Expression of activation-induced cytidine deaminase (AID) is crucial for immunoglobulin V gene somatic hypermutations (SHMs) and immunoglobulin class switch recombinations (CSRs).1 Expression of AID is associated with the germinal center (GC) reaction and is not expressed in naive B cells.2 AID expression requires CD40 signaling and interleukin 4,3,4 likely to be encountered in the lymphoid tissues. Previously, we and others have shown that AID mRNA in B-cell non-Hodgkin lymphomas was confined to GC-derived lymphomas.5,6 Recently, Babbage et al7 reported AID mRNA by reverse transcriptase-polymerase chain reaction (RT-PCR) in circulating tumor cells from 17 of 18 mantle cell lymphoma (MCL) patients. Because no AID mRNA was detected in blood cells of healthy donors, they concluded that AID expression in MCL is a tumor-related activation phenomenon.
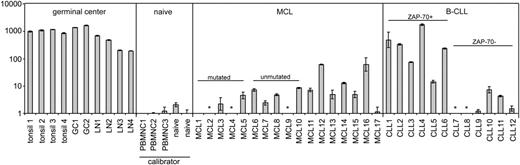
By use of Taqman quantitative RT-PCR, we detected AID expression in tissue samples from 14 of 17 MCL patients, but with the exception of 2 cases, the level was very low (Figure 1). In reactive tonsils, lymph nodes, and tonsillar GC cells expression was greater than 100-fold higher. Compared with circulating naive B cells, AID expression was on average 2-fold higher in MCL tissue samples. The AID expression levels were comparable in 5 IGVH-mutated MCL cases versus 5 IGVH-unmutated MCL cases (SHM cutoff 2%), concordant with the study by Babbage et al.7
AID mRNA expression levels in control tissue, purified GC and naive B cells, MCL tissue, and B-CLL tissue. All MCL and B-CLL lymph node tissue samples were analyzed using standard histology and immunohistochemistry including CD5, CD23, and cyclin D1. Tissue samples contained less than 5% CD3+ T cells. Pre-existent germinal centers were not present in the studied MCL and B-CLL tissue samples upon histologic examination. By immunohistochemistry there were no CD10+ germinal center cells present. For all MCL cases, t(11;14) was confirmed by fluorescence in situ hybridization. Expression of ZAP-70, a surrogate marker for germ line IGVH status in B-CLL,8,9 was assessed by immunohistochemistry. Taqman quantitative RT-PCR for AID (Applied Biosystems, Foster City, CA; Assay-on-demand Hs00221068_m1; this primer set detects all known alternative splice variants) and TATA-box binding protein (TBP) as endogenous control was performed on reactive tonsils and lymph nodes (LN), purified GC and naive B cells, and tissue samples from MCL patients and B-CLL patients. By dividing the mean threshold cycle (Ct) from triplicate AID measurements by the mean threshold cycle from triplicate TBP measurements, the mean ΔCt was calculated. The ΔΔCt values were calculated using the mean ΔCt of the 2 naive B-cell samples and the 3 healthy donor peripheral blood mononuclear (PBMNC) cell samples as a calibrator. The expression factor difference and range were calculated by the following formulas: 2-ΔΔCt (mean factor difference); 2-(ΔΔCt-ΔCt SD) and 2-(ΔΔCt+ΔCt SD) (error bars indicate range factor difference). The factor difference conversion of the ΔΔCt and range are depicted in the graph in a logarithmic fashion. * indicates not detectable.
AID mRNA expression levels in control tissue, purified GC and naive B cells, MCL tissue, and B-CLL tissue. All MCL and B-CLL lymph node tissue samples were analyzed using standard histology and immunohistochemistry including CD5, CD23, and cyclin D1. Tissue samples contained less than 5% CD3+ T cells. Pre-existent germinal centers were not present in the studied MCL and B-CLL tissue samples upon histologic examination. By immunohistochemistry there were no CD10+ germinal center cells present. For all MCL cases, t(11;14) was confirmed by fluorescence in situ hybridization. Expression of ZAP-70, a surrogate marker for germ line IGVH status in B-CLL,8,9 was assessed by immunohistochemistry. Taqman quantitative RT-PCR for AID (Applied Biosystems, Foster City, CA; Assay-on-demand Hs00221068_m1; this primer set detects all known alternative splice variants) and TATA-box binding protein (TBP) as endogenous control was performed on reactive tonsils and lymph nodes (LN), purified GC and naive B cells, and tissue samples from MCL patients and B-CLL patients. By dividing the mean threshold cycle (Ct) from triplicate AID measurements by the mean threshold cycle from triplicate TBP measurements, the mean ΔCt was calculated. The ΔΔCt values were calculated using the mean ΔCt of the 2 naive B-cell samples and the 3 healthy donor peripheral blood mononuclear (PBMNC) cell samples as a calibrator. The expression factor difference and range were calculated by the following formulas: 2-ΔΔCt (mean factor difference); 2-(ΔΔCt-ΔCt SD) and 2-(ΔΔCt+ΔCt SD) (error bars indicate range factor difference). The factor difference conversion of the ΔΔCt and range are depicted in the graph in a logarithmic fashion. * indicates not detectable.
A salient discordance between IGVH mutational status and AID expression has been reported for B-cell chronic lymphocytic leukemia (B-CLL), although AID expression levels in B-CLL cells remained well below that of GC cells.10 In that study, AID was determined in circulating B-CLL cells. In the tissue compartment, B-CLL cells are organized into “pseudo-follicles,” in which scattered CD4+ T cells capable of delivering signals involved in AID expression are present. We therefore also quantified AID mRNA expression in tissue samples from B-CLL patients (n = 12). Four of 6 cases expressing zeta-associated protein 70 (ZAP-70), thus presumably containing a low number of mutations,9 showed high expression of AID comparable to reactive tonsils and lymph nodes. This expression was much higher than in MCL tissues. In 6 cases without ZAP-70 expression, AID expression was on average 300-fold lower compared with tonsils and lymph nodes. These results underscore the inverse correlation between IGVH mutational status/ZAP-70 expression and AID expression in B-CLL. Whether AID expression in unmutated/ZAP-70-expressing B-CLL is related to CSR activity remains to be established.
It is conceivable that AID expression is induced in the tissue compartment of ZAP-70-expressing B-CLL and wanes in the circulating tumor cells. We conclude that with the exception of a very few cases, AID expression is very low in MCL and is not related to IGVH mutational status, whereas in tissue samples from B-CLL patients, high AID expression levels are related to ZAP-70 expression.
Dynamics of activation-induced cytidine deaminase expression in t(11;14) mantle cell lymphoma
In normal immune responses, location is not quite everything but it is a critical factor. How malignant B cells behave in different sites is beginning to be addressed. Two important mechanisms, somatic mutation in IgV genes and class switch, generally, but not invariably, occur in germinal centers (GCs). For both there is a prerequisite for the enzyme, activation-induced cytidine deaminase (AID). In B-cell tumors, therefore, it is important to assess changes in AID expression in tissues compared with blood.
The letter from Guikema et al draws attention to the fact that both mantle cell lymphoma (MCL) and B-cell chronic lymphocytic leukemia (B-CLL) occupy tissue and blood compartments and raises the question of differential expression of AID at these sites within tumor clones. They approach MCL only from tissue and confirm our findings from blood that AID is expressed in both unmutated (UM) and mutated (MUT) MCL at levels higher than in normal circulating or naive B cells. They demonstrate overall a lower level of AID expression in MCL compared with tonsil tissue. However, since somatic mutation and isotype switch are likely to be highly activated in infected tonsils, AID levels are very high at that site. Heterogeneity of expression between cases of MCL is striking, with very few negative cases and others showing elevated levels, some markedly. Although our approach was semiquantitative, we had also observed significant variation in levels of AID transcripts between cases.1 The possibility that AID expression in MCL is linked to isotype switch events, but apparently not to somatic mutation, was raised by our study, although this correlation was not seen in all cases. The role of the up-regulated AID therefore remains unclear.
Guikema et al also look more closely at B-CLL where AID expression is elevated, mainly in the zeta-associated protein of 70 kDa (ZAP-70)-positive subset. They make the point that B-CLL in tissue has remarkably high levels of AID. However, in the absence of matched B-CLL cells from the blood compartment, indications that levels of AID transcripts can be higher than in circulating B-CLL cells remain unproven.2,3 AID expression, displaying intraclonal heterogeneity in circulating B-CLL,4 has again been linked to isotype switch activity.5,6
Clearly, maturational events in normal B cells occur mainly in tissue sites, particularly in the germinal center of the lymph node, but possibly also in other locations. In tumors that occupy blood and tissue, we need to analyze both. Blood can provide purified tumor cells, avoiding contamination with other cells producing the same molecules. However, tissue can tell us more about the influence of the local environment on the tumor clone, which will have relevance for tumor behavior. The expression of AID here has potential for modulating this behavior, as dysregulated AID activity could lead to hypermutation of additional genes to drive clonal evolution, particularly in those cells already bearing genetic abnormalities, such as chromosomal translocations in the immunoglobulin H (IgH) switch region.7,8
Supported by The Leukaemia Research Fund (United Kingdom).
Correspondence: Surinder S. Sahota, LRF Senior Scientist, Molecular Immunology Group, Cancer Sciences Division, Southampton University Hospitals, Southampton, United Kingdom; e-mail: s.s.sahota@soton.ac.uk.
Supported by the Dutch Cancer Society (NKB2000-2207) and the European Community BIOMED program (QLG1-CT-2000-00687).

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal