Abstract
Proteasome inhibitors, a novel class of chemotherapeutic agents, enhance the antitumor efficacy of anthracyclines in vitro and in vivo. We therefore sought to determine the maximum tolerated dose (MTD) and dose-limiting toxicities of bortezomib and pegylated liposomal doxorubicin (PegLD). Bortezomib was given on days 1, 4, 8, and 11 from 0.90 to 1.50 mg/m2 and PegLD on day 4 at 30 mg/m2 to 42 patients with advanced hematologic malignancies. Grade 3 or 4 toxicities in at least 10% of patients included thrombocytopenia, lymphopenia, neutropenia, fatigue, pneumonia, peripheral neuropathy, febrile neutropenia, and diarrhea. The MTD based on cycle 1 was 1.50 and 30 mg/m2 of bortezomib and PegLD, respectively. However, due to frequent dose reductions and delays at this level, 1.30 and 30 mg/m2 are recommended for further study. Pharmacokinetic and pharmacodynamic studies did not find significant drug interactions between these agents. Antitumor activity was seen against multiple myeloma, with 8 of 22 evaluable patients having a complete response (CR) or near-CR, including several with anthracycline-refractory disease, and another 8 having partial responses (PRs). One patient with relapsed/refractory T-cell non-Hodgkin lymphoma (NHL) achieved a CR, whereas 2 patients each with acute myeloid leukemia and B-cell NHL had PRs. Bortezomib/PegLD was safely administered in this study with promising antitumor activity, supporting further testing of this regimen.
Introduction
The ubiquitin-proteasome pathway (UPP) is responsible for the majority of eukaryotic intracellular protein turnover.1 Coordinated UPP function is essential to many cellular processes including mitosis, processing and degradation of transcription factors and short-lived proteins, and removal of misfolded or damaged proteins.1 Inhibitors of the proteasome induce apoptosis preferentially in transformed cells while sparing normal cells in several model systems2 including non-Hodgkin lymphoma (NHL) and multiple myeloma (MM). An especially strong preclinical rationale has been developed in MM, where proteasome inhibition with bortezomib3 blocked nuclear factor–κ B (NF-κB) activation-induced bone marrow stroma–derived interleukin-6 production, inhibiting myeloma growth.4 Other sequelae of NF-κB blockade included down-regulation of antiapoptotic B-cell lymphoma/leukemia-2 (Bcl-2) protein, up-regulation of proapoptotic Bcl associated X protein (Bax),5 and inhibition of adhesion molecules.6 Proteasome inhibitors also suppressed p44/42 mitogen-activated protein kinase signaling,4 activated caspase-8– and -9–dependent apoptotic pathways,7 and induced p53 and c-Jun N-terminal kinase.8
Bortezomib3 (VELCADE, formerly PS-341), the first proteasome inhibitor to enter the clinic, was well-tolerated in phase 1 studies of patients with hematologic malignancies.9 Dose-limiting toxicities (DLTs) included thrombocytopenia, hyponatremia, hypokalemia, fatigue, and malaise. Evidence of antitumor activity was seen in 9 of 9 patients with plasma cell dyscrasias, including 1 durable complete response (CR). A multicenter phase 2 trial in heavily pretreated patients with relapsed/refractory MM used bortezomib at 1.3 mg/m2 on days 1, 4, 8, and 11, followed by a 10-day rest every 21-day cycle.10 Reduction of disease burden by at least 50% was seen in 27% of 193 patients with a 10% CR rate, including 4% who met the stringent European Group for Blood and Marrow Transplant CR criteria.11 These findings led to the approval of bortezomib for myeloma patients who had received at least 2 prior regimens and progressed on the last of these. Results from a randomized phase 3 study comparing bortezomib with high-dose dexamethasone have also been presented.12 Patients in the bortezomib arm experienced a longer time to progression and had a superior overall and 1-year survival at the interim analysis.
The proteasome is also a rational target for chemosensitization, and modulation of its function enhances the efficacy of chemotherapeutics.2,13 DNA-damaging agents including anthracyclines induce an NF-κB–driven antiapoptotic program, involving the Bcl-2 and inhibitor of apoptosis protein families, that was blocked by proteasome inhibition.14 These inhibitors may overcome drug resistance by interfering with P-glycoprotein15 and by inducing cleavage of Bcl-2.16 Conversely, anthracyclines suppress proteasome inhibitor–mediated induction of the antiapoptotic mitogen-activated protein kinase phosphatase 1 (MKP-1).17 Further supporting the promise of proteasome inhibitor/anthracycline combinations, in vitro studies have shown synergistic proapoptotic effects and the ability to overcome anthracycline resistance.18,19
To begin to evaluate the possibility that a proteasome inhibitor/anthracycline regimen could have enhanced clinical activity, we studied the maximum tolerated dose (MTD), DLTs, pharmacodynamics (PDs), and pharmacokinetics (PKs) of bortezomib in combination with pegylated liposomal doxorubicin (PegLD; Doxil/Caelyx). PegLD was used because it was anticipated that many patients would have had prior anthracycline-based therapy, and there was evidence that PegLD may have less cardiac toxicity.20 Also, the prolonged half-life (t1/2) of doxorubicin in the liposomal preparation allowed maximal overlap between the 2 agents with a convenient dosing schedule. Finally, PegLD was the only anthracycline for which there was in vivo data showing enhanced antitumor efficacy in combination with bortezomib.21 In this report we present the results of this study as well as preliminary evidence of antitumor activity.
Patients, materials, and methods
Patient selection
Patients with a confirmed hematologic malignancy who had relapsed or been refractory to at least 1 standard therapy, or who could not receive conventional therapy, or for whom no standard was available were candidates for this study. Inclusion criteria were as follows: (a) Karnofsky performance status of at least 60%; (b) age 18 years or older; (c) expected survival 2 months or longer; (d) no major surgery, radiation, or chemotherapy in the previous 3 weeks and no immunotherapy in the previous 4 weeks; (e) absence of active infection; (f) adequate hepatic function, with total bilirubin level less than 1.2 times the upper limit of normal (ULN) and aspartate and alanine aminotransferase levels less than 2.5 times the ULN; and (g) adequate coagulation function, with a prothrombin time and activated partial thromboplastin time of less than 1.5 times the ULN. Adequate bone marrow function was a white blood cell count of at least 2 × 109/L (2000/μL), absolute neutrophil count of at least 1 × 109/L (1000/μL), hemoglobin level of at least 80 g/L (8.0 g/dL), and a platelet count of at least 50 × 109/L (50 000/μL). Patients who were transfusion- or growth factor–dependent were allowed, provided these values could be achieved with transfusion. A prior doxorubicin exposure of 400 mg/m2 or less was required, and adequate cardiovascular function was defined as no ischemia, new conduction system abnormalities, class 3 or 4 New York Heart Association congestive heart failure, or myocardial infarction in the previous 6 months. A 50% left ventricular ejection fraction (LVEF) was initially required but later amended to 45%. Initially, the required serum creatinine level was 132.6 μM (1.5 mg/dL) or less but this was later amended to 221.0 μM (2.5 mg/dL) or less, or an estimated creatinine clearance level of at least 0.50 mL/s (30 mL/min).22 Exclusion criteria included the following: (a) pregnancy or breast feeding; (b) known human immunodeficiency virus seropositivity or active viral hepatitis; (c) known hypersensitivity to standard or liposomal doxorubicin preparations or boron-containing compounds; and (d) serious medical or psychiatric conditions that could interfere with treatment. All patients practiced effective birth control and gave written informed consent in accordance with federal and institutional guidelines before treatment. This study was approved by the University of North Carolina at Chapel Hill's Institutional Review Board, and performed according to the tenets of the Helsinki protocol.
Study design
This phase 1 trial was designed to determine the MTD and DLTs of bortezomib and PegLD and to study bortezomib PDs and doxorubicin PKs, whereas secondary objectives included obtaining preliminary response data. Toxicities were assessed using the National Cancer Institute (NCI) Common Toxicity Criteria version 2.0, with leukemia adverse events (AEs) criteria being applied to patients with known or suspected marrow involvement. DLT was defined based on toxicities during cycle 1 as a grade 4 hematologic toxicity or a grade 3 toxicity in any other system except alopecia. Grade 4 neutropenia was not a DLT unless it was associated with fever or lasted longer than 5 days, and nausea, vomiting, and diarrhea were not DLTs if they could be managed with standard antiemetic and antidiarrheal medications. MTD was intended to be defined as the highest dose studied for which the incidence of DLTs was less than 33%. It was anticipated that at the next higher dose level at least 2 patients out of the initial group of up to 6 would have experienced a DLT.
Bortezomib was dosed in mg/m2, and a modified Fibonacci escalation was used with dose steps of 0.90, 1.05, 1.20, 1.30, 1.40, and 1.50 mg/m2. For dose escalation, 3 evaluable patients had to complete cycle 1 without a DLT. If one DLT was seen then an additional 3 evaluable patients were accrued, and further escalation occurred if no additional DLTs were seen. Additional patients were accrued once the recommended dose had been identified to confirm the safety of this dose.
Drug administration
Millennium Pharmaceuticals (Cambridge, MA) provided bortezomib as a sterile, lyophilized powder in vials with mannitol, which was reconstituted with normal saline to a drug concentration of 1 mg/mL. Bortezomib was administered by intravenous push over 3 to 5 seconds on days 1, 4, 8, and 11, followed by a 10-day rest period, with this 3-week period comprising 1 cycle. PegLD was purchased through the University of North Carolina at Chapel Hill Memorial Hospital Pharmacy from Tibotec Therapeutics (Bridgewater, NJ), handled as directed by the package insert and material safety data sheet, and administered as a 1-hour infusion at 30 mg/m2 on day 4 of each cycle, 1 hour after that day's bortezomib. Day 4 was chosen to allow collection of proteasome inhibition data on days 1 and 4 in the presence of bortezomib alone, allowing each patient to serve as a control, and data on days 8 and 11 with both drugs present. Treatment days could be changed by up to 24 hours providing there was at least a 72-hour span between consecutive bortezomib doses.
Response criteria
For patients with nonmyelomatous hematologic malignancies, CR was defined as total resolution of measurable disease parameters, whereas a partial response (PR) was defined as a 50% or greater resolution without the appearance of new disease. Minor responses (MRs) were those in which disease decreased by at least 25% but less than 50%, and stable disease comprised between a less than 25% resolution but a 25% or greater increase in measurable parameters, both without new disease. Progressive disease was defined as an increase of greater than 25% in measurable disease parameters. For patients with MM, the European Group for Blood and Marrow Transplantation criteria were used to define responses.11
Pharmacodynamics and pharmacokinetics
Bortezomib rapidly exits the intravascular compartment. Therefore, standard PK parameters do not adequately guide dosing, and a PD assay measuring the percentage proteasome inhibition was used to provide a more relevant characterization.23 Blood samples were collected at baseline and 1 hour after bortezomib for measurement of 20S-proteasome activity during cycle 1. PegLD PKs were studied by collecting blood samples at baseline and at 1, 24, and 96 hours and 7, 14, and 18 days after PegLD administration. Doxorubicin released from the liposomal preparation was evaluated by high-performance liquid chromatography.24,25 Compartmental and noncompartmental analysis was conducted using WinNonlin Professional software, version 3.2 (Pharsight Corporation, Mountain View, CA).
Results
Patients
A total of 42 patients with advanced hematologic malignancies were enrolled and treated between December 2001 and September 2004. These patients received a total of 208 cycles of bortezomib and PegLD, with a median of 4 cycles per patient (range, 1-11 cycles). Demographic characteristics, diagnoses, performance status, and prior therapies for this population are detailed in Table 1.
Patient characteristics
Patient characteristic . | No. (%)* . |
|---|---|
| Total no. of patients | 42 |
| Sex | |
| Female | 19 (45) |
| Male | 23 (55) |
| Age, y | |
| Mean | 59 |
| Range | 35-82 |
| Race | |
| African American | 7 (17) |
| Asian American | 1 (2) |
| Caucasian American | 33 (79) |
| Other | 1 (2) |
| Diagnoses | |
| Acute myeloid leukemia | 5 (12) |
| Chronic lymphocytic leukemia | 2 (5) |
| Hairy cell leukemia | 1 (2) |
| Hodgkin disease | 2 (5) |
| Multiple myeloma | 24 (57) |
| Myelodysplasia with excess blasts | 1 (2) |
| Non-Hodgkin lymphoma | 7 (17) |
| Karnofsky performance status | |
| 100 | 3 (7) |
| 90-80 | 32 (76) |
| 70-60 | 7 (17) |
| Prior therapy | |
| Chemotherapy | 40 (95) |
| Median number of regimens | 5 |
| Range | 0-14 |
| Radiation therapy | 16 (38) |
| Marrow or stem cell transplantation | 11 (26) |
Patient characteristic . | No. (%)* . |
|---|---|
| Total no. of patients | 42 |
| Sex | |
| Female | 19 (45) |
| Male | 23 (55) |
| Age, y | |
| Mean | 59 |
| Range | 35-82 |
| Race | |
| African American | 7 (17) |
| Asian American | 1 (2) |
| Caucasian American | 33 (79) |
| Other | 1 (2) |
| Diagnoses | |
| Acute myeloid leukemia | 5 (12) |
| Chronic lymphocytic leukemia | 2 (5) |
| Hairy cell leukemia | 1 (2) |
| Hodgkin disease | 2 (5) |
| Multiple myeloma | 24 (57) |
| Myelodysplasia with excess blasts | 1 (2) |
| Non-Hodgkin lymphoma | 7 (17) |
| Karnofsky performance status | |
| 100 | 3 (7) |
| 90-80 | 32 (76) |
| 70-60 | 7 (17) |
| Prior therapy | |
| Chemotherapy | 40 (95) |
| Median number of regimens | 5 |
| Range | 0-14 |
| Radiation therapy | 16 (38) |
| Marrow or stem cell transplantation | 11 (26) |
Values in this column are number of patients, and parenthetical values are percentages, except when indicated otherwise in the row headings.
Dose escalation
The first patient cohort received bortezomib at 0.90 mg/m2/dose and 30 mg/m2 of PegLD. At this dose step, the first patient developed DLTs including grade 3 diarrhea, hypotension, confusion, syncope, and suspected aspiration pneumonia. These occurred in the setting of poorly controlled Crohn disease with self-administration of deodorized tincture of opium, and all were resolved with medical management. An additional 5 patients were enrolled with no further DLTs, leading to dose escalation of bortezomib to 1.05 mg/m2. One patient in this cohort with rapidly progressive MM and a high paraprotein level developed signs of hyperviscosity including headaches and was removed from the study to undergo plasmapheresis and exchange. Since completion of cycle 1 was required for determination of DLTs and concurrent plasmapheresis was an exclusion factor for protocol therapy, this patient was replaced and no DLTs were noted at this level. Among patients at the 1.20 mg/m2 dose, there was 1 episode of grade 3 intestinal obstruction found by computed tomography (CT) to be due to a volvulus around pre-existing abdominal adhesions, which resolved spontaneously and was considered not drug related. This patient did not complete the full first cycle and was replaced, with no DLTs at this level. For the 1.30 mg/m2 dose step, the first 2 patients accrued could not complete cycle 1 due to 1 case each of progressive acute myeloid leukemia (AML) and NHL. These patients were replaced and no DLTs were initially noted in this cohort, though subsequently an episode of grade 3 hypertension was identified. After dose escalation of bortezomib to 1.40 mg/m2, grade 4 neutropenia was seen in a single patient with plasma cell leukemia at this level without other DLTs. Finally, constipation of grade 3 severity was seen at 1.50 mg/m2 in 1 patient, again without other DLTs, concluding the planned dose escalations.
According to the initial protocol definition of MTD based on cycle 1, bortezomib at 1.50 mg/m2 and PegLD at 30 mg/m2 met these criteria. Further bortezomib dose escalation was not pursued since this would have exceeded its known single-agent MTD.26 Due to the frequent need for dose delays and dose reductions in later cycles at this level, however, and at 1.40 mg/m2, as described in “Adverse events,” bortezomib at 1.30 mg/m2 and PegLD at 30 mg/m2 were chosen for further testing. An additional 11 patients were accrued to gain further experience, among whom 3 developed grade 3 or 4 events, including 1 episode each of febrile neutropenia, peripheral neuropathy, and hyponatremia with bowel obstruction, which resolved with correction of the hyponatremia. Of the final total of 42 patients treated, 6 were at the 0.90 mg/m2 bortezomib dose step, 4 each received 1.05 and 1.20 mg/m2, 16 were treated with 1.30 mg/m2/dose, and 6 each received 1.40 and 1.50 mg/m2/dose.
Adverse events
Adverse events were documented in all patients at all dose levels, with 158 different event types and a total of 1284 occurrences. Among those events seen in at least 10% of patients irrespective of grade or relationship to drug therapy, listed in Table 2, fatigue was most common, with the next most frequent constitutional symptoms being anorexia and weight loss. Hematologic toxicities were prominent with thrombocytopenia, lymphopenia, and anemia, all seen in at least 50% of patients. Gastrointestinal events included nausea, constipation, and diarrhea, whereas frequent neurologic events included peripheral neuropathy, headache, back pain, and myalgias. Additional toxicities of note included rash as well as hand-foot syndrome (HFS; also known as palmar plantar erythrodysesthesia [PPE]), mucositis, cough, and bruising or ecchymosis.
All adverse events occurring in at least 10% of patients, N = 42
Adverse event . | Incidence, n (%) . |
|---|---|
| Fatigue | 37 (88) |
| Thrombocytopenia | 29 (69) |
| Lymphopenia | 27 (64) |
| Nausea | 27 (64) |
| Constipation | 25 (60) |
| Peripheral neuropathy | 23 (55) |
| Anemia | 22 (52) |
| Neutropenia | 19 (45) |
| Anorexia | 18 (43) |
| Rash | 17 (40) |
| Diarrhea | 16 (38) |
| Mucositis | 14 (33) |
| Headache | 10 (24) |
| Cough | 10 (24) |
| Back pain | 9 (21) |
| Weight loss | 9 (21) |
| PPE/HFS* | 9 (21) |
| Bruising or ecchymosis | 9 (21) |
| Myalgia | 8 (19) |
| Fever | 8 (19) |
| Sinusitis | 8 (19) |
| Pneumonia | 8 (19) |
| Dyspnea | 7 (17) |
| Dysgeusia | 6 (14) |
| Extremity edema | 6 (14) |
| Arthralgia | 5 (12) |
| Depression | 5 (12) |
| Hypokalemia | 5 (12) |
| Hypotension | 5 (12) |
| Febrile neutropenia | 4 (10) |
| Vomiting | 4 (10) |
| Bloating | 4 (10) |
| Insomnia | 4 (10) |
| Gastroesophageal reflux | 4 (10) |
| Tachycardia | 4 (10) |
| Flulike symptoms | 4 (10) |
| Urinary tract infection | 4 (10) |
| Abdominal pain | 4 (10) |
Adverse event . | Incidence, n (%) . |
|---|---|
| Fatigue | 37 (88) |
| Thrombocytopenia | 29 (69) |
| Lymphopenia | 27 (64) |
| Nausea | 27 (64) |
| Constipation | 25 (60) |
| Peripheral neuropathy | 23 (55) |
| Anemia | 22 (52) |
| Neutropenia | 19 (45) |
| Anorexia | 18 (43) |
| Rash | 17 (40) |
| Diarrhea | 16 (38) |
| Mucositis | 14 (33) |
| Headache | 10 (24) |
| Cough | 10 (24) |
| Back pain | 9 (21) |
| Weight loss | 9 (21) |
| PPE/HFS* | 9 (21) |
| Bruising or ecchymosis | 9 (21) |
| Myalgia | 8 (19) |
| Fever | 8 (19) |
| Sinusitis | 8 (19) |
| Pneumonia | 8 (19) |
| Dyspnea | 7 (17) |
| Dysgeusia | 6 (14) |
| Extremity edema | 6 (14) |
| Arthralgia | 5 (12) |
| Depression | 5 (12) |
| Hypokalemia | 5 (12) |
| Hypotension | 5 (12) |
| Febrile neutropenia | 4 (10) |
| Vomiting | 4 (10) |
| Bloating | 4 (10) |
| Insomnia | 4 (10) |
| Gastroesophageal reflux | 4 (10) |
| Tachycardia | 4 (10) |
| Flulike symptoms | 4 (10) |
| Urinary tract infection | 4 (10) |
| Abdominal pain | 4 (10) |
Palmar plantar erythrodysesthesia/hand-foot syndrome.
Among the grade 3 or 4 AEs occurring in any cycle, thrombocytopenia was the most commonly reported and occurred in 18 patients (43%; Table 3). Of the 2 episodes of lower gastrointestinal bleeding, only 1 was in a patient with grade 3 thrombocytopenia; both patients had a prior history of bleeding episodes and there were no other significant hemorrhages. Lymphopenia was seen in 17 patients followed by neutropenia in 7, which was complicated by fever in 4, of whom 3 had either AML or myelodysplasia. Additional infectious complications included sinusitis in 2 patients, 3 patients had fever without neutropenia and infection, and pneumonia occurred in 6 patients. Fatigue was a prominent grade 3 AE, occurring in 6 patients, whereas anorexia was seen in 2. Treatment-emergent peripheral neuropathy to a severity of grade 3 or 4 occurred in 5 patients, all of whom were either removed from study or had dose delays and reductions. Notable gastrointestinal events included diarrhea in 4 patients and bowel obstruction in 3, with 2 of the latter being due to volvulus around pre-existing adhesions, as well as 1 episode of constipation with abdominal pain. All of the latter 3 patients were able to restart therapy after resolution of their obstruction without recurrent gastrointestinal difficulties. Additional grade 3 or 4 events that occurred in 1 patient each, and were at least possibly drug related, included a PegLD-related allergic reaction, anemia, ataxia, atrial fibrillation, confusion, dehydration, deep vein thrombosis, Varicella zoster reactivation, Clostridium difficile infection, hypokalemia or hyponatremia, impotence, mental status changes, pleuritic chest pain, pleural effusion, PPE/HFS, rash, and tachycardia. Listeria monocytogenes bacteremia occurred in 1 patient with indolent NHL during cycle 2 at the 1.40 mg/m2 dose level of bortezomib. This patient had received 14 prior lines of therapy including 4 fludarabine-based regimens, which are associated with Listeria infections in patients with chronic lymphocytic leukemia, and was able to resume therapy subsequently without further infectious complications.
All adverse events of intensity of at least grade 3 occurring in at least 2 patients
. | Bortezomib dose level, mg/m2 . | . | . | . | . | . | . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adverse event . | 0.90, N = 6, n (%) . | 1.05, N = 4, n (%) . | 1.20, N = 4, n (%) . | 1.30, N = 16, n (%) . | 1.40, N = 6, n (%) . | 1.50, N = 6, n (%) . | Total, N = 42, n (%) . | ||||||
| Thrombocytopenia | 1 (17) | 3 (75) | 1 (25) | 8 (50) | 3 (50) | 2 (33) | 18 (43) | ||||||
| Lymphopenia | 1 (17) | 3 (75) | 0 | 7 (44) | 2 (33) | 4 (67) | 17 (40) | ||||||
| Neutropenia | 1 (17) | 0 | 1 (25) | 1 (6) | 3 (50) | 1 (17) | 7 (17) | ||||||
| Fatigue | 0 | 1 (25) | 1 (25) | 2 (13) | 1 (17) | 1 (17) | 6 (14) | ||||||
| Pneumonia | 2 (33) | 0 | 0 | 2 (13) | 0 | 2 (33) | 6 (14) | ||||||
| Peripheral neuropathy | 0 | 1 (25) | 0 | 1 (6) | 1 (17) | 2 (33) | 5 (12) | ||||||
| Febrile neutropenia | 1 (17) | 1 (25) | 1 (25) | 1 (6) | 0 | 1 (17) | 4 (10) | ||||||
| Diarrhea | 1 (17) | 0 | 0 | 1 (6) | 0 | 2 (33) | 4 (10) | ||||||
| Bowel obstruction | 0 | 0 | 2 (50) | 1 (6) | 0 | 0 | 3 (7) | ||||||
| Syncope | 1 (17) | 0 | 0 | 0 | 1 (17) | 1 (17) | 3 (7) | ||||||
| Fever | 1 (17) | 0 | 0 | 1 (6) | 0 | 1 (17) | 3 (7) | ||||||
| Anorexia | 0 | 0 | 0 | 2 (13) | 0 | 0 | 2 (5) | ||||||
| Dyspnea | 0 | 0 | 0 | 1 (6) | 0 | 1 (17) | 2 (5) | ||||||
| Hypotension | 1 (17) | 0 | 0 | 1 (6) | 0 | 0 | 2 (5) | ||||||
| Lower gastrointestinal bleeding | 1 (17) | 0 | 0 | 0 | 0 | 1 (17) | 2 (5) | ||||||
| Sinusitis | 1 (17) | 0 | 0 | 1 (6) | 0 | 0 | 2 (5) | ||||||
. | Bortezomib dose level, mg/m2 . | . | . | . | . | . | . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adverse event . | 0.90, N = 6, n (%) . | 1.05, N = 4, n (%) . | 1.20, N = 4, n (%) . | 1.30, N = 16, n (%) . | 1.40, N = 6, n (%) . | 1.50, N = 6, n (%) . | Total, N = 42, n (%) . | ||||||
| Thrombocytopenia | 1 (17) | 3 (75) | 1 (25) | 8 (50) | 3 (50) | 2 (33) | 18 (43) | ||||||
| Lymphopenia | 1 (17) | 3 (75) | 0 | 7 (44) | 2 (33) | 4 (67) | 17 (40) | ||||||
| Neutropenia | 1 (17) | 0 | 1 (25) | 1 (6) | 3 (50) | 1 (17) | 7 (17) | ||||||
| Fatigue | 0 | 1 (25) | 1 (25) | 2 (13) | 1 (17) | 1 (17) | 6 (14) | ||||||
| Pneumonia | 2 (33) | 0 | 0 | 2 (13) | 0 | 2 (33) | 6 (14) | ||||||
| Peripheral neuropathy | 0 | 1 (25) | 0 | 1 (6) | 1 (17) | 2 (33) | 5 (12) | ||||||
| Febrile neutropenia | 1 (17) | 1 (25) | 1 (25) | 1 (6) | 0 | 1 (17) | 4 (10) | ||||||
| Diarrhea | 1 (17) | 0 | 0 | 1 (6) | 0 | 2 (33) | 4 (10) | ||||||
| Bowel obstruction | 0 | 0 | 2 (50) | 1 (6) | 0 | 0 | 3 (7) | ||||||
| Syncope | 1 (17) | 0 | 0 | 0 | 1 (17) | 1 (17) | 3 (7) | ||||||
| Fever | 1 (17) | 0 | 0 | 1 (6) | 0 | 1 (17) | 3 (7) | ||||||
| Anorexia | 0 | 0 | 0 | 2 (13) | 0 | 0 | 2 (5) | ||||||
| Dyspnea | 0 | 0 | 0 | 1 (6) | 0 | 1 (17) | 2 (5) | ||||||
| Hypotension | 1 (17) | 0 | 0 | 1 (6) | 0 | 0 | 2 (5) | ||||||
| Lower gastrointestinal bleeding | 1 (17) | 0 | 0 | 0 | 0 | 1 (17) | 2 (5) | ||||||
| Sinusitis | 1 (17) | 0 | 0 | 1 (6) | 0 | 0 | 2 (5) | ||||||
Drug-related AEs frequently influenced decisions about both dose timing as well as the absolute dose administered. Doses of either bortezomib, PegLD, or both could be delayed by up to 2 weeks to allow recovery from a toxicity, and dose reductions of either or both could also be performed. Since an MTD could not be identified according to the DLTs seen in cycle 1, the frequency of dose delays and reductions was used to determine a recommended dose. Delays were particularly frequent above the 1.30 mg/m2 level (Table 4), occurring in 5 of 6 patients treated at either 1.40 mg/m2 or 1.50 mg/m2 of bortezomib. Moreover, 44% and 32% of cycles delivered at the 1.40 mg/m2 or 1.50 mg/m2 levels, respectively, were delayed compared with only 10% to 25% at the lower steps. The frequency of dose reductions of bortezomib or PegLD was also related to the absolute bortezomib dose level (Table 5), with 3 reductions being needed at 1.50 mg/m2 and 1 at 1.40 mg/m2, whereas none were needed among the initial patient cohort at 1.30 mg/m2. Table 6 indicates the distribution of grade 3 or 4 AEs in the 1.30 mg/m2 dose cohort according to the cycle of therapy. Thus, 1.30 mg/m2 of bortezomib and 30 mg/m2 of PegLD is recommended for further study as a regimen that can be administered for several cycles with an acceptable risk of dose delays or reductions.
Therapy-related dose delays in patients receiving bortezomib and PegLD
Bortezomib dose level, mg/m2 . | Patients with at least 1 delay, n, (%) . | Cycles with at least 1 delay,*n, (%) . | Reasons for dose delays that were at least possibly attributable to therapy with bortezomib/PegLD (n) . |
|---|---|---|---|
| 0.90 | 3/6 (50) | 7/34 (21) | Febrile neutropenia (2), Varicella zoster (1), neutropenia (1), neutropenia and fatigue (1), hypotension (1), atrial fibrillation (1) |
| 1.05 | 3/4 (75) | 5/27 (19) | Febrile neutropenia (1), thrombocytopenia (1), neutropenia and thrombocytopenia (1), PPE/HFS (1), fatigue and neuropathy (1) |
| 1.20 | 1/4 (25) | 2/21 (10) | Mucositis and rash (1), fatigue (1) |
| 1.30 | 2/5 (40) | 4/20 (25) | Fatigue (2), thrombocytopenia (1), neutropenia (1), fatigue and anorexia (1) |
| 1.40 | 5/6 (83) | 14/32 (44) | Thrombocytopenia (13), thrombocytopenia and neutropenia (1), and neutropenia (1) |
| 1.50 | 5/6 (83) | 11/34 (32) | Neutropenia (3), leg weakness (2), diarrhea (2), diarrhea with fatigue (1), diarrhea with worsening neuropathy (1), PPE/HFS (1), myalgias (1) |
Bortezomib dose level, mg/m2 . | Patients with at least 1 delay, n, (%) . | Cycles with at least 1 delay,*n, (%) . | Reasons for dose delays that were at least possibly attributable to therapy with bortezomib/PegLD (n) . |
|---|---|---|---|
| 0.90 | 3/6 (50) | 7/34 (21) | Febrile neutropenia (2), Varicella zoster (1), neutropenia (1), neutropenia and fatigue (1), hypotension (1), atrial fibrillation (1) |
| 1.05 | 3/4 (75) | 5/27 (19) | Febrile neutropenia (1), thrombocytopenia (1), neutropenia and thrombocytopenia (1), PPE/HFS (1), fatigue and neuropathy (1) |
| 1.20 | 1/4 (25) | 2/21 (10) | Mucositis and rash (1), fatigue (1) |
| 1.30 | 2/5 (40) | 4/20 (25) | Fatigue (2), thrombocytopenia (1), neutropenia (1), fatigue and anorexia (1) |
| 1.40 | 5/6 (83) | 14/32 (44) | Thrombocytopenia (13), thrombocytopenia and neutropenia (1), and neutropenia (1) |
| 1.50 | 5/6 (83) | 11/34 (32) | Neutropenia (3), leg weakness (2), diarrhea (2), diarrhea with fatigue (1), diarrhea with worsening neuropathy (1), PPE/HFS (1), myalgias (1) |
The data shown include only the first cohort of patients accrued to allow determination of the MTD and DLTs and do not include delays in the 11 patients added at 1.30 mg/m2 for further experience. Dose delays occurring in any cycle are shown.
The fraction shown is the number of unique cycles delayed/total number of cycles given to all patients at this dose level.
Dose reductions in patients receiving bortezomib and PegLD
Bortezomib dose level, mg/m2 . | Drug reduced (dose in mg/m2) . | Cycle and day* . | Reason . |
|---|---|---|---|
| 1.05 | PegLD (to 22.5) | c4, d4 | PPE/HFS2 |
| 1.30† | Bortezomib (to 1.05) | c4, d1 | Decreased quality of life |
| 1.30† | Bortezomib (to 1.05) | c5, d1 | Peripheral neuropathy |
| 1.40 | Bortezomib (to 1.05) | c3, d1 | Thrombocytopenia |
| 1.50 | Bortezomib (to 1.30) | c3, d8 | Peripheral neuropathy |
| 1.50 | Bortezomib (to 1.05) | c4, d8 | Diarrhea and fatigue |
| 1.50 | Bortezomib (to 1.30) | c6, d8 | Diarrhea and nausea |
| 1.50 | Bortezomib (to 1.30) | c3, d1 | Peripheral neuropathy |
| 1.50 | PegLD (to 22.5) | c3, d1 | PPE/HFS |
Bortezomib dose level, mg/m2 . | Drug reduced (dose in mg/m2) . | Cycle and day* . | Reason . |
|---|---|---|---|
| 1.05 | PegLD (to 22.5) | c4, d4 | PPE/HFS2 |
| 1.30† | Bortezomib (to 1.05) | c4, d1 | Decreased quality of life |
| 1.30† | Bortezomib (to 1.05) | c5, d1 | Peripheral neuropathy |
| 1.40 | Bortezomib (to 1.05) | c3, d1 | Thrombocytopenia |
| 1.50 | Bortezomib (to 1.30) | c3, d8 | Peripheral neuropathy |
| 1.50 | Bortezomib (to 1.05) | c4, d8 | Diarrhea and fatigue |
| 1.50 | Bortezomib (to 1.30) | c6, d8 | Diarrhea and nausea |
| 1.50 | Bortezomib (to 1.30) | c3, d1 | Peripheral neuropathy |
| 1.50 | PegLD (to 22.5) | c3, d1 | PPE/HFS |
The cycle (c) and day (d) at which reductions were pursued are indicated after the respective abbreviations. In the first case at 1.50, a patient had 2 separate bortezomib dose reductions, whereas in the last case, both bortezomib and PegLD were reduced concomitantly.
Both dose reductions at 1.30 mg/m2 occurred in the expanded cohort at this level and these data therefore did not contribute to the determination of a dose recommended for further study.
Adverse events of at least grade 3 intensity occurring in patients at the recommended dose level of bortezomib and PegLD
Cycle no. (n)* . | Adverse event (n)† . |
|---|---|
| 1 (16) | Detailed in the section, “Adverse events” |
| 2 (10) | Allergic reaction (1), hypertension (1), hyponatremia (1), neutropenia (1), pneumonia (1), lymphopenia (2), thrombocytopenia (4) |
| 3 (10) | Hypertension (1), hyponatremia (1), peripheral neuropathy/impotence (1), pneumonia (1), lymphopenia (5), thrombocytopenia (5) |
| 4 (9) | Clostridium difficile infection (1), confusion (1), diarrhea (1), hypertension (1), sinusitis (1), vein thrombosis (1), peripheral neuropathy (2), thrombocytopenia (4), lymphopenia (5) |
| 5 (6) | Diarrhea (1), fever (1), thrombocytopenia (2), lymphopenia (3) |
| 6 (6) | Anorexia (1), thrombocytopenia (1), fatigue (1), lymphopenia (3) |
| 7 (3) | Diarrhea (1), lymphopenia (1) |
| 8 (2) | Lymphopenia (2) |
Cycle no. (n)* . | Adverse event (n)† . |
|---|---|
| 1 (16) | Detailed in the section, “Adverse events” |
| 2 (10) | Allergic reaction (1), hypertension (1), hyponatremia (1), neutropenia (1), pneumonia (1), lymphopenia (2), thrombocytopenia (4) |
| 3 (10) | Hypertension (1), hyponatremia (1), peripheral neuropathy/impotence (1), pneumonia (1), lymphopenia (5), thrombocytopenia (5) |
| 4 (9) | Clostridium difficile infection (1), confusion (1), diarrhea (1), hypertension (1), sinusitis (1), vein thrombosis (1), peripheral neuropathy (2), thrombocytopenia (4), lymphopenia (5) |
| 5 (6) | Diarrhea (1), fever (1), thrombocytopenia (2), lymphopenia (3) |
| 6 (6) | Anorexia (1), thrombocytopenia (1), fatigue (1), lymphopenia (3) |
| 7 (3) | Diarrhea (1), lymphopenia (1) |
| 8 (2) | Lymphopenia (2) |
Number of patients treated who were at risk for an adverse event.
Number of patients treated who developed the indicated event.
Seven patients treated on this study died within 30 days of their last protocol-directed therapy. This included 3 patients with AML, 2 with NHL, and 1 each with atypical hairy cell leukemia and Hodgkin disease. Six of these were judged to have died from disease progression or a progression-associated complication. The only exception occurred in an 82-year-old with AML who developed shortness of breath during cycle 3, and chest radiography showed bilateral alveolar infiltrates without cardiogenic edema. He was treated for community-acquired pneumonia with oral and then intravenous antibiotics but worsened both clinically and radiographically. Further diagnostic measures were not pursued at the request of the family and the patient died of respiratory failure. Another 7 patient deaths occurred beyond 30 days from the last dose of bortezomib or PegLD, all due to disease progression.
Pharmacodynamics and pharmacokinetics
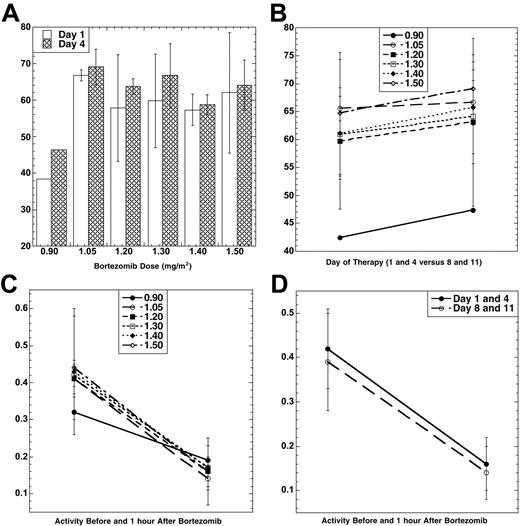
Bortezomib PD was evaluated using an ex vivo assay of the 20S-proteasome.23 The mean percent inhibition 1 hour after each bortezomib dose compared with the pretreatment baseline (Figure 1A) was lowest at 0.90 mg/m2. Among the doses from 1.05 to 1.50 mg/m2, however, inhibition was comparable, and no significant difference was noted between days 1 and 4. To study if PegLD could impact upon bortezomib-induced effects, proteasome inhibition was compared on days 1 and 4 (in the presence only of bortezomib) with days 8 and 11 (when bortezomib and PegLD were present; Figure 1B). Mean proteasome inhibition on days 8 and 11 (67.4%) was higher than that measured on days 1 and 4 (63.7%) by an absolute value of 3.4% (P = .02 for 2-sample t test). The effect of bortezomib and PegLD on the specific activity of the chymotryptic proteasome protease was also evaluated. Specific activity decreased with bortezomib (Figure 1C), with baseline and 1 hour posttherapy activities being comparable across the 1.05 to 1.50 mg/m2/dose range. Finally, the mean specific activity at baseline and 1 hour after dosing was studied on days 1 and 4 and compared with days 8 and 11. This activity declined in both situations by approximately the same amount, 0.26 on days 1 and 4 versus 0.25 on days 8 and 11 (P = .80 for 2-sample t test). Thus, the bortezomib-induced decline of the specific activity of the proteasome did not depend on whether PegLD was present.
Pharmacodynamics of bortezomib and PegLD. (A) Inhibition of the chymotryptic activity of the 20S-proteasome by bortezomib is shown as a function of the administered dose level (in mg/m2). The mean percentage inhibition 1 hour after each dose compared with the pretreatment baseline is shown, along with the standard deviation (SD), for days 1 (□) and 4 (▤). Data were available from only 1 patient at the 0.90 mg/m2 dose level due to an error in processing the other 5 patient's samples. (B) The 20S-proteasome inhibition (± SD) induced by bortezomib alone on days 1 and 4 is compared with mean inhibition on days 8 and 11, when both bortezomib and PegLD are present. The mean percentage inhibition 1 hour after each dose on days 1 and 4 for all patients at each dose level (•, 0.90 mg/m2; ○, 1.05 mg/m2; ▪, 1.20 mg/m2; □, 1.30 mg/m2; ♦, 1.40 mg/m2; ⋄, 1.50 mg/m2) is compared with the mean percentage inhibition on days 8 and 11. (C) Specific activity (mean ± SD) of the chymotrypsin-like proteasome protease is shown at baseline on day 1 and then 1 hour after bortezomib for each of the 6 dose levels of this proteasome inhibitor studied (symbols indicate same dose levels as in panel B). The units for specific activity are picomoles of fluorescent chromophore released per second per milligram of total protein. (D) Proteasome activity is shown at baseline and 1 hour after dosing with either bortezomib alone (mean specific activity ± SD on days 1 and 4, •) or bortezomib in the presence of PegLD (mean specific activity ± SD on days 8 and 11, ○).
Pharmacodynamics of bortezomib and PegLD. (A) Inhibition of the chymotryptic activity of the 20S-proteasome by bortezomib is shown as a function of the administered dose level (in mg/m2). The mean percentage inhibition 1 hour after each dose compared with the pretreatment baseline is shown, along with the standard deviation (SD), for days 1 (□) and 4 (▤). Data were available from only 1 patient at the 0.90 mg/m2 dose level due to an error in processing the other 5 patient's samples. (B) The 20S-proteasome inhibition (± SD) induced by bortezomib alone on days 1 and 4 is compared with mean inhibition on days 8 and 11, when both bortezomib and PegLD are present. The mean percentage inhibition 1 hour after each dose on days 1 and 4 for all patients at each dose level (•, 0.90 mg/m2; ○, 1.05 mg/m2; ▪, 1.20 mg/m2; □, 1.30 mg/m2; ♦, 1.40 mg/m2; ⋄, 1.50 mg/m2) is compared with the mean percentage inhibition on days 8 and 11. (C) Specific activity (mean ± SD) of the chymotrypsin-like proteasome protease is shown at baseline on day 1 and then 1 hour after bortezomib for each of the 6 dose levels of this proteasome inhibitor studied (symbols indicate same dose levels as in panel B). The units for specific activity are picomoles of fluorescent chromophore released per second per milligram of total protein. (D) Proteasome activity is shown at baseline and 1 hour after dosing with either bortezomib alone (mean specific activity ± SD on days 1 and 4, •) or bortezomib in the presence of PegLD (mean specific activity ± SD on days 8 and 11, ○).
Pharmacokinetic parameters of PegLD in the presence of bortezomib were determined by detection of doxorubicin released from pegylated liposomes.24,25 The peak plasma concentration of doxorubicin after single-dose administration, area under the concentration time curve, total plasma body clearance (CL), volume of distribution, and t1/2 were determined at each dose level and for the entire cohort (Table 7). There was no significant trend detected for a change in these parameters with the bortezomib dose, which in this study did not therefore impact on the PKs of PegLD.
Doxorubicin pharmacokinetics
Pharmacokinetic parameter . | Mean for all dose levels, n = 35 . |
|---|---|
| Cmax, mg/mL | 18.0 ± 4.4 (11.1-29.9) |
| AUC, (mg/mL) × h | 2010 ± 802 (535-3600) |
| CL, mL/h | 33.6 ± 20.0 (12.0-123.61) |
| Vss, mL | 3090 ± 1150 (468-6020) |
| t1/2, h | 79.9 ± 29.5 (27.0-167.0) |
Pharmacokinetic parameter . | Mean for all dose levels, n = 35 . |
|---|---|
| Cmax, mg/mL | 18.0 ± 4.4 (11.1-29.9) |
| AUC, (mg/mL) × h | 2010 ± 802 (535-3600) |
| CL, mL/h | 33.6 ± 20.0 (12.0-123.61) |
| Vss, mL | 3090 ± 1150 (468-6020) |
| t1/2, h | 79.9 ± 29.5 (27.0-167.0) |
All values are expressed as the mean ± the standard deviation. The range is shown in parentheses. Cmax indicates peak plasma concentration after single dose administration; AUC, area under the concentration time curve; CL, total plasma body clearance; Vss, volume of distribution at steady state; and t1/2, half-life.
Antitumor activity
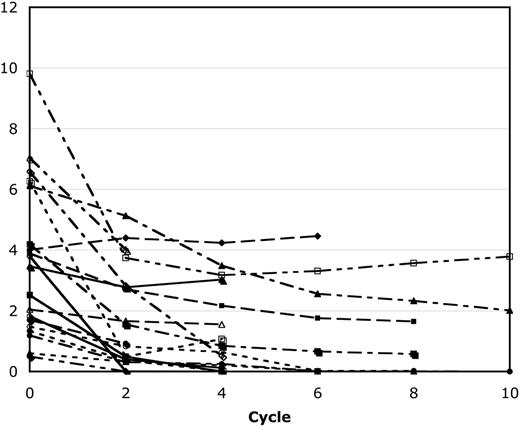
Twenty-four MM patients enrolled onto this study, 22 of whom completed at least 2 cycles of therapy, which was required to be evaluable for a response. One inevaluable patient was removed because of the DLT at 0.90 mg/m2, whereas the other was removed to undergo plasmapheresis and exchange, as detailed previously. Figure 2 shows the paraprotein response for all patients with a detectable serum monoclonal protein treated with bortezomib/PegLD. Among the 22 evaluable patients, 5 achieved a CR as defined by the European Group for Blood and Marrow Transplantation criteria.11 Another 3 patients had a near-CR, with a trace residual paraprotein on immunofixation. All but one of these occurred at or below the recommended dose level, with CR seen in 2 patients at 1.05 mg/m2 and 1 each at the 0.90, 1.30, and 1.50 mg/m2 doses, whereas near-CR was seen in 1 patient each at 1.05, 1.20, and 1.30 mg/m2. Among patients with a CR or near-CR, 2 achieved their best response within 2 cycles, 3 attained this within 4 cycles, 2 reached this by cycle 6, whereas 1 did not do so until cycle 10. Only 2 of these patients required dose reductions during their therapy, including 1 at the 1.05 mg/m2 dose step, whose PegLD was reduced by 25% due to PPE/HFS, and 1 at 1.50 mg/m2, whose bortezomib was reduced to 1.30 mg/m2 due to neuropathy. An additional 8 patients had PRs, 5 patients had either an MR or stable disease, and only 1 patient who completed the first 2 cycles of therapy progressed. Thirteen MM patients had previously received anthracycline-based regimens at some point and were reported to have progression, stable disease, or an initial response followed by either progression or stable disease. Four of these had a CR, 1 had a near-CR, and 3 had PRs after bortezomib/PegLD.
Response of patients with secretory multiple myeloma to bortezomib and PegLD. The serum monoclonal protein level of all 19 patients with secretory multiple myeloma who were evaluable for a response is shown both at baseline and after every 2 cycles of therapy with bortezomib and PegLD. Out of the total of 22 evaluable patients, 2 with light chain only disease and 1 with nonsecretory disease are excluded from this graph. Also, 2 of these patients received a total of 11 cycles but then withdrew when bortezomib was approved by the Food and Drug Administration to receive additional treatment with their primary oncologist, and their results are shown only through cycle 10.
Response of patients with secretory multiple myeloma to bortezomib and PegLD. The serum monoclonal protein level of all 19 patients with secretory multiple myeloma who were evaluable for a response is shown both at baseline and after every 2 cycles of therapy with bortezomib and PegLD. Out of the total of 22 evaluable patients, 2 with light chain only disease and 1 with nonsecretory disease are excluded from this graph. Also, 2 of these patients received a total of 11 cycles but then withdrew when bortezomib was approved by the Food and Drug Administration to receive additional treatment with their primary oncologist, and their results are shown only through cycle 10.
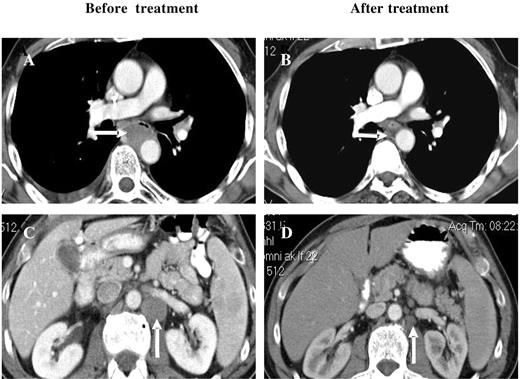
Among the other disease categories, 7 patients with NHL were enrolled, 5 were evaluable for a response, and 2 of these with B-cell NHL had PRs. One patient with relapsed/refractory peripheral T-cell NHL achieved a CR after 6 cycles of bortezomib/PegLD (Figure 3). There were also 5 patients with AML treated, 2 of whom received at least 2 cycles, whereas the other 3 experienced disease progression or progression-related toxicities that precluded further treatment. One of the 2 evaluable patients had newly diagnosed AML and was not a candidate for standard therapies. After 2 cycles he achieved a PR by the circulating blast count, which was reduced from a baseline of 7% (absolute blast count 2688) to 1% (107), though he remained transfusion dependent. This patient developed pneumonitis during cycle 3 as detailed earlier, a confirmatory bone marrow was not performed, and he did not receive additional therapy. The second AML patient had relapsed disease after multiple prior induction regimens as well as autologous peripheral blood stem cell transplantation (auto-PBSCT). An initial bone marrow showed hypercellularity with 19% blasts, which after 2 cycles became normocellular with only 4% blasts though a clonal cytogenetic abnormality persisted, and she did require intermittent platelet and red blood cell transfusions. Treatment was interrupted due to febrile neutropenia and disseminated Varicella zoster, and upon resolution of these the patient's disease had progressed. Reinitiation of bortezomib/PegLD again converted her hypercellular marrow, showing 28% involvement after 4 cycles to a normocellular marrow with 5% blasts.
Response of a patient with peripheral T-cell NHL to bortezomib and PegLD. This 64-year-old male with peripheral T-cell non-Hodgkin lymphoma was initially treated with 6 cycles of chemotherapy with cyclophosphamide, adriamycin, vincristine, and prednisone followed by involved field radiation to the mediastinum, resulting in a complete remission. After his disease relapsed he received salvage chemotherapy with ifosfamide, carboplatin, and etoposide, and autologous peripheral blood stem cell transplantation, again resulting in a remission. Upon relapse he received 2 cycles of the histone deacetylase inhibitor depsipeptide with disease progression and then received 6 cycles of bortezomib and PegLD. A complete remission was documented by computed tomography, with resolution of both mediastinal (compare panels A and B) and abdomino-pelvic adenopathy (compare panels C and D) and confirmed by positron emission tomography. Arrows indicate areas of peri-aortic adenopathy in the mediastinum (A) and abdomen (C) that have resolved after therapy (B and D, respectively).
Response of a patient with peripheral T-cell NHL to bortezomib and PegLD. This 64-year-old male with peripheral T-cell non-Hodgkin lymphoma was initially treated with 6 cycles of chemotherapy with cyclophosphamide, adriamycin, vincristine, and prednisone followed by involved field radiation to the mediastinum, resulting in a complete remission. After his disease relapsed he received salvage chemotherapy with ifosfamide, carboplatin, and etoposide, and autologous peripheral blood stem cell transplantation, again resulting in a remission. Upon relapse he received 2 cycles of the histone deacetylase inhibitor depsipeptide with disease progression and then received 6 cycles of bortezomib and PegLD. A complete remission was documented by computed tomography, with resolution of both mediastinal (compare panels A and B) and abdomino-pelvic adenopathy (compare panels C and D) and confirmed by positron emission tomography. Arrows indicate areas of peri-aortic adenopathy in the mediastinum (A) and abdomen (C) that have resolved after therapy (B and D, respectively).
Discussion
The multicatalytic proteinase complex, or proteasome, has been validated as a novel target for cancer therapy with the approval of the potent, specific inhibitor bortezomib for MM patients with at least 2 prior therapies who progressed on the last of these. Preclinical studies also strongly support proteasome inhibition as a rational approach for chemosensitization and overcoming chemotherapy resistance. This prompted us to evaluate the combination of bortezomib and PegLD, which in this study was administered safely to patients with advanced hematologic malignancies, most of whom were heavily pretreated. According to the protocol-specified definition, bortezomib at 1.50 mg/m2 with 30 mg/m2 of PegLD met criteria for MTD. However, to define a regimen that could be administered with a lower likelihood of dose delays and dose reductions, 1.30 mg/m2 of bortezomib and 30 mg/m2 of PegLD are recommended for further study.
Grade 3 or 4 AEs in at least 10% of patients included thrombocytopenia (43%), lymphopenia (40%), neutropenia (17%), fatigue (14%), pneumonia (14%), peripheral neuropathy (12%), febrile neutropenia (10%), and diarrhea (10%). In a previous phase 2 study of single-agent bortezomib in MM,10 thrombocytopenia of similar severity occurred in 31%, lymphopenia in 55% (personal written communication, D.-L.E., Millennium Pharmaceuticals, July 26, 2004), fatigue in 12%, neuropathy in 12%, and diarrhea in 8%. Neutropenia, febrile neutropenia, and pneumonia were increased with the current regimen, but 10 of 17 such episodes occurred at levels above the 1.30 mg/m2 bortezomib dose used in the phase 2 trial and 11 occurred in patients with diseases other than MM, such as AML. From the standpoint of all adverse events, those seen in greater than 50% of patients in the current study included fatigue, thrombocytopenia, lymphopenia, nausea, constipation, peripheral neuropathy, and anemia. Of these, only fatigue was seen in as many as 50% of patients in the aforementioned phase 2 study. This comparison suggests that at least some of the grade 3 or 4 toxicities in the current trial were bortezomib related and may not have been significantly potentiated by the addition of PegLD. Some of the less severe grade 1 or 2 adverse events did seem to be increased with the combination, however, including nausea and constipation. Therefore, the possibility that PegLD enhances the toxicity of bortezomib certainly deserves further study. A phase 3 trial comparing bortezomib to bortezomib with PegLD in patients with relapsed/refractory MM is currently underway and will allow this question to be answered more fully.
PegLD has been used in patients with hematologic malignancies predominantly in combination with other agents. One recent phase 2 study of newly diagnosed MM patients, for example, used PegLD at the same dose intensity of 10 mg/m2/wk with vincristine and dexamethasone (DVd).27 Grade 3 and 4 toxicities included neutropenia (30%), anemia (21%), PPE/HFS (21%), mucositis (12%), and thrombocytopenia (9%). The incidence of most of these toxicities in the current study was lower with the exception of thrombocytopenia, which was increased. Also, febrile neutropenia was not seen with DVd, though of the 4 episodes seen with bortezomib/PegLD only 1 occurred in a patient with MM. Overall the data suggest that, other than thrombocytopenia, bortezomib generally does not augment most PegLD-related toxicities. However, larger studies in a more homogeneous population of patients from the standpoint of their diagnosis, prior therapy, and bortezomib/PegLD dosing regimen are needed to evaluate this possibility more fully. With regard to other toxicities of anthracyclines, liposomal preparations may induce less cardiac toxicity.20 In the current study, patients were excluded if they had a prior doxorubicin exposure of greater than 400 mg/m2. Those patients who exceeded this level while on study were evaluated every 2 to 4 cycles with serial radionuclide ventriculography and 2 had a decrease in their LVEF of greater than 10%. Both patients were clinically asymptomatic but taken off study, and 1 received a follow-up within 2 months that revealed a return of their LVEF to the pretreatment baseline.
Some studies suggested that anthracyclines bound the proteasome which then acted as a carrier for their entry into cellular nuclei,28 raising the possibility of an interaction between the 2 drugs. Pharmacodynamic analyses determined if PegLD impacted upon bortezomib-induced proteasome inhibition (Figure 1). Each patient serving as a control in that data obtained with bortezomib alone on days 1 and 4 was compared with data on days 8 and 11, when both drugs were present together. Bortezomib induced a higher percentage proteasome inhibition on days 8 and 11 than on days 1 and 4, and this trend did reach statistical significance. Its absolute magnitude was modest, however, and it is likely that the lower baseline values of specific activity on days 8 and 11 explain the higher percentage inhibition on those days. Further study may therefore be needed to fully determine if an interaction is occurring, and the current results do not rule out the possibility that, for example, binding of doxorubicin causes a small allosteric shift that makes the proteasome more accessible to bortezomib. Pharmacokinetic studies were also performed evaluating the disposition of doxorubicin from its pegylated, liposomal preparation (Table 6). Results from these revealed parameters that were comparable to those seen in previous studies of single-agent PegLD. In one study of patients with solid tumors receiving PegLD,29 for example, t1/2 was 79 hours, CL was 40 mL/h, and volume of distribution at steady-state (Vss) was 3.9 L. Moreover, there was no trend for a change in any of the PK parameters with an increasing bortezomib dose (not shown). The current study was not designed to have each patient serve as her/his own control for PegLD PKs, and either such a design or a randomized study would be needed to evaluate this question fully. However, taken together, these findings suggest that bortezomib does not alter the PKs of PegLD, and PegLD impacts only weakly, if at all, upon the PDs of bortezomib.
Evidence of antitumor efficacy was obtained in this study against AML, with 1 PR in a patient with newly diagnosed disease and a second durable, reproducible PR in a patient with multiple relapsed leukemia. Responses were also seen in this trial in patients with NHL, including 2 PRs in B-cell indolent lymphomas. These may well be a reflection of the known single-agent activity of bortezomib against follicular NHL.9,30,31 More notably, 1 patient with multiply relapsed/refractory peripheral T-cell NHL achieved a CR of 9-months duration. In addition, 1 MM patient with cutaneous involvement with mycosis fungoides noted improvement in his lesions on therapy, which flared again when bortezomib/PegLD was discontinued. These data support in vitro and in vivo studies that suggest that proteasome inhibition is a rational strategy against T-cell diseases32 and support further testing of bortezomib or bortezomib/PegLD in this population.
Antitumor activity was seen predominantly against MM, with an overall response rate of 73% (CR+near-CR+PR). Eight patients had a CR or near-CR, and this 36% rate is higher than the 10% documented in a phase 2 trial of single-agent bortezomib.10 Limited follow-up is available about time to progression, which was not an end point on the current study, but the available information suggests that these remissions were durable, ranging from 2 to greater than 16 months. Also of note is the 38% CR+near-CR rate in myeloma patients with previously anthracycline nonresponsive disease. These results must certainly be interpreted cautiously, given the small sample size, but do suggest the possibility that bortezomib may enhance sensitivity to anthracyclines and overcome anthracycline resistance in patients in vivo. Preliminary results from other phase 1 combination trials suggest that bortezomib may sensitize and abrogate resistance to melphalan33 and thalidomide34 as well. The current results support further testing of the bortezomib/PegLD regimen in MM, and in this regard a pilot phase 2 study is being conducted through the Cancer and Leukemia Group B for patients with previously untreated disease. While additional studies of stem cell effects are still needed, 2 patients from the current trial underwent auto-PBSCT after successful mobilization and engrafted normally, suggesting that bortezomib/PegLD can be given safely prior to transplantation. These ongoing phase 2 and 3 studies will determine the potential role of bortezomib/PegLD in initial therapy for multiple myeloma and also in patients with relapsed and/or refractory disease.
Prepublished online as Blood First Edition Paper, December 30, 2004; DOI 10.1182/blood-2004-07-2911.
Supported in part by Millennium Pharmaceuticals, the General Clinical Research Centers Program of the Division of Research Resources, National Institutes of Health (NIH; RR00046), the University of North Carolina at Chapel Hill Lineberger Comprehensive Cancer Center Support Grant (NIH P30-CA16086), and by grants from the Leukemia and Lymphoma Society (R6206-02, R.Z.O.) and the National Institutes of Health (RO1 CA102278, R.Z.O.).
Several of the authors (J.A., D.-L.E., E.G.T., and M.G.) are, or at the time of this study were, employed by a company (Millennium Pharmaceuticals, Inc) whose product (bortezomib) was studied in the present work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors would like to thank Drs David Schenkein and Fabio Benedetti for assistance with both the trial and the manuscript. This study as a whole is dedicated to the patients whose participation made it possible.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal