Abstract
Patients with T-cell acute lymphoblastic leukemias (T-ALLs) within the Leucémies Aiguës Lymphoblastiques de l'Adulte-94 (LALA-94) prospective trial were treated with a 4-drug per 4-week induction, with intermediate-dose cytarabine and mitoxantrone salvage treatment for patients not achieving complete remission (CR) in 1 course. Only the latter received allografts, if possible, thus providing an informative setting for assessing early response. Representative patients with T-ALL (91 patients) were classified into surface T-cell receptor (TCR)–expressing T-ALL patients (TCRαβ+ or TCRγδ+), pre-αβ T-ALL patients (cTCRβ+, TCR–), and immature (IM) cTCRβ–, TCR– T-ALL patients; 81 patients underwent genotyping for SIL-TAL1, CALM-AF10, HOX11, and HOX11L2. Overall, CR was obtained in 81 (89%) patients; relapse rate was 62% at 4 years and overall survival (OS) rate was 38%. CR rate was significantly lower in IM T-ALL patients after 1 course (45% vs 87%; P < .001) and after salvage (74% vs 97%; P = .002), with the latter inducing a higher rate of CR (9 [64%] of 14) than initial induction. Once CR was obtained, cumulative relapse rates were similar for IM, pre-αβ, and TCR+ T-ALL patients (P = .51), but were higher in HOX11L2 (83%) and SIL-TAL1 (82%) T-ALL patients compared with other genetic subgroups (48%; P = .05). This was associated with an inferior OS for HOX11L2 T-ALLs (13% vs 47% in HOX11L2-T-ALLs; P = .009). The majority of patients with HOX11 T-ALL underwent allografting, predominantly in second CR, but were not associated with a superior OS. Both TCR and genotypic stratification can therefore contribute to risk-adapted management of adult T-ALLs.
Introduction
Approximately 15% of pediatric and 25% of adult patients with acute lymphoblastic leukemia (ALL) belong to the T lymphoid lineage. The prognosis in children is relatively good, with 5-year survival rates of 60% to 70% compared with only 50% for adults.1,2 T-ALLs correspond to a heterogeneous group of acute leukemias arrested at various stages of development. Several recent studies demonstrate that a significant proportion of T-ALLs can be identified by their oncogenic deregulated pathways.3,4 Approximately half exclusively demonstrate 1 of the 4 HOX11, HOX11L2, SIL-TAL1, or CALM-AF10 abnormalities.4 Chiaretti and colleagues5 recently used gene profiling to suggest differences between adult and childhood T-ALLs, and we have demonstrated that stage of maturation arrest and the incidence of oncogenic abnormalities varies considerably with age.4 The clinical impact of oncogene deregulation has been reported for childhood T-ALLs,3,6-9 but much less data are available for adult cases.5,10
We have described a T-cell receptor (TCR)–based classification of cytoplasmic (c)CD3+, CD7+ T-ALL that allows division into “mature” TCRαβ- or TCRγδ-expressing cases, pre-αβ T-ALLs which express cytoplasmic TCRβ (cTCRβ), RAG-1, and pTα in the absence of sCD3/TCR and, as such, are likely to express a pre-TCR and immature (IM)–, TCR-, and cTCRβ-negative T-ALLs.11 The incidence of pre-αβ T-ALLs does not vary with age, whereas IM T-ALLs are more frequent in adults and TCR+ T-ALLs more frequent in children.4 Approximately half of adult T-ALLs demonstrate SIL-TAL1 or CALM-AF10 fusion transcripts or deregulated expression of HOX11 or HOX11L2, in a mutually exclusive fashion.4 CALM-AF10 was restricted to γδ-lineage T-ALLs,12 HOX11 and SIL-TAL1 to αβ lineage T-ALLs,3,4,13 and HOX11L2 to an intermediate αβ/γδ category.4 It has been reported that HOX11+ adult T-ALLs as well as pediatric T-ALLs with rearrangements involving chromosome 10q24 have a better prognosis,6,10,14 although the favorable prognosis of HOX11 within adult T-ALLs, as assessed by transcriptional profiling, has been contested.5 In pediatric T-ALLs, the prognostic impact of HOX11L2 deregulation remains uncertain,8,9 and its impact in adult T-ALLs, as for SIL-TAL1 and CALM-AF10, has not been evaluated.
In the prospective French multicenter LALA-94 protocol, patients with T-ALL were a priori considered as having relatively good-risk disease, based on analysis of our preceding LALA-87 protocol.1 As a consequence, those with a human leukocyte antigen (HLA)–identical family donor were not programmed to receive allogeneic stem cell transplantation (SCT) in first remission, unless 2 courses were required to achieve this first remission. This particular setting favors assessment of the impact of initial T-ALL features on relapse incidence, since only a minority of patients were considered as eligible for SCT in first remission. We have therefore analyzed the clinical impact of a TCR/genetic-based classification of adult T-ALLs treated within the LALA-94 protocol.
Patients, materials, and methods
LALA-94 trial design
Between 1994 and 2002, the LALA-94 multicenter prospective randomized trial included 1000 patients aged 15 to 55 years, of which 26% were diagnosed with T-ALL. Approval was obtained in May 1994 for these studies within the LALA-94 trial from the institutional review board (IRB) of Hospices Civils de Lyon, Lyon, France. Informed consent was provided according to the Declaration of Helsinki and signed by all included patients. At diagnosis, both T- and B-lineage ALLs were randomized to a 4-week, 4-drug induction course consisting of prednisone (60 mg/d from day 1 to day 7 and from day 15 to day 21), vincristine (2 mg total dose at days 1, 8, 15, and 22), cyclophosphamide (750 mg/m2/d at day 1 and day 8), and daunorubicin (30 mg/m2/d from day 1 to day 3 and from day 15 to day 16) or idarubicin (9 mg/m2/d from day 1 to day 3 and day 8). Patients were then allocated to standard- or high-risk groups according to diagnostic characteristics and initial response to chemotherapy. Standard-risk T-ALL was defined as patients without central nervous systems (CNS) involvement achieving complete remission (CR) after 1 course of induction chemotherapy, regardless of initial leucocytosis. These patients were then randomized between intensive and standard consolidation. On the basis of this protocol, patients with an HLA-identical related donor were not programmed to receive allogeneic SCT in first remission, unless 2 courses were needed to achieve first remission. High-risk T-ALL patients, defined as those not achieving CR after 1 course of induction, underwent salvage therapy followed by genetic randomization for allogeneic SCT or a second randomization between autologous SCT and intensive consolidation, as for standard-risk patients in the absence of a sibling donor. The salvage therapy was identical to the intensive consolidation arm and was based on intermediate-dose cytarabine (1 g/m2 per 12 hours from days 1 to 4) and mitoxantrone (10 mg/m2/d from days 3 to 5).
Patients
Analysis was restricted to 91 T-ALL patients with diagnostic material available among the 181 T-ALL patients included before 2000. Their CR rate (89%) and outcome (3-year overall survival [OS] and disease-free survival [DFS] of 36% [25%-47%] and 44% [32.5%-54%], respectively) did not differ significantly from those of all T-ALL patients included during the same period (3-year OS, 42%; DFS, 31%). Early deaths were defined as those occurring prior to evaluation of response to initial induction and those occurring within 1 month of randomization. Median follow-up of the 91 patients was 4 years. All 91 T-ALL patients were classified on the basis of expression of a surface TCR and or cTCRβ, as assessed with the βF1 antibody on permeabilized cells.11 In addition, 81 patients underwent molecular genotyping for SIL-TAL1, CALM-AF10, and quantification of HOX11 and HOX11L2, with 69 patients also undergoing quantification of LMO1/2, LYL1 and TAL1.4,12 Cytogenetic data for these patients will be presented in detail in a future study.
Early death during induction occurred in 4 patients. Eleven received allografts in first CR, whereas 16 received allografts in second CR. It must, however, be noted that 5 of the 11 patients who received allografts in first CR actually reached CR after the first induction course (protocol violation). The number of patients who received allogeneic SCT either in first or second CR is detailed in Table 1, with respect to their immunophenotype and genotypic subsets.
Response to induction therapy and patients who received allogeneic SCT according to TCR and genotypic subsets
. | Patients, N (%) . | CR after 1 course (%) . | Early death . | CR after salvage . | Overall CR rate (%) . | SCT in 1st CR¶ . | SCT in 2nd CR¶ . | Total SCT CR, %¶ . |
|---|---|---|---|---|---|---|---|---|
| All patients | 91 | 66 (73) | 4 | 15/21 | 81 (89) | 11 (5/6) | 16 (15/1) | 30 |
| TCR subsets* | ||||||||
| Immature | 31 (34) | 14 (45)‡ | 3 | 9/14 | 23 (74)‡ | 4 (1/3) | 2 (1/1) | 19 |
| Pre-αβ | 29 (32) | 28 (97)‡ | 0 | 1/1 | 29 (100)§ | 2 (2/0) | 9 (9/0) | 40 |
| TCR-αβ+ | 18 (20) | 14 (78) | 1 | 3/3 | 17 (94) | 2 (1/1) | 1 (1/0) | 17 |
| TCR-αβ+ | 13 (14) | 10 (77) | 0 | 2/3 | 12 (92) | 3 (1/2) | 4 (4/0) | 54 |
| Genotypic subsets† | ||||||||
| CALM-AF10 | 8 (10) | 4 (50) | 0 | 3/4 | 7 (87.5) | 2 (1/1) | 1 (0/1) | 38 |
| SIL-TAL1 | 11 (14) | 11 (100)§ | 0 | — | 11 (100) | 0 | 3 (3/0) | 27 |
| HOX11 | 11 (14) | 11 (100)§ | 0 | — | 11 (100) | 2 (2/0) | 4 (4/0) | 54 |
| HOX11L2 | 10 (12) | 5 (50) | 3 | 1/2 | 6 (60)§ | 1 (0/1) | 2 (2/0) | 30 |
| TAL1 | 22 (32) | 18 (82) | 0 | 3/4 | 21 (95) | 4 (2/2) | 2 (2/0) | 27 |
| LYL | 8 (12) | 3 (37.5)∥ | 1 | 2/4 | 5 (62.5)∥ | 3 (2/1) | 0 | 27 |
| LMO2 | 15 (22) | 7 (47)∥ | 1 | 4/7 | 11 (73) | 1 (0/1) | 2 (2/0) | 20 |
. | Patients, N (%) . | CR after 1 course (%) . | Early death . | CR after salvage . | Overall CR rate (%) . | SCT in 1st CR¶ . | SCT in 2nd CR¶ . | Total SCT CR, %¶ . |
|---|---|---|---|---|---|---|---|---|
| All patients | 91 | 66 (73) | 4 | 15/21 | 81 (89) | 11 (5/6) | 16 (15/1) | 30 |
| TCR subsets* | ||||||||
| Immature | 31 (34) | 14 (45)‡ | 3 | 9/14 | 23 (74)‡ | 4 (1/3) | 2 (1/1) | 19 |
| Pre-αβ | 29 (32) | 28 (97)‡ | 0 | 1/1 | 29 (100)§ | 2 (2/0) | 9 (9/0) | 40 |
| TCR-αβ+ | 18 (20) | 14 (78) | 1 | 3/3 | 17 (94) | 2 (1/1) | 1 (1/0) | 17 |
| TCR-αβ+ | 13 (14) | 10 (77) | 0 | 2/3 | 12 (92) | 3 (1/2) | 4 (4/0) | 54 |
| Genotypic subsets† | ||||||||
| CALM-AF10 | 8 (10) | 4 (50) | 0 | 3/4 | 7 (87.5) | 2 (1/1) | 1 (0/1) | 38 |
| SIL-TAL1 | 11 (14) | 11 (100)§ | 0 | — | 11 (100) | 0 | 3 (3/0) | 27 |
| HOX11 | 11 (14) | 11 (100)§ | 0 | — | 11 (100) | 2 (2/0) | 4 (4/0) | 54 |
| HOX11L2 | 10 (12) | 5 (50) | 3 | 1/2 | 6 (60)§ | 1 (0/1) | 2 (2/0) | 30 |
| TAL1 | 22 (32) | 18 (82) | 0 | 3/4 | 21 (95) | 4 (2/2) | 2 (2/0) | 27 |
| LYL | 8 (12) | 3 (37.5)∥ | 1 | 2/4 | 5 (62.5)∥ | 3 (2/1) | 0 | 27 |
| LMO2 | 15 (22) | 7 (47)∥ | 1 | 4/7 | 11 (73) | 1 (0/1) | 2 (2/0) | 20 |
Criteria for positivity are given in “Patients, materials, and methods.” — indicates not applicable.
All 91 patients were studied and patients from each subset were compared with those from the other 3 subsets.
HOX11, HOX11L2, SIL-TAL1, and CALM-AF10 testing was performed in 81 of 91 patients; patients from each subset were compared to all other patients tested. LYL, LMO2, and TAL1 testing was performed in 69 patients.
P < .01.
P < .05.
P < .10.
Parenthetical values indicate numbers of patients who achieved CR after 1 induction or 1 induction followed by 1 salvage course, respectively.
Statistical analysis
Patient characteristics and CR rates were compared using the Fisher exact test and median comparisons by the Mann-Whitney test. Cumulative incidence of relapse was measured from the date of first CR achievement until first relapse, with deaths in first CR and allogeneic SCT in first CR considered as competing events and compared by the Gray test.15 Overall survival was measured from the date of randomization until the date of death or last contact. For overall survival estimations and comparisons, all patients who received allografts in first CR were censored at the date of transplantation. Overall survival was estimated by the Kaplan Meier method,16 then compared by the log-rank test.17 Survival comparisons were adjusted for covariates using the Cox model and tested by the likelihood ratio test.18 For each T-ALL subset, comparisons were generally performed using the whole population of tested patients not belonging to that subset as control. Given that no differences in outcome were observed within the postremission randomization arms,19 comparisons were not adjusted for postremission treatment arms. Failure time data were analyzed at the reference date of January 2004. Type I error was fixed at the 5% level. All tests were 2-tailed. Statistical analysis was performed on Stata 7.0 (College Station, TX) and R 1.9.0 (http://www.r-project.org/) software packages.
Results
Clinical and biological features of T-ALLs
All cases were defined as T-ALL on classical immunophenotypic criteria, as defined by the European Group for the Immunological Characterization of Leukemias (EGIL)20 ; all expressed cytoplasmic/surface (c/s) CD3 and CD7. Detection of surface TCR and/or cTCRβ expression allowed separation of T-ALLs into 4 categories: TCRαβ+ T-ALLs (20%); TCRγδ+ T-ALLs (14%), TCR–, βF1+, pre-αβ T-ALLs (32%), and TCR– βF1-IM T-ALLs (34%). The immunophenotypic and genotypic characteristics of the different categories are reported in Table 2 and the clinical characteristics of the 91 patients in Table 3. There were 74 men and 17 women, but with no difference in the sex ratio in the immunophenotypic or genotypic subcategories. Mediastinal involvement was observed in 57%. Median white blood cell (WBC) count was significantly lower in patients with IM T-ALLs (P = .001) and higher in those with mature TCRαβ+ ALL (P = .017).
Immunophenotype and genotype of LALA-94 patients classified by TCR status
. | Immature 31 . | Pre-αβ 29 . | TCR-αβ 18 . | TCR-αβ 13 . | Total 91 . |
|---|---|---|---|---|---|
| Patients, N (%) | 31 (34%) | 29 (32%) | 18 (20%) | 13 (14%) | 91 (100%) |
| Phenotype, % | |||||
| CD34 | 58 | 11 | 50 | 23 | 37 |
| CD13/33 | 23 | 7 | 24 | 17 | 15 |
| CD5 | 87 | 97 | 100 | 92 | 93 |
| CD2 | 47 | 100 | 83 | 31 | 68 |
| CD4/8 DN | 74 | 7 | 22 | 15 | 34 |
| CD4/8 DP | 3 | 86 | 61 | 31 | 45 |
| CD4 or CD8 | 23 | 7 | 17 | 54 | 21 |
| CD1a | 20 | 81 | 53 | 31 | 47 |
| CD10 | 30 | 39 | 44 | 46 | 38 |
| TdT | 80 | 100 | 83 | 100 | 90 |
| Genetic abnormalities, n | |||||
| Analyzed for MG | 29 | 28 | 13 | 11 | 81 |
| CALM-AF10 | 5 | 0 | 0 | 3 | 8 |
| HOX11L2 | 6 | 1 | 2 | 1 | 10 |
| SIL-TAL1 | 1 | 4 | 5 | 0 | 10 |
| HOX11 | 2 | 8 | 0 | 1 | 11 |
| MG-negative | 15 | 15 | 6 | 6 | 42 |
| Analyzed for TAL1, LMO2, and LYL | 24 | 25 | 12 | 8 | 69 |
| TAL1 | 6 | 9 | 7 | 0 | 22 |
| LMO2 | 9 | 4 | 1 | 1 | 15 |
| LYL | 5 | 1 | 1 | 1 | 8 |
. | Immature 31 . | Pre-αβ 29 . | TCR-αβ 18 . | TCR-αβ 13 . | Total 91 . |
|---|---|---|---|---|---|
| Patients, N (%) | 31 (34%) | 29 (32%) | 18 (20%) | 13 (14%) | 91 (100%) |
| Phenotype, % | |||||
| CD34 | 58 | 11 | 50 | 23 | 37 |
| CD13/33 | 23 | 7 | 24 | 17 | 15 |
| CD5 | 87 | 97 | 100 | 92 | 93 |
| CD2 | 47 | 100 | 83 | 31 | 68 |
| CD4/8 DN | 74 | 7 | 22 | 15 | 34 |
| CD4/8 DP | 3 | 86 | 61 | 31 | 45 |
| CD4 or CD8 | 23 | 7 | 17 | 54 | 21 |
| CD1a | 20 | 81 | 53 | 31 | 47 |
| CD10 | 30 | 39 | 44 | 46 | 38 |
| TdT | 80 | 100 | 83 | 100 | 90 |
| Genetic abnormalities, n | |||||
| Analyzed for MG | 29 | 28 | 13 | 11 | 81 |
| CALM-AF10 | 5 | 0 | 0 | 3 | 8 |
| HOX11L2 | 6 | 1 | 2 | 1 | 10 |
| SIL-TAL1 | 1 | 4 | 5 | 0 | 10 |
| HOX11 | 2 | 8 | 0 | 1 | 11 |
| MG-negative | 15 | 15 | 6 | 6 | 42 |
| Analyzed for TAL1, LMO2, and LYL | 24 | 25 | 12 | 8 | 69 |
| TAL1 | 6 | 9 | 7 | 0 | 22 |
| LMO2 | 9 | 4 | 1 | 1 | 15 |
| LYL | 5 | 1 | 1 | 1 | 8 |
The proportion of patients expressing each immunophenotypic marker is stated, as is the number of patients with a given genetic marker within each TCR-defined category. DP indicates double positive; DN, double negative; and MG, molecular genetic (ie, HOX11, HOX11L2, CALM-AF10, SIL-TAL1).
Clinical features according to TCR and genotypic subsets
. | Patients, N . | Sex ratio, F/M . | Median age, y (range) . | Median WBC, 109/L (range) . | Mediastinal involvement, %* . |
|---|---|---|---|---|---|
| All patients | 91 | 17/74 | 29 (16-55) | 68 (0.5-624) | 57 |
| TCR subsets† | |||||
| Immature | 31/91 | 6/25 | 29 (17-52) | 21.7 (0.5-281)‡ | 53 |
| Pre-αβ | 29/91 | 5/24 | 34 (16-50) | 70.5 (1.1-368) | 59 |
| TCR-αβ+ | 18/91 | 3/15 | 29 (16-55) | 120 (7.6-624)§ | 47 |
| TCR-αβ+ | 13/91 | 3/10 | 28 (21-39) | 100 (37-152) | 75 |
| Genotypic subsets∥ | |||||
| CALM-AF10 | 8/81 | 1/7 | 28.5 (23-43) | 23.4 (2.2-105)§ | 75 |
| SIL-TAL1 | 11/81 | 2/9 | 22 (16-45)‡ | 169 (15.9-624)‡ | 50 |
| HOX11 | 11/81 | 2/9 | 36 (17-47) | 70.5 (5.1-260) | 73 |
| HOX11L2 | 10/81 | 3/7 | 27 (17-52) | 21.9 (7.8-299) | 30¶ |
| TAL1 | 22/69 | 2/20 | 25.5 (16-50)¶ | 96 (1.1-620)¶ | 50 |
| LYL | 8/69 | 1/7 | 31.5 (21-52) | 33 (0.5-177) | 62.5 |
| LMO2 | 15/69 | 3/12 | 26 (16-52) | 31 (0.5-256) | 38 |
. | Patients, N . | Sex ratio, F/M . | Median age, y (range) . | Median WBC, 109/L (range) . | Mediastinal involvement, %* . |
|---|---|---|---|---|---|
| All patients | 91 | 17/74 | 29 (16-55) | 68 (0.5-624) | 57 |
| TCR subsets† | |||||
| Immature | 31/91 | 6/25 | 29 (17-52) | 21.7 (0.5-281)‡ | 53 |
| Pre-αβ | 29/91 | 5/24 | 34 (16-50) | 70.5 (1.1-368) | 59 |
| TCR-αβ+ | 18/91 | 3/15 | 29 (16-55) | 120 (7.6-624)§ | 47 |
| TCR-αβ+ | 13/91 | 3/10 | 28 (21-39) | 100 (37-152) | 75 |
| Genotypic subsets∥ | |||||
| CALM-AF10 | 8/81 | 1/7 | 28.5 (23-43) | 23.4 (2.2-105)§ | 75 |
| SIL-TAL1 | 11/81 | 2/9 | 22 (16-45)‡ | 169 (15.9-624)‡ | 50 |
| HOX11 | 11/81 | 2/9 | 36 (17-47) | 70.5 (5.1-260) | 73 |
| HOX11L2 | 10/81 | 3/7 | 27 (17-52) | 21.9 (7.8-299) | 30¶ |
| TAL1 | 22/69 | 2/20 | 25.5 (16-50)¶ | 96 (1.1-620)¶ | 50 |
| LYL | 8/69 | 1/7 | 31.5 (21-52) | 33 (0.5-177) | 62.5 |
| LMO2 | 15/69 | 3/12 | 26 (16-52) | 31 (0.5-256) | 38 |
WBC indicates white blood cell count.
Criteria for positivity are given in “Patients, materials, and methods.”
All the 91 patients were studied and patients from each subset were compared with those from the other 3 subsets.
P < .01.
P < .05.
HOX11, HOX11L2, SIL-TAL1, and CALM-AF10testing was performed in 81 of the 91 patients and patients from each subset were compared with all other patients tested. LYL, LMO2, and TAL1 testing was performed in 69 patients only and patients from each subset were compared with all other patients tested.
P < .10.
Within genetic subgroups, median WBC count was lower in cases with CALM-AF10 (P = .03) and higher in those with SIL-TAL1 rearrangement (P = .007). The 11 patients with SIL-TAL1+ ALL were younger (P = .04) and the HOX11 patients tended to be older (P = .16). Mediastinal involvement appeared less frequent in patients with HOX11L2+ T-ALLs (30%), without reaching statistical significance. TCRγδ development is, at least partially, extrathymic. It is therefore noteworthy that mediastinal involvement was, if anything, more frequent in patients with TCRγδ+ T-ALLs or CALM-AF10+ T-ALLs (75%), which are restricted to the TCRγδ lineage.12 TAL1 expression correlated with SIL-TAL1 but was not restricted to these cases (7 of the 8 SIL-TAL1+ cases were TAL1+, compared with 15 of the 73 SIL-TAL1– cases; P < .001), as previously shown.3,4 Trends for a younger median age and a higher initial WBC count were also observed in patients with TAL1+ ALL (P = .07 by Mann-Whitney; Table 3).
Response to induction therapy
Rates of response to induction therapy are shown in Table 1. Sixty-six patients (73%) reached CR after the first induction course. Fifteen additional patients eventually entered CR after a salvage course, leading to an overall CR rate of 89%. Patients with pre-αβ ALL were very sensitive to induction therapy, with a 97% CR rate after 1 course (versus 61% in other patients; P < .001 by the Fisher exact test) and a 100% overall CR rate (versus 84% in other patients; P = .03). Conversely, CR rate after 1 course was significantly lower in patients with immature T-ALLs (45% versus 87% in other patients; P < .001). This was related to a higher rate of patients alive with resistant disease rather than to early deaths. A high proportion of these patients (9 [64%] of 14) entered CR after the salvage course combining anthracycline and intermediate-dose cytarabine but the overall CR rate remained lower in this patient subset (74% versus 97% in other patients; P = .002). Thus, a higher proportion of patients went into remission with salvage compared with initial induction therapy.
Among genetic subtypes, all patients with SIL-TAL1+ or HOX11+ T-ALLs reached CR after the first induction course (P = .03 when compared with other patients). The poor CR rate obtained in patients with HOX11L2+ ALL (60%) is more difficult to interpret because of the occurrence of 3 early deaths in this small subset of 10 patients. Finally, trends for lower response rates after induction therapy were noted in patients with LYL+ or LMO2+ T-ALLs (Table 1), in keeping with their higher incidence of expression in IM T-ALLs.4
Cumulative incidence of relapse
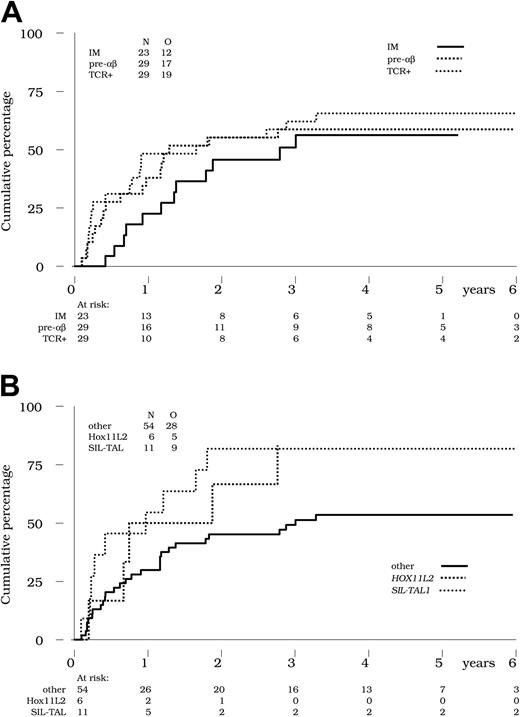
For statistical reasons, and since there were no differences in outcome between these 2 subgroups, we analyzed the TCRαβ and TCRγδ subgroups together within the TCR+ category (Table 4). Of the 81 patients achieving an initial CR, the cumulative incidence of relapse in the presence of 2 competing events (death or allogeneic SCT in first CR) was estimated at 60% at 4 years. No significant difference in the cumulative incidence of relapse was observed in the CR patients from the immature (56% at 4 years), pre-αβ (59% at 4 years), and TCR+ (66% at 4 years) subsets, respectively (P = .51 by the Gray test; Figure 1A and Table 4).
Relapse and overall survival by TCR status or genotype
. | Cumulative incidence of relapse, 4-y estimates, % (95% CI) . | Overall survival, 4-y estimates, % (95% CI) . |
|---|---|---|
| All patients | 60 (50-71) | 44 (33-54) |
| TCR subsets | ||
| Immature | 56 (36-76) | 30 (14-49) |
| Pre-αβ | 59 (42-77) | 59* (38-75) |
| TCR+ | 66 (49-82) | 30 (14-48) |
| Genotype subsets | ||
| CALM-AF10 | 57 (27-90) | 42 (6-77) |
| SIL-TAL1 | 82† (56-97) | 45 (17-71) |
| HOX11 | 45 (22-77) | 56 (20-80) |
| HOX11L2 | 83† (48-99) | 11‡ (1-40) |
| Others | 55 (39-71) | 45 (28-61) |
| TAL1 | 62 (42-82) | 39 (18-60) |
| LYL | NE | NE |
| LMO2 | 68 (42-85) | 29 (8-55) |
. | Cumulative incidence of relapse, 4-y estimates, % (95% CI) . | Overall survival, 4-y estimates, % (95% CI) . |
|---|---|---|
| All patients | 60 (50-71) | 44 (33-54) |
| TCR subsets | ||
| Immature | 56 (36-76) | 30 (14-49) |
| Pre-αβ | 59 (42-77) | 59* (38-75) |
| TCR+ | 66 (49-82) | 30 (14-48) |
| Genotype subsets | ||
| CALM-AF10 | 57 (27-90) | 42 (6-77) |
| SIL-TAL1 | 82† (56-97) | 45 (17-71) |
| HOX11 | 45 (22-77) | 56 (20-80) |
| HOX11L2 | 83† (48-99) | 11‡ (1-40) |
| Others | 55 (39-71) | 45 (28-61) |
| TAL1 | 62 (42-82) | 39 (18-60) |
| LYL | NE | NE |
| LMO2 | 68 (42-85) | 29 (8-55) |
NE indicates not estimated.
P = .02 by the log-rank test.
P = .05 by the Gray test.
P = .003 by the log-rank test.
Cumulative incidence of relapse. (A) Incidence of relapse by TCR status. Cumulative incidence of relapse is indicated in the 23, 29, and 29 CR patients with IM (solid line), pre-αβ (bold dotted line), and TCR+ ALL (thin dotted line), respectively. No significant difference was observed between these 3 subsets (P = .51 by the Gray test). (B) Incidence of relapse by genetic abnormalities. Cumulative incidence of relapse was higher in the 11 CR patients with SIL-TAL1+ ALL (thin dotted line; 82% at 4 years) and in the 6 CR patients with HOX11L2+ ALL (bold dotted line; 83% at 4 years) compared with the 54 other CR patients tested (solid line; 53% at 4 years) (P = .05 by the Gray test).
Cumulative incidence of relapse. (A) Incidence of relapse by TCR status. Cumulative incidence of relapse is indicated in the 23, 29, and 29 CR patients with IM (solid line), pre-αβ (bold dotted line), and TCR+ ALL (thin dotted line), respectively. No significant difference was observed between these 3 subsets (P = .51 by the Gray test). (B) Incidence of relapse by genetic abnormalities. Cumulative incidence of relapse was higher in the 11 CR patients with SIL-TAL1+ ALL (thin dotted line; 82% at 4 years) and in the 6 CR patients with HOX11L2+ ALL (bold dotted line; 83% at 4 years) compared with the 54 other CR patients tested (solid line; 53% at 4 years) (P = .05 by the Gray test).
Within genetic subsets, no significant difference in the cumulative incidence of relapse was seen in the HOX11+ (45% at 4 years), CALM-AF10+ (57% at 4 years), TAL1+ (62% at 4 years), or LMO2+ (68% at 4 years) patient subsets. Conversely, a higher estimated cumulative incidence of relapse was observed in patients with HOX11L2+ T-ALLs (83% at 4 years) and in those with SIL-TAL1+ T-ALLs (82% at 4 years), compared with 54% in patients negative for these markers (P = .05 by the Gray test; Figure 1B). Of note, these differences remain significant after adjustment for the WBC count (< 100 × 109/L versus ≥ 100 × 109/L). The number of CR patients with LYL+ T-ALL was too low to allow for estimation in this subset.
Overall survival
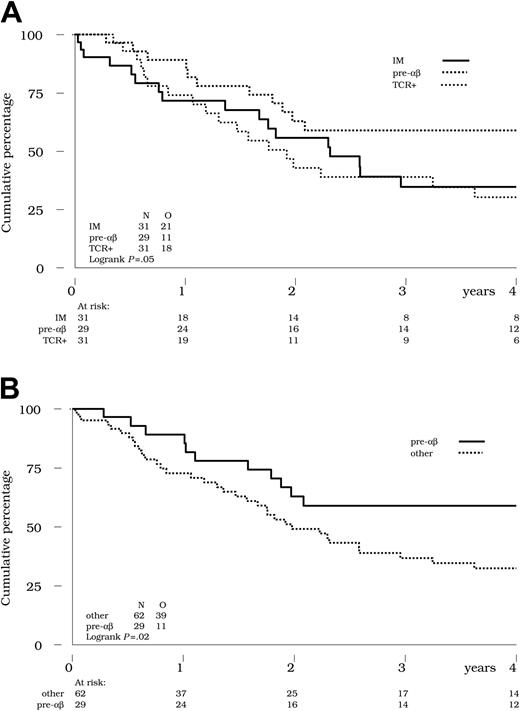
At 4 years, the estimated OS of the entire cohort of 91 patients was 44% (see Table 4 for confidence intervals). According to TCR status, the 4-year OS was 30% in the immature subset, 59% in pre-αβ and 30% in the TCR+ subset (Table 4 and Figure 2A). The difference in favor of the pre-αβ subset compared with the 2 other subsets was statistically significant (Figure 2B; P = .02 by the log-rank test), even after adjustment for the WBC count (< 100 × 109/L versus ≥ 100 × 109/L; P = .02 using the Cox model). Given that the incidence of relapse was not lower in this pre-αβ subset and that the postrelapse survival was as poor (data not shown), this observation probably reflects the better response to induction therapy noted in these patients, or potentially a higher rate of allograft in CR2. It is noteworthy (Table 1) that the proportion of patients undergoing SCT was higher in the pre-αβ, HOX11, and TCRγδ+ subsets.
Overall survival by TCR status. (A) Overall survival was not different in patients with IM (solid line) compared with TCR+ T-ALL (thin dotted line). Bold dotted line indicates pre-αβ ALL. (B) Pre-αβ ALL (solid line) vs all others (dotted line). Survival was significantly better in the pre-αβ subset compared with the 62 other patients with either IM or TCR+ ALL (59% versus 32% at 4 years; P = .02 by the log-rank test).
Overall survival by TCR status. (A) Overall survival was not different in patients with IM (solid line) compared with TCR+ T-ALL (thin dotted line). Bold dotted line indicates pre-αβ ALL. (B) Pre-αβ ALL (solid line) vs all others (dotted line). Survival was significantly better in the pre-αβ subset compared with the 62 other patients with either IM or TCR+ ALL (59% versus 32% at 4 years; P = .02 by the log-rank test).
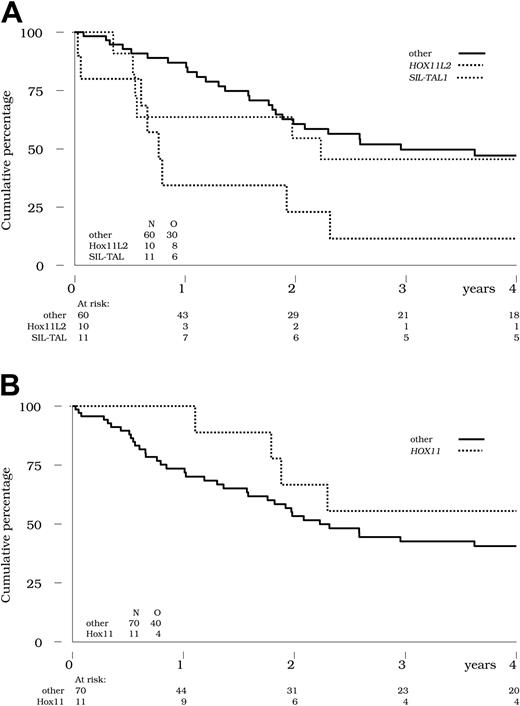
The lower CR rate and the higher cumulative incidence of relapse previously mentioned in patients with HOX11L2+ ALL translated into a poor OS (11% at 4 years; Figure 3A), compared with 47% in all other patients (P = .003 by the log-rank-test). This difference remained significant after adjustment for WBC count (< 100 × 109/L versus ≥ 100 ×109/L; P = .007 using the Cox model). Despite the aforementioned higher incidence of relapse, overall survival was not inferior in patients with SIL-TAL1+ T-ALL (Figure 3A).
Overall survival by genetic abnormalities. (A) Overall survival is indicated in the 10 patients with HOX11L2+ (bold dotted line) and the 11 patients with SIL-TAL1+ T-ALL (thin dotted line) compared with the 60 other patients tested (solid line). Survival was significantly worse in the HOX11L2+ subset (11% at 4 years; P = .003 by the log-rank test), but not in the SIL-TAL1+ subset (45% at 4 years; P = .67 by the log-rank test), compared with other patients (47% at 4 years). (B) HOX11 expression. Overall survival is indicated in the 11 patients with HOX11+ (dotted line) compared with the 70 other patients tested (solid line). No significant difference was observed between these 2 subsets (P = .21 by the log-rank test).
Overall survival by genetic abnormalities. (A) Overall survival is indicated in the 10 patients with HOX11L2+ (bold dotted line) and the 11 patients with SIL-TAL1+ T-ALL (thin dotted line) compared with the 60 other patients tested (solid line). Survival was significantly worse in the HOX11L2+ subset (11% at 4 years; P = .003 by the log-rank test), but not in the SIL-TAL1+ subset (45% at 4 years; P = .67 by the log-rank test), compared with other patients (47% at 4 years). (B) HOX11 expression. Overall survival is indicated in the 11 patients with HOX11+ (dotted line) compared with the 70 other patients tested (solid line). No significant difference was observed between these 2 subsets (P = .21 by the log-rank test).
Finally, it must be noted that in this adult series, the 11 patients with HOX11+ T-ALLs did not show a significantly superior OS (56% at 4 years) compared with all other patients tested (41% at 4 years, P = .21 by the log-rank-test; Figure 3B). Six of these 11 patients underwent SCT, predominantly in second remission.
Discussion
Therapeutic decisions are increasingly based on biological parameters determined at diagnosis or during the early response to induction. We have recently used a TCR-based classification to demonstrate differences in stage of maturation arrest, lineage restriction, and genotype within the heterogeneous group of disorders collectively classified as T-ALL.4,11,12 In this manuscript we have compared this classification with a genetic one in order to identify subgroups of adult T-ALLs that may benefit from specific management.
Immature T-ALLs are characterized by a low presenting WBC count and a low CR rate to classical ALL-type induction, with a superior response to cytarabine and anthracycline myeloid-type salvage therapy. Unlike B-cell precursor ALL, high WBC count does not identify poor-risk T-ALL in children2 or adults.1 This is in keeping with the low WBC count seen in IM T-ALLs and the genetic subgroups with which these immature T-ALLs are associated. WBC count did not have prognostic impact on relapse rate or survival within the present series (data not shown). Although the proportion of IM T-ALL patients achieving CR is lower than other subsets, relapse risk and OS are not inferior. This is likely to, at least partially, reflect the fact that these patients were stratified for allograft within the LALA-94 protocol. This subgroup is common in adult T-ALL patients, occurring in one third of the present series, but it is also found in approximately 20% of T-ALL patients aged 10 to 20 years and 8% of those younger than 10 years.4 It can be identified rapidly by flow-cytometric detection of cTCRβ on permeabilized cells and can therefore be used for early therapeutic stratification, including at initial induction. While initial corticosensitivity was not assessed within the LALA-94 protocol, given the immature stage of maturation arrest prior to beta-selection, it is likely that a significant proportion of IM T-ALLs are corticoresistant.
With regard to genetic subsets, IM T-ALLs include predominantly HOX11L2 and CALM-AF10 T-ALLs with 3′ AF10 break-points. We have previously shown that the majority of immature ALs with the latter form of CALM-AF10 fusion transcripts are of poor prognosis, when analyzed retrospectively,12 but the small number of CALM-AF10 patients in the present study, combined with their heterogeneous stage of maturation arrest (5 IM, 3 TCRγδ) and type of fusion transcript (4 5′ and 4 3′ breakpoints) limits detailed analysis. It will obviously be important to evaluate these cases prospectively. They represent approximately 10% of adult T-ALLs and can be detected by RT-PCR or fluorescence in situ hybridization (FISH), although determination of the type of fusion transcript requires PCR analysis.12
HOX11L2 expression was of poor prognosis with the LALA-94 protocol, as a result of failure to respond to both initial and salvage induction and an increased relapse rate for patients achieving CR. Allograft in first CR is unlikely to improve the survival of this subgroup significantly, since only 60% achieve CR after salvage and of the 6 patients in this category, 3 underwent allograft. These patients present with a low WBC count, relatively infrequent mediastinal involvement, and a heterogeneous but characteristic stage of maturation arrest intermediate to the TCRαβ and TCRγδ lineages.4 Little data concerning HOX11L2 deregulation in adult T-ALLs are available. Mauviaux et al21 reported 0 of 8 adult cases and Ferrando et al10 reported 2 (4%) of 52. We demonstrated a 16% incidence in adolescents and 11% in patients with T-ALL over 20 years in a series of 173 cases.4 HOX11L2 expression has been reported to be of poor prognosis within a small number of pediatric T-ALL patients from St Jude's Total Therapy X1-X111,10 and a subgroup of patients from the French acute lymphoblastic leukemia (FRALLE) protocol,8 but not within the pediatric European Organization for Research and Treatment of Cancer (EORTC) 58881/58951 protocol.9 Our data are keeping with the former pediatric studies. If this is confirmed in independent prospective adult studies, these patients could benefit from more intensive chemotherapeutic regimens or innovative therapy.
Several biological characteristics of IM T-ALLs suggest that they are similar to acute myeloid leukemia (AML) despite cCD3 and CD7 expression. Both frequently express CD34, CD13, and/or CD33,11 and demonstrate complex caryotypes (data not shown) and fusion transcripts rather than Ig/TCR rearrangements.12 Whether they should be treated on AML- or ALL-type protocols merits reflection. Transcriptional profiling of this group will also help identify particular subgroups that may require specific management. We identified a similar incidence of LMO2 expression (22%) to Ferrando et al10 (31%) and, in keeping with them, did not identify any particular clinical features in this subgroup. LYL1 is less frequent in adult than pediatric T-ALL patients4 but does not present distinguishing features compared with IM T-ALL patients in general, when LYL1 expression is most common. We only identified 4 out of 67 LMO1-expressing T-ALL patients, compared with 7 out of 52 patients.10 The small number of cases precluded analysis of this subgroup. While it was not possible to perform multivariate analysis with the limited number of patients available, it will clearly be important to identify the response to treatment with the genetic subgroups comprising IM T-ALL patients.
Pre-αβ T-ALL patients presented with an intermediate WBC count, immunophenotypic features of cortical thymocytes, and a 100% CR rate. This reflects the fact that these T-ALL patients probably express a pre-TCR11 and are likely to be arrested during the process of β-selection, with consequent corticosensitivity. The high CR rate translates into a higher OS, which needs to be interpreted after taking into account the genetic subcategories that comprise this group; predominantly HOX11 and, to a lesser extent, SIL-TAL1.
HOX11/10q24 T-ALLs are reported to be of relatively good prognosis in pediatric T-ALL patients.6,14 Ferrando et al10 identified 16 (31%) cases with HOX11 expression from a series of 52 adult T-ALL patients, treated on 2 different therapeutic trials, demonstrated a favorable outcome for this subgroup, and suggested that these patients may be at greater risk from the treatment-related mortality of allografting. This is all the more striking since overexpression is more common in adult (18% of patients older than 20 years) than pediatric T-ALL patients (4% in patients younger than 10 years4 ) and is associated with older patients among adult T-ALL patients.4,10 HOX11 patients did not demonstrate significant improvement in OS within the present trial, in keeping with results obtained in the Gruppo Italiano Malattie Ematologiche dell'Adulto (GIMEMA) 0496 adult ALL trial.5 The relapse rate in HOX11 T-ALL patients in the present series was 50% and the majority underwent SCT, predominantly in second CR, suggesting that, as for initial CR, the CR2 rate is relatively high. The different prognostic impact of HOX11 expression may reflect therapeutic differences but may also result from different real-time quantitative (RQ)–PCR cut-off criteria. Several studies have reported T-ALL patients with 2 to 3 log lower levels of HOX11 expression, and Kees et al6 identified 2 distinct categories of HOX11+ cases (20% with high-level expression and 29% with low-level expression). The 31% incidence detected by Ferrando et al10 is higher than the 14% identified here or the 18% incidence reported by transcriptional profiling.5 It is possible that these low-level positive cases, which we considered negative, identify a good prognostic subgroup within the Eastern Cooperative Oncology Group (ECOG) and Cancer and Leukemia Group B (CALGB) patients.10
As for pre-αβ and HOX11 T-ALL patients, SIL-TAL1 T-ALL patients also demonstrated a 100% CR rate, including after the first course. These patients, however, demonstrated a high relapse rate, similar to that observed in HOX11L2 T-ALL patients. Unlike the latter, this did not translate into an inferior OS. Although the small number of patients limits valid interpretation, it does suggest that these cases are salvaged by chemotherapy followed by allogeneic SCT performed in second remission. Given the high relapse rate in this group, it may be worth considering allografting in first remission in these cases. We did not find the same increased incidence of relapse in patients with TAL1+, SIL-TAL1– T-ALLs, suggesting that the relapse rate may not correlate directly with TAL1 overexpression. SIL-TAL1 expression is due to the TAL1d deletion in approximately 80% of cases.22,23 TAL1d is reported to be associated with a higher WBC count but to have no clear prognostic impact in pediatric patients with T-ALL, although it was associated with a trend for improved event-free survival.24 Its prognosis in adult patients with T-ALL has to date been difficult to assess, due to the low incidence of TAL1d.25 We confirm the leucocytosis and absence of impact on OS, but additionally suggest that that these patients may relapse more frequently while remaining responsive to salvage therapy.
Patients with TCR+ T-ALL represent a heterogeneous group, with both TCRαβ and TCRγδ and CALM-AF10, HOX11L2, and SIL-TAL1 cases. It is consequently difficult to analyze response to treatment within this subgroup. It is, however, noteworthy that TCRγδ patients demonstrate similar leucocytosis and mediastinal involvement to patients with TCRαβ T-ALL. TCRγδ cells comprise less than 1% of thymocytes. This population may either be at increased risk of malignant transformation, or the TCRγδ-expressing T-ALLs may differ from physiologic TCRγδ thymocytes.
Phenotypical classification based on TCR expression (cTCRβ or surface TCRαβ/γδ) differs from the EGIL classification, which is primarily based on CD1a expression as a way of identifying a “cortical” phenotype, regardless of TCR status.20 Evaluation of the clinical impact of EGIL status in 69 of 91 LALA-94 patients demonstrated that 6 patients with IM were CD1a+ and 5 patients with pre-αβ were CD1a–. IM TCR status was superior to CD1a negativity in identifying sCD3/TCR-negative patients who did not go into CR after 1 induction course (data not shown) but predictive power for a higher cumulative incidence of relapse was similar in the IM and CD1a– categories. Controversial data has been reported for the favorable or neutral prognostic impact of CD1a, CD10, or CD2 expression, once again predominantly in pediatric patients with T-ALL.2,26-31 Less data are available for adult patients with T-ALL: CD10+ cases have been reported to be associated with a better DFS in the LALA-87 study,1 and Chiaretti et al5 have shown that high-level CD2 transcripts by RQ-PCR quantification, which correlate with protein expression, are associated with long remission. We suggest that addition of cTCRβ assessment to diagnostic panels will improve our capacity to stratify adult patients with T-ALL when used in combination with a genotypic classification including at least HOX11, HOX11L2, SIL-TAL1, and CALM-AF10. These parameters need to be evaluated in independent prospective trials.
Prepublished online as Blood First Edition Paper, January 6, 2005; DOI 10.1182/blood-2004-09-3666.
Supported by the Fondation contre la leucémie de la Fondation de France, the Ligue Nationale Contre le Cancer, the Association de la Recherche sur le Cancer (ARC), and the Biomed-2 BMH4-CT98-3936 concerted action.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank all the biologists and clinicians of the LALA group, particularly N. Dastugue and E. Kuhlienne (Toulouse), F.-X. Mahon and C. Bilhou-Nabera (Bordeaux), M. Lafage (Marseille), S. Hayette (Lyon), X. Troussard (Caen), I. Radford-Weiss and F. Valensi (Paris, Necker), F. Davi (Paris, La Pitié-Salpêtrière), C. Bayle and A.-L. Bennaceur (Institut Gustave-Roussy, Villejuif), B. Lenormand (Rouen), M. Dupont (Montpellier), C. Preudhomme (Lille), F. Picard (Paris, Cochin), and B. Soulea (Percy) for providing T-ALL samples and results.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal