inhibition of ST4 cells in vitro. (A) ST4 cells (0.25 × 106/mL) were cultured in triplicate in round-bottom 96-well plates in complete medium alone (▪) or in complete medium plus 1000 U/mL IFN-γ (□) in the presence of serially increasing doses of DFO (from 0 to 10 μM). After 48 hours, [3H]TdR uptake was measured. The results are expressed as the arithmetic mean of cpm plus or minus the standard deviation (SD). A representative experiment of 3 independently performed is shown. (B) ST4 cells were cultured in complete medium in the presence of 10 μM DFO with (gray histogram) or without (open histogram) 1000 U/mL IFN-γ for 24 hours. Apoptosis was measured using Tunel assay. Tunel staining profile of ST4 cells cultured in complete medium was used to set the marker of nonapoptotic cells (M). A representative experiment of 3 independently performed is shown.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/105/8/10.1182_blood-2004-07-2686/6/m_zh80080577010006.jpeg?Expires=1770029673&Signature=T91m8Ldjs5DK8oMlWzcISnYu1R7jA7sqAxMl6blqpdjifl4ka9R1o0SU1X3oxucmhL1VVHyE2RRIzUhNMcff0HMg1muv4ZBveD7GSKeavciyLhnYA3jFkod~I4CVpnMjawbzGc1q1E03IiDGVkIsryrdyRXiMbMbbLfuHJDAvTVNysRXT9ZliSvaLG9VUUPY4cMDAaH1xfBh0mtxyqIohA09zVIh6foyw0Y4YjCjctvwDCpYEjYuRtxj2-wJBVTQVh6yeZy3MuEl~jtZe7yNFeGdmEmnC1RUlu34VvdW3Pw6pXJzGFSDWgdfvAz-Dv0HfA7Xy3B8x~XZw-Yn-lDFvg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal