Abstract
Inactivation of the murine tissue factor (TF) gene or tissue factor pathway inhibitor 1 (TFPI) gene results in embryonic lethality, indicating that both are required for embryonic development. We have shown that expression of low levels of TF from a transgene (hTF) rescues TF-null embryos. However, low-TF mice (mTF–/–/hTF+) have hemostatic defects in the uterus, placenta, heart, and lung. In this study, we hypothesized that the death of TFPI–/– embryos was due to unregulated TF/FVIIa activity and that the hemostatic defects in low-TF mice were due to insufficient TF expression. Therefore, we attempted to rescue TFPI–/– embryos by reducing TF expression, and to restore hemostasis in low-TF mice by abolishing TFPI expression. Intercrossing TFPI+/–/mTF+/–/hTF+/– mice generated close to the expected number of TFPI–/–/low-TF mice at weaning age from 128 offspring, indicating rescue of TFPI–/– embryos from embryonic lethality. Conversely, a decrease in TFPI levels dose-dependently prolonged the survival of low-TF mice and rescued the hemorrhagic defects in the lung and placenta but not in the heart or uterus. These results indicate that the correct balance between TF and TFPI in different organs is required to maintain hemostasis during embryonic development and in adult mice.
Introduction
Tissue factor (TF) is the primary cellular initiator of blood coagulation.1 After vessel injury, the TF/factor VIIa (FVIIa) complex activates factor X and factor IX, which leads to the generation of thrombin and fibrin deposition.1 TF/FVIIa activity is regulated by the Kunitz-type proteinase inhibitor, tissue factor pathway inhibitor 1 (TFPI), via a factor Xa–dependent feedback pathway.2
Disruption of the TF gene results in a high rate of mortality of TF–/– embryos at embryonic day (E) 10.5, which appears to be due to a combination of hemostatic defects and a defective yolk sac vasculature.3-5 In addition, placentas of TF–/– embryos beyond E13.5 contain blood pools consistent with impaired hemostasis.6 TF+/– mice are normal. Similar defects in the yolk sac vasculature were observed in factor V–/–, prothrombin–/–, and protease-activated receptor-1 (PAR-1)–/– embryos.7-11 These data suggest that TF-dependent thrombin generation and PAR-1 signaling contributes to the maintenance of the yolk sac vasculature.12,13
We found that murine TF-null embryos were rescued by expression of human TF from a transgene (hTF).14 Rescued mice (mTF–/–/hTF+/o) expressed low levels of TF in all tissues (∼ 1% of wild-type TF levels) and were designated “low-TF” mice. Low-TF mice have shorter lifespans compared with wild-type mice, in part, due to fatal lung hemorrhages.15 In addition, low-TF mice exhibit fatal postpartum hemorrhages, blood pools in the placenta, and cardiac fibrosis beyond 3 months of age.6,15 These results suggest that low-TF mice have impaired hemostasis in the uterus, placenta, lung, and heart.
Inactivation of the TFPI gene results in complete intrauterine lethality.16 Sixty percent of TFPI–/– embryos die between E9.5 and E11.5 and the remainder die during late gestation. Histologic analysis of TFPI–/– embryos revealed abnormalities in the yolk sac and placenta.16 The appearance of fibrin deposition in the liver, intravascular thrombosis, and hemorrhages in TFPI–/– embryos surviving beyond E12.5 suggested that unregulated TF/FVIIa activity led to a consumptive coagulopathy and secondary bleeding.16 TFPI+/– mice are normal and have plasma TFPI activity approximately 50% that of wild-type mice. It has been shown that FVII deficiency rescued the embryonic lethality of TFPI–/– embryos.17 However, TFPI–/–/FVII–/– neonates died shortly after birth due to FVII deficiency.
Recent studies have shown that there are 2 forms of TFPI in the mouse transcribed from the same gene.18,19 TFPIα contains 3 Kunitz domains and binds to the surface of cells via a glycosylphosphatidylinositol (GPI) anchor. The second form, TFPIβ, contains Kunitz domains 1 and 2 fused to an intrinsic GPI anchor. Both TFPIα and TFPIβ mRNAs are expressed at E7.0 but only TFPIα mRNA persists until E17.0.18 In adult mice, TFPIα and TFPIβ mRNAs are expressed in various tissues, including the heart and lung.18,20
In this study, we determined if low levels of TF rescue the embryonic lethality in TFPI–/– embryos by intercrossing TFPI+/–/mTF+/–/hTF+/– mice. We found that reducing TF expression completely rescued TFPI–/– embryos from embryonic lethality and generated viable adult mice. This permitted analysis of the effect of TFPI deficiency on the hemostatic defects in the uterus, placenta, heart, and lung of low-TF mice. The absence of TFPI reduced the number of blood pools in the placenta and abolished fatal lung hemorrhages in low-TF mice. However, a deficiency in TFPI did not affect uterine hemostasis or cardiac fibrosis in low-TF mice. Therefore, the correct levels of TF and TFPI are required to maintain hemostasis during development and in adult mice.
Materials and methods
Mice
All studies were approved by The Scripps Research Institute Animal Care and Use Committee and comply with the National Institutes of Health guidelines. Low-TF and TFPI+/– mice on a C57BL/6 background were used.14,16 We used the notation hTF+/– for mice defined by breeding to contain a single copy of the human minigene and hTF+/o for mice that contain either 1 or 2 copies of the human minigene. The functional levels of TF in mTF–/–/hTF+/o mice on a C57BL/6 background were 1.67% ± 0.47% (n = 3) in the brain, 3.40% ± 0.02% (n = 3) in the lung, and 0.86% ± 0.09% (n = 3) in the heart compared with levels of TF in wild-type mice. Due to the very low levels of TF in low-TF mice, we could not detect differences in TF activity between mice containing 1 or 2 copies of the minigene.
Placental blood pools
The surface view of each placenta was photographed and the number and size of blood pools was given an arbitrary score between 0 and 5 by 2 investigators blinded to the genotypes.
Histology
Tissues were fixed in a 10% zinc formalin solution and embedded in paraffin. Sections (3 μM) were stained with hematoxylin and eosin (H&E), Prussian Blue, or Masson Trichrome. All photomicrographs were taken with an Olympus BX60 microscope equipped with an Olympus UPlanFI 4 ×/0.10, 20 ×/0.50, 40 ×/0.75, and 100 ×/1.30 objective lenses (Olympus, Melville, NY) and a digital DVC 1310C camera (DVC, Austin, TX).
Immunohistochemistry and TUNEL assay
Placentas were fixed in a 10% zinc formalin solution and embedded in paraffin. Tissue sections were incubated overnight with a rabbit antihuman fibrinogen antibody (1:5000; Dako, Carpinteria, CA) and this was detected using a Rabbit Vectastatin Elite ABC kit and NovaRED (Vector Laboratories, Burlingame, CA). The presence of apoptotic cells in placental sections was analyzed using a transferase-mediated deoxyuridine triphosphate nick end labeling (TUNEL) assay (ApopTag Plus Peroxidase in situ Apoptosis Detection Kit; Serologicals, Temecula, CA). The tissue was counterstained with Mayer hematoxylin (Sigma-Aldrich, St Louis, MO).
Northern blotting
Total RNA was isolated from various tissues. Levels of TFPIα and TFPIβ mRNAs were determined by Northern blotting using a radiolabeled full-length TFPI cDNA probe. The blot was rehybridized with the housekeeping gene, glyceraldehyde 3-phosphate dehydrogenase (GAPDH). Bands were visualized by autoradiography.
Data analysis
Statistical significance of the distribution of genotypes was analyzed by Chi-square test with 10 degrees of freedom. Log-rank analysis was used to determine statistical significance of the survival of the different mice. The percentage of fibrosis of heart sections is presented as a mean plus or minus standard deviation (SD). Statistical analysis of the cardiac fibrosis data was performed using an unpaired Student t test. Differences were considered to be statistically significant at P values less than .05.
Results
Characterization of TFPI–/– embryos and placentas
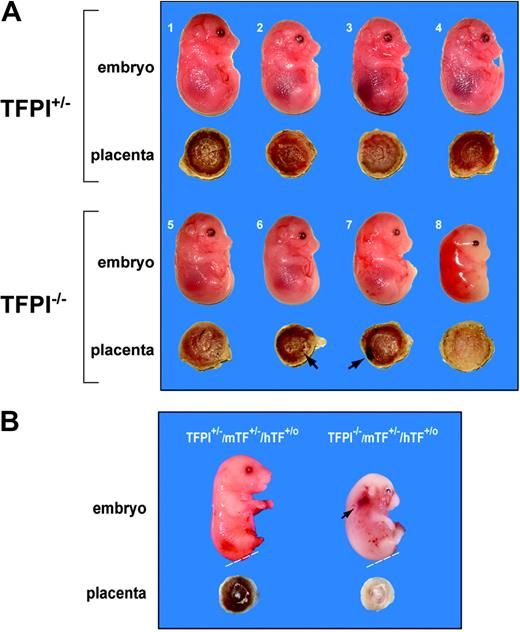
A previous study reported that 60% of TFPI–/– embryos die between E9.5 and E11.5 and the remainder (40%) die during the late gestational period.16 Of 233 pups born, none were TFPI–/– pups. This study was performed on a mixed genetic background. Similarly, we found that TFPI+/– intercrosses on a C57BL/6 background failed to produce live TFPI–/– pups from 100 mice analyzed at weaning age, although 2 stillborn TFPI–/– pups were recovered. Timed breedings between TFPI+/– mice were performed to generate embryos and placentas of defined age. In 3 litters of TFPI+/– crosses, we observed 7 TFPI+/+, 12 TFPI+/–, and 4 TFPI–/– E14.5 embryos. In this small group of 23 embryos, TFPI–/– embryos were present at 70% of the expected frequency. Of these 4 TFPI–/– embryos, 3 were alive and one was dead (Figure 1A). The 3 live TFPI–/– embryos were grossly normal but one (no. 7) was pale with signs of hemorrhage (Figure 1A). The dead TFPI–/– embryo (no. 8) was growth-retarded and undergoing resorption (Figure 1A). A second dead E15.5 TFPI–/– embryo was observed from a cross between a TFPI+/–/low-TF male (Table 1) and a TFPI+/–/mTF+/–/hTF+/o female (Figure 1B). This TFPI–/– embryo was growth-retarded and had numerous hemorrhages. Our data are consistent with the original study showing that 100% of TFPI–/– embryos die during gestation.16
Analysis of TFPI–/– embryos and placentas. (A) We observed 4 TFPI+/– embryos (nos. 1-4) and 4 TFPI–/– embryos (nos. 5-8) at E14.5 from the same litter in a cross between TFPI+/– mice. Embryos 1 to 7 were alive and embryo 8 was dead. Hemorrhages were observed on the surface of placentas no. 6 and no. 7 (arrows) and placenta no. 8 was white. (B) Two E15.5 embryos. The TFPI+/–/mTF+/–/hTF+/o embryo and placenta was normal, whereas the TFPI–/–/mTF+/–/hTF+/o embryo was growth-retarded with signs of hemorrhage (arrow) and had a white placenta. The lower leg and/or tail were removed for genotyping (dashed line).
Analysis of TFPI–/– embryos and placentas. (A) We observed 4 TFPI+/– embryos (nos. 1-4) and 4 TFPI–/– embryos (nos. 5-8) at E14.5 from the same litter in a cross between TFPI+/– mice. Embryos 1 to 7 were alive and embryo 8 was dead. Hemorrhages were observed on the surface of placentas no. 6 and no. 7 (arrows) and placenta no. 8 was white. (B) Two E15.5 embryos. The TFPI+/–/mTF+/–/hTF+/o embryo and placenta was normal, whereas the TFPI–/–/mTF+/–/hTF+/o embryo was growth-retarded with signs of hemorrhage (arrow) and had a white placenta. The lower leg and/or tail were removed for genotyping (dashed line).
Genotype of 128 offspring, at 4 weeks of age, generated by intercrossing TFPI+/–/mTF+/–/hTF+/– mice
. | Genotype . | . | . | . | . | . | . | ||
|---|---|---|---|---|---|---|---|---|---|
| Group . | TFPI . | mTF . | hTF . | Expected, % . | Observed, % . | Observed, no. . | Obs/E, % . | ||
| 1 | +/+ | +/+ | +/o | 6.25 | 10.15 | 13 | 162 | ||
| 2 | +/+ | +/+ | - | 2.08 | 1.56 | 2 | 75 | ||
| 3 | +/+ | +/- | +/o | 12.5 | 10.15 | 13 | 81 | ||
| 4 | +/+ | +/- | - | 4.17 | 0.78 | 1 | 19 | ||
| 5* | +/+ | -/- | +/o | 6.25 | 6.25 | 8 | 100 | ||
| 6 | +/+ | -/- | - | 0 | 0 | 0 | - | ||
| 7 | +/- | +/+ | +/o | 12.5 | 17.96 | 23 | 144 | ||
| 8 | +/- | +/+ | - | 4.17 | 3.13 | 4 | 75 | ||
| 9 | +/- | +/- | +/o | 25 | 28.13 | 36 | 113 | ||
| 10 | +/- | +/- | - | 8.33 | 7.03 | 9 | 84 | ||
| 11* | +/- | -/- | +/o | 12.5 | 10.15 | 13 | 81 | ||
| 12 | +/- | -/- | - | 0 | 0 | 0 | - | ||
| 13 | -/- | +/+ | +/o | 0 | 0 | 0 | - | ||
| 14 | -/- | +/+ | - | 0 | 0 | 0 | - | ||
| 15 | -/- | +/- | +/o | 0 | 0 | 0 | - | ||
| 16 | -/- | +/- | - | 0 | 0 | 0 | - | ||
| 17* | -/- | -/- | +/o | 6.25 | 4.69 | 6 | 75 | ||
| 18 | -/- | -/- | - | 0 | 0 | 0 | - | ||
. | Genotype . | . | . | . | . | . | . | ||
|---|---|---|---|---|---|---|---|---|---|
| Group . | TFPI . | mTF . | hTF . | Expected, % . | Observed, % . | Observed, no. . | Obs/E, % . | ||
| 1 | +/+ | +/+ | +/o | 6.25 | 10.15 | 13 | 162 | ||
| 2 | +/+ | +/+ | - | 2.08 | 1.56 | 2 | 75 | ||
| 3 | +/+ | +/- | +/o | 12.5 | 10.15 | 13 | 81 | ||
| 4 | +/+ | +/- | - | 4.17 | 0.78 | 1 | 19 | ||
| 5* | +/+ | -/- | +/o | 6.25 | 6.25 | 8 | 100 | ||
| 6 | +/+ | -/- | - | 0 | 0 | 0 | - | ||
| 7 | +/- | +/+ | +/o | 12.5 | 17.96 | 23 | 144 | ||
| 8 | +/- | +/+ | - | 4.17 | 3.13 | 4 | 75 | ||
| 9 | +/- | +/- | +/o | 25 | 28.13 | 36 | 113 | ||
| 10 | +/- | +/- | - | 8.33 | 7.03 | 9 | 84 | ||
| 11* | +/- | -/- | +/o | 12.5 | 10.15 | 13 | 81 | ||
| 12 | +/- | -/- | - | 0 | 0 | 0 | - | ||
| 13 | -/- | +/+ | +/o | 0 | 0 | 0 | - | ||
| 14 | -/- | +/+ | - | 0 | 0 | 0 | - | ||
| 15 | -/- | +/- | +/o | 0 | 0 | 0 | - | ||
| 16 | -/- | +/- | - | 0 | 0 | 0 | - | ||
| 17* | -/- | -/- | +/o | 6.25 | 4.69 | 6 | 75 | ||
| 18 | -/- | -/- | - | 0 | 0 | 0 | - | ||
This cross will generate 27 different genotypes. Mice with 1 or 2 copies of the hTF transgene (hTF+/o) were combined, giving 18 different groups. The absence of the transgene is indicated as a minus (-).
Obs indicates observed; E, expected.
Groups 5, 11, and 17 have wild-type, 50%, and no TFPI on a low-TF background, respectively.
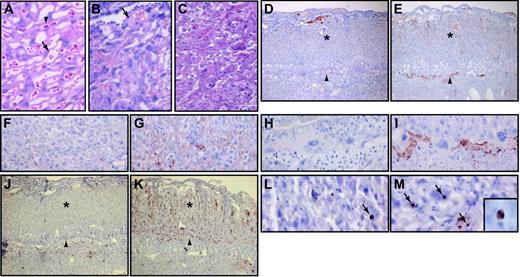
Macroscopic analysis of the placentas of the 3 live TFPI–/– embryos revealed blood pools on the surface of placentas no. 6 and no. 7 (Figure 1A). Histologic analysis of the placentas demonstrated that the large blood pool in placenta no. 7 contained maternal blood and was located on the surface of the labyrinthine zone (not shown). The placenta of the dead TFPI–/– embryo was white and bloodless (Figure 1A). The placental labyrinthine zone contains a complex vascular network of embryonic vessels and maternal lacunae. The different vascular structures can be identified by the presence of large nucleated red blood cells in the embryonic vessels and small nonnucleated red blood cells in maternal lacunae (Figure 2A). Histologic analysis of the labyrinthine zone of a placenta from a live TFPI–/– embryo at E14.5 revealed disruption of the vascular network and thrombosis (Figure 2B). The placenta of the dead TFPI–/– embryo showed an almost complete loss of vascular channels (Figure 2C). We observed increased fibrin deposition within the labyrinthine zone and at the interface between the placenta and the decidua in TFPI–/– placentas (Figure 2D-I). In addition, there was a dramatic increase in the number of apoptotic cells in this placenta compared with TFPI+/– placentas (Figure 2J-M). Similar results were observed with the E15.5 TFPI–/–/mTF+/–/hTF+/o placenta (not shown). These results indicate that TFPI deficiency leads to increased fibrin deposition, thrombosis, hemorrhage, and cell death in late gestational placentas.
Histologic analysis of TFPI–/– placentas. E14.5 placentas from wild-type (A), as well as live (B) and dead (C) TFPI–/– embryos were analyzed by H&E. Embryonic nucleated red blood cells (arrowhead) and maternal red blood cells (arrow) were observed in the wild-type placenta. The placenta of the live TFPI–/– embryo contained thrombi within the labyrinthine zone (arrow). The labyrinthine zone of the placenta of the dead TFPI–/– embryo was disrupted. Fibrin staining of placentas of TFPI+/– (D, F, H) and the dead TFPI–/– (E, G, I) embryos. TUNEL staining of apoptotic cells in placentas of TFPI+/– (J, L) and the dead TFPI–/– embryo (K, M). Arrowheads (D-E, J-K) indicate the trophoblast giant cell layer.Asterisks (D-E, J-K) indicate the labyrinthine zone. Arrows in panels L and M indicate TUNEL-positive cells. Original magnification, × 40 (D-E, J-K), × 400 (A-C, F-I, L-M), and × 1000 (inset in panel M showing nuclear condensation in a TUNEL-positive apoptotic cell).
Histologic analysis of TFPI–/– placentas. E14.5 placentas from wild-type (A), as well as live (B) and dead (C) TFPI–/– embryos were analyzed by H&E. Embryonic nucleated red blood cells (arrowhead) and maternal red blood cells (arrow) were observed in the wild-type placenta. The placenta of the live TFPI–/– embryo contained thrombi within the labyrinthine zone (arrow). The labyrinthine zone of the placenta of the dead TFPI–/– embryo was disrupted. Fibrin staining of placentas of TFPI+/– (D, F, H) and the dead TFPI–/– (E, G, I) embryos. TUNEL staining of apoptotic cells in placentas of TFPI+/– (J, L) and the dead TFPI–/– embryo (K, M). Arrowheads (D-E, J-K) indicate the trophoblast giant cell layer.Asterisks (D-E, J-K) indicate the labyrinthine zone. Arrows in panels L and M indicate TUNEL-positive cells. Original magnification, × 40 (D-E, J-K), × 400 (A-C, F-I, L-M), and × 1000 (inset in panel M showing nuclear condensation in a TUNEL-positive apoptotic cell).
Generation of TFPI–/–/mTF–/–/hTF+/o mice
TFPI+/–/mTF+/–/hTF+/– mice were generated by breeding low-TF male mice with TFPI+/– female mice. Next, we analyzed 128 offspring at weaning age from intercrosses between these TFPI+/–/mTF+/–/hTF+/– mice (Table 1). It was assumed that 7 of the 18 genotypes listed in Table 1 would not survive to weaning age because of the embryonic lethality associated with either TF or TFPI deficiency. We hypothesized that all the others would have equal survivability, including TFPI–/– mice with low levels of TF. Consistent with our hypothesis, we observed 11 of the 18 possible genotypes that can result from such a cross (Table 1). The observed frequencies of all 11 viable genotypes were not different from the expected frequencies by Chi-square test assuming the null hypothesis (P = .247). We have no evidence of neonatal deaths of any of the genotypes. Importantly, the number of TFPI–/–/low-TF mice generated was not statistically different from the expected frequency. Similarly, we observed TFPI+/+/low-TF mice and TFPI+/–/low-TF mice at the expected frequencies. In a separate control breeding, TFPI+/–/mTF+/– intercrosses produced 86 progeny at weaning age but no TFPI–/–/mTF–/– mice were observed (not shown). With this group size, the absence of TFPI–/–/mTF–/– mice was statistically significant (P = .034), assuming the null hypothesis. Twenty additional TFPI–/–/low-TF mice were generated from breedings of TFPI+/–/mTF+/–/hTF+/o and TFPI+/–/mTF+/–/hTF– mice (4 mice), as well as from TFPI–/–/low-TF males that were bred with TFPI+/–/mTF+/–/hTF+/o females (16 mice). These mice were used to analyze the effect of TFPI deficiency on hemostasis in the uterus, placenta, heart, and lung.
Expression of TFPIα and TFPIβ mRNAs in mouse tissues
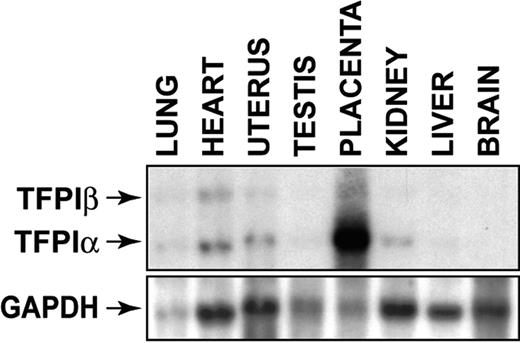
We determined the levels of TFPIα and TFPIβ mRNA in different mouse tissues. A high level of TFPIα mRNA was observed in the placenta relative to the other tissues. TFPIα mRNA was also expressed in the heart, uterus, kidney, lung, testis, and liver but not in the brain (Figure 3). TFPIβ mRNA was detected in heart, lung, and uterus (Figure 3). This pattern of TFPI gene expression suggests that the absence of TFPI may differentially affect the hemostatic defects in the placenta, uterus, lung, and heart of low-TF mice.
Tissue distribution of TFPIα and TFPIβ mRNAs in mouse tissues. Levels of TFPIα and TFPIβ mRNAs in various tissues were determined by Northern blotting using 10 μg RNA for each tissue. Loading was assessed by reprobing the blot with a GAPDH probe. TFPIα mRNA is 1.3 kb and TFPIβ mRNA is 4.0 kb.
Tissue distribution of TFPIα and TFPIβ mRNAs in mouse tissues. Levels of TFPIα and TFPIβ mRNAs in various tissues were determined by Northern blotting using 10 μg RNA for each tissue. Loading was assessed by reprobing the blot with a GAPDH probe. TFPIα mRNA is 1.3 kb and TFPIβ mRNA is 4.0 kb.
Analysis of placentas expressing different levels of TFPI and TF
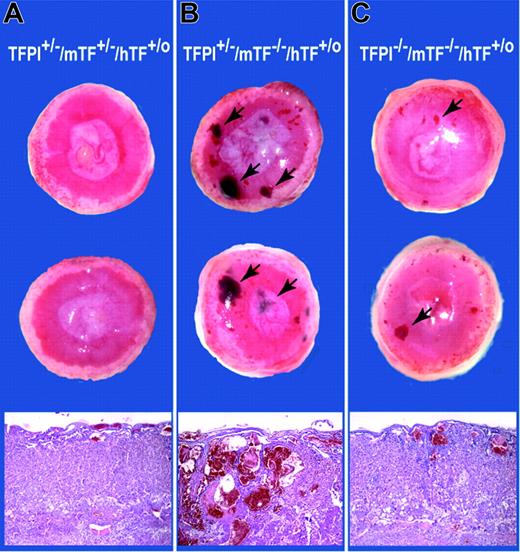
The absence of TFPI in the placenta is associated with loss of the vascular network, whereas low levels of TF lead to the formation of blood pools. We analyzed placentas expressing different levels of TFPI and TF to determine if these phenotypes could be corrected by rebalancing TFPI and TF expression. Twenty-one E15.5 placentas derived from crosses between TFPI–/–/low-TF male mice and TFPI+/–/mTF+/–/hTF+/o female mice were analyzed. We observed embryos with the following 4 genotypes: TFPI+/–/mTF+/–/hTF+/o (n = 6); TFPI+/–/low TF (n = 7); TFPI–/–/mTF+/–/hTF+/o (n = 2); TFPI–/–/low TF (n = 6). Placentas of TFPI+/–/mTF+/–/hTF+/o embryos were normal (Figure 4A). Placentas of TFPI+/–/low-TF embryos had large blood pools throughout the labyrinthine zone (Figure 4B). Strikingly, placentas of TFPI–/–/low-TF mice had a reduced number and size of the blood pools and a normal vascular network (Figure 4C). Quantitation of the blood pools using an arbitrary scoring system between 0 and 5 indicated that the mean score of TFPI+/–/low-TF mice (3.43 ± 1.27; n = 7) was significantly higher (P = .004) than that of TFPI–/–/low-TF mice (1.08 ± 0.97; n = 6). These data indicate that a deficiency of TFPI reduces the number of blood pools observed in low-TF placentas. In addition, low levels of TF rescue the vascular defect in TFPI–/– placentas.
Analysis of placentas containing different levels of TFPI and TF. Representative E15.5 placentas from a cross between a TFPI–/–/low-TF male and a TFPI+/–/mTF+/–/hTF+/o female. Macroscopic surface views of placentas are shown. (A) TFPI+/–/mTF+/–/hTF+/o placentas. (B) TFPI+/–/mTF–/–/hTF+/o placentas. (C) TFPI–/–/mTF–/–/hTF+/o placentas. Blood pools in panels B and C are shown with arrows. Histologic analysis (H&E) of the placentas is shown. Original magnification, × 40.
Analysis of placentas containing different levels of TFPI and TF. Representative E15.5 placentas from a cross between a TFPI–/–/low-TF male and a TFPI+/–/mTF+/–/hTF+/o female. Macroscopic surface views of placentas are shown. (A) TFPI+/–/mTF+/–/hTF+/o placentas. (B) TFPI+/–/mTF–/–/hTF+/o placentas. (C) TFPI–/–/mTF–/–/hTF+/o placentas. Blood pools in panels B and C are shown with arrows. Histologic analysis (H&E) of the placentas is shown. Original magnification, × 40.
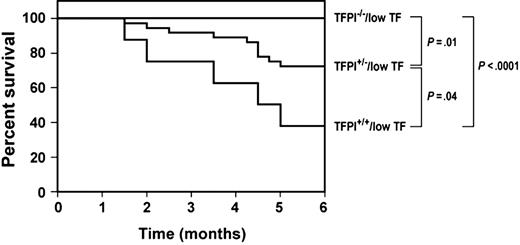
Survival of low-TF mice with different levels of TFPI
To determine if different levels of TFPI affect the survival of low-TF mice, we monitored mice over a 6-month period. All TFPI–/–/low-TF mice (n = 21) survived this period, whereas 28% (10 of 36) of TFPI+/–/low-TF mice and 63% (5 of 8) of TFPI+/+/low-TF mice died during this period (Figure 5). The differences in survival between each of the 3 groups were statistically significant (Figure 5), indicating that the effect of TFPI levels on the survival of low-TF mice was dose dependent. Previously, it was shown that TFPI+/– mice have plasma TFPI activity approximately 50% that of wild-type mice.16 No deaths of TFPI–/–/low-TF mice were observed up to 15 months of age. These results indicate that TFPI deficiency significantly prolongs the survival of low-TF mice.
Survival of low-TF mice with different levels of TFPI. We determined the survival of low-TF mice containing either wild-type levels of TFPI (TFPI+/+; n = 8), 50% levels of TFPI (TFPI+/–; n = 36), or no TFPI (TFPI–/–; n = 21). All 3 groups exhibited a statistically significant difference in survival.
Survival of low-TF mice with different levels of TFPI. We determined the survival of low-TF mice containing either wild-type levels of TFPI (TFPI+/+; n = 8), 50% levels of TFPI (TFPI+/–; n = 36), or no TFPI (TFPI–/–; n = 21). All 3 groups exhibited a statistically significant difference in survival.
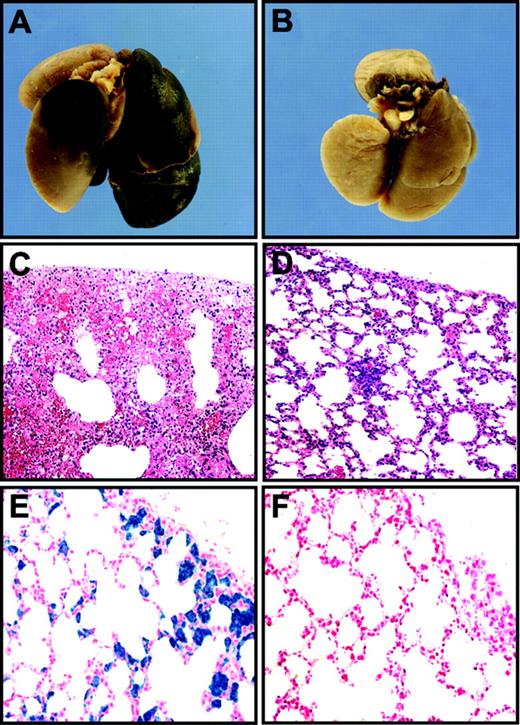
Effect of TFPI deficiency on hemostasis in the lungs and hearts of low-TF mice
Autopsies of 3 TFPI+/+/low-TF mice that were recovered from the survival experiment revealed extensive bleeding in their lungs (Figure 6A,C). This strongly suggested that lung hemorrhage is a major cause of death of low-TF mice. Lungs of euthanized TFPI–/–/low-TF mice were normal (Figure 6B,D). We analyzed the lungs of euthanized TFPI+/+/low-TF mice to determine if there is additional evidence of impaired hemostasis. Histologic analysis of lungs of 6-month-old TFPI+/+/low-TF mice showed brown deposits throughout the lungs in the alveolar septa in 9 of 10 mice (not shown). Prussian Blue staining demonstrated that these deposits contained iron (Figure 6E), indicating that they represented hemosiderin deposits. Hemosiderin is produced by phagocytic digestion of hematin, which is most likely derived from erythrocytes hemorrhaging into the lungs. In contrast, lungs of TFPI–/–/low-TF mice either contained no hemosiderin or very low levels of hemosiderin (6 mice; Figure 6F). These results suggest that the absence of TFPI restores hemostasis in the lung by increasing functional levels of TF. Attempts to show increased functional levels of TF in lungs from TFPI–/–/low-TF mice compared with TFPI+/+/low-TF mice were not successful due to the very low levels of TF present in the samples.
Analysis of lungs of low-TF mice with different levels of TFPI. Low-TF mice with wild-type levels of TFPI frequently died of lung hemorrhages (A, C) and also had hemosiderin deposition in their lungs (E). In contrast, no hemorrhages or hemosiderin deposits were observed in lungs of low-TF mice with no TFPI (B, D, F). Panels A and B show macroscopic views of lungs. Panels C and D were stained with H&E. Panels E and F were stained with Prussian Blue. Original magnifications of the panels were × 40 (C and D) and × 400 (E and F). PanelsA, C, and E show lungs from TFPI+/+/low-TF mice and panels B, D, and F show tissues from TFPI–/–/low-TF mice.
Analysis of lungs of low-TF mice with different levels of TFPI. Low-TF mice with wild-type levels of TFPI frequently died of lung hemorrhages (A, C) and also had hemosiderin deposition in their lungs (E). In contrast, no hemorrhages or hemosiderin deposits were observed in lungs of low-TF mice with no TFPI (B, D, F). Panels A and B show macroscopic views of lungs. Panels C and D were stained with H&E. Panels E and F were stained with Prussian Blue. Original magnifications of the panels were × 40 (C and D) and × 400 (E and F). PanelsA, C, and E show lungs from TFPI+/+/low-TF mice and panels B, D, and F show tissues from TFPI–/–/low-TF mice.
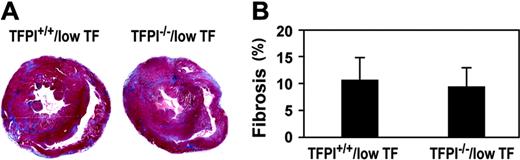
Low-TF mice exhibit progressive cardiac fibrosis, which appears to be secondary to impaired hemostasis in the heart.15 We analyzed the amount of cardiac fibrosis in 6-month-old, low-TF mice with either wild-type levels of TFPI or no TFPI. We observed no differences in the amount of cardiac fibrosis in the 2 groups of mice (P = .56; Figure 7). This result indicates that the absence of TFPI does not affect the cardiac phenotype of low-TF mice.
Cardiac fibrosis in low-TF mice with different levels of TFPI. Mice were euthanized at 6 months of age and hearts analyzed for fibrosis by staining with Masson trichrome. (A) Representative cross sections. Normal myocardium stains red and fibrotic tissue stains blue. (B) Quantitation of the fibrosis was determined using NIH Image Software. Data are presented as mean plus or minus SD from 8 TFPI+/+/low-TF mice and 7 TFPI–/–/low-TF mice.
Cardiac fibrosis in low-TF mice with different levels of TFPI. Mice were euthanized at 6 months of age and hearts analyzed for fibrosis by staining with Masson trichrome. (A) Representative cross sections. Normal myocardium stains red and fibrotic tissue stains blue. (B) Quantitation of the fibrosis was determined using NIH Image Software. Data are presented as mean plus or minus SD from 8 TFPI+/+/low-TF mice and 7 TFPI–/–/low-TF mice.
Effect of TFPI deficiency on uterine hemostasis in low-TF mice
Low-TF mice have impaired uterine hemostasis that results in fatal hemorrhage during gestation and postpartum.6 We analyzed the effect of TFPI deficiency on uterine hemostasis by analyzing the outcome of pregnancies in low-TF female mice with different levels of TFPI bred with C57BL/6 male mice. The following rates of fatal postpartum hemorrhages were observed: TFPI+/+/low TF, 18% (3 of 17); TFPI+/–/low TF, 29% (4 of 14); and TFPI–/–/low TF, 17% (2 of 12). The fact that we still observe fatal hemorrhages in TFPI–/–/low-TF mice suggests that a deficiency in TFPI does not rescue the uterine hemostasis defect of low-TF mice.
Discussion
Our results demonstrate that a low level of TF expression quantitatively rescues the intrauterine lethality of TFPI–/– embryos. This result agrees with a recent study showing that FVII deficiency rescues the embryonic lethality of TFPI–/– embryos.17 However, it is likely that a low level of maternal FVII from the TFPI+/–/FVII+/– mothers permits the survival of the TFPI–/–/FVII–/– embryos.13 TFPI–/– embryos have fibrin deposition in the liver and older TFPI–/– embryos (E14.5-E18.5) often have numerous hemorrhages (Figure 1).17 These observations are consistent with the notion that an absence of TFPI leads to unregulated TF/FVIIa activity, which leads to a consumptive coagulopathy and secondary bleeding.
The death of 60% of TFPI–/– embryos between E9.5 and E11.5 is likely due to a yolk sac defect. Interestingly, TF–/–, factor V–/–, prothrombin–/–, and PAR-1–/– embryos also have a yolk sac defect.7-11 This has led to the idea that TF-dependent thrombin generation and PAR-1 signaling is required for the maintenance of the yolk sac vasculature.12,13 Paradoxically, both an increase or a decrease in TF/FVIIa activity due to deficiencies in TFPI and TF, respectively, may result in a yolk sac defect due to dysregulated thrombin–PAR-1 signaling. A consumption of coagulation factors in TFPI–/– embryos at E9.5 to E11.5 may lead to a reduction in thrombin–PAR-1 signaling.
We and others noted various abnormalities in the placentas of older TFPI–/– embryos (Figures 1 and 2).16 We found that placentas of live TFPI–/– embryos at E14.5 had thrombi in the labyrinthine zone and hemorrhages on the placental surface. Placentas of dead TFPI–/– embryos at E14.5 and E15.5 exhibited increased fibrin deposition and numerous apoptotic cells within the labyrinthine zone. These data again suggest that excess TF/FVIIa activity in the placenta leads to fibrin deposition and thrombosis with secondary hemorrhages. Importantly, reducing levels of TF expression rescued the placental defects observed in TFPI–/– embryos.
We found that TFPI–/–/low-TF mice were generated at the expected frequency from TFPI+/–/mTF+/–/hTF+/– intercrosses and that these mice appear to have normal lifespans. In contrast, TFPI–/–/FVII–/– neonates die shortly after birth due to FVII deficiency.17 The survival of TFPI–/–/low-TF mice permitted us to examine the effect of TFPI deficiency on the hemostatic defects in low-TF mice. Previously, we reported that low-TF mice have fatal postpartum hemorrhage, blood pools in the placenta, cardiac hemorrhage and fibrosis, and acute lung hemorrhages.6,14,15 TFPI deficiency did not significantly change the frequency of fatal postpartum hemorrhage of low-TF mice and did not decrease the degree of cardiac fibrosis. In contrast, TFPI deficiency decreased the number and size of blood pools in low-TF placentas and abolished fatal lung hemorrhages of low-TF mice. The effect of TFPI deficiency on the survival of low-TF mice was dose dependent.
High levels of TFPIα mRNA are expressed in the placenta with lower levels in the uterus, heart, and lung (Figure 3). Low levels of TFPIβ mRNA are also detected in the lung, heart, uterus, and placenta. These results suggest that loss of TFPI would most dramatically impact TF activity in the placenta because this tissue expresses very high levels of TFPI. This is consistent with our data showing that TFPI deficiency reduces blood pools in low-TF placentas. It is more difficult to explain why the absence of TFPI rescues the hemostatic defect in the lung but not the heart or uterus of low-TF mice because these 3 tissues express similar levels of TFPIα and TFPIβ mRNAs. We propose that the uterus, heart, and lung may require different thresholds of TF activity to maintain hemostasis. If the TF activity in the lung of low-TF mice is close to this threshold, then the absence of TFPI may be sufficient to increase TF activity to above the level required to maintain hemostasis. In contrast, the level of TF activity in the uterus and heart may remain below the critical threshold required for hemostasis in these tissues despite the absence of TFPI. Indeed, we have generated a line of mice expressing 10% levels of human TF in the heart and these mice exhibit cardiac fibrosis similar to that of low-TF mice (not shown).
Recently, Rosenberg and Aird21 proposed a model of tissue-specific anticoagulation. The differential expression of the 3 major anticoagulant pathways, TFPI, antithrombin, and thrombomodulin/activated protein C, in different vascular beds suggested that some pathways were more important than others in controlling coagulation in a given organ. Consistent with this idea, TFPI and thrombomodulin are expressed at different levels in different tissues.22 For instance, in humans the expression of TFPI mRNA in the placenta is greater than lung and greater than heart, whereas thrombomodulin mRNA in the heart is greater than lung and greater than placenta.22 Moreover, functional studies in mice heterozygous for thrombomodulin revealed increased fibrin deposition in the heart and lung but not in kidney or brain.23 In TFPI–/– embryos, fibrin deposition was observed in the liver, whereas in antithrombin–/– embryos, fibrin was found in the heart and liver.16,24 These reports and data from the current study support this model of tissue-specific anticoagulation.
Prepublished online as Blood First Edition Paper, December 14, 2004; DOI 10.1182/blood-2004-09-3724.
Supported by a grant from the National Institutes of Health.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We would like to acknowledge M. Szeto and T. Thinnes for technical assistance, C. Johnson for preparing the manuscript, and Dr G. Broze for providing TFPI+/– mice.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal