Abstract
G12/13 or Gq signaling pathways activate platelet GPIIb/IIIa when combined with Gi signaling. We tested whether combined Gi and Gz pathways also cause GPIIb/IIIa activation and compared the signaling requirements of these events. Platelet aggregation occurred by combined stimulation of Gi and Gz pathways in human platelets and in P2Y1-deficient and Gαq-deficient mouse platelets, confirming that the combination of Gi and Gz signaling causes platelet aggregation. When Gi stimulation was combined with Gz stimulation, there was a small mobilization of intracellular calcium. Chelation of intracellular calcium decreased the extent of this platelet aggregation, whereas it abolished the Gq plus Gi-mediated platelet aggregation. Costimulation of Gi plus Gz pathways also caused thromboxane generation that was dependent on outside-in signaling and was inhibited by PP2, a Src family tyrosine kinase inhibitor. Src family tyrosine kinase inhibitors also inhibited platelet aggregation and decreased the PAC-1 binding caused by costimulation of Gi and Gz signaling pathways in aspirin-treated platelets. However, Src family kinase inhibitors did not affect Gq plus Gi-mediated platelet aggregation. We conclude that the combination of Gi plus Gz pathways have different requirements than Gq plus Gi pathways for calcium and Src family kinases in GPIIb/IIIa activation and thromboxane production.
Introduction
Adenosine diphosphate (ADP) is contained in the dense granules of platelets and is released upon platelet activation by agonists such as collagen, thrombin, and thromboxane A2. Released ADP plays a significant role in the potentiation of many platelet responses, including GPIIb/IIIa activation, 1 dense granule secretion,2 and platelet procoagulant activity,3-5 though ADP itself also causes platelet aggregation6 and thromboxane A2 generation.7 While the potentiating effects of ADP have been realized, the signaling mediators for ADP-induced platelet activation still are being identified.
ADP stimulates platelet aggregation by signaling through the P2Y1 and P2Y12 receptors.1,8-10 Stimulation of the Gq-coupled P2Y1 receptor leads to phospholipase Cβ (PLCβ) activation and subsequent intracellular calcium mobilization.11 Increased intracellular calcium mobilization causes platelet shape change through a process of filopodial extension and actin reorganization.12,13 The P2Y12 receptor couples to Gi and leads to the inhibition of adenylyl cyclase14-17 but also activates PI-3 kinase,18 RapIb,19,20 Akt,21-24 and G-protein–coupled inwardly rectifying potassium channels (GIRKS).25 It is the signaling downstream of this receptor that is predominantly responsible for the potentiation of many platelet responses.26-31
Platelets contain members of the Gq, Gi, Gs, and G12/13 families of heterotrimeric G proteins.10,32 Heterotrimeric G proteins contain α, β, and γ subunits and are classified based on the α subunit.33 We previously have shown that costimulation of Gq and Gi signaling pathways downstream of the P2Y1 and P2Y12 receptors, respectively, leads to platelet aggregation.34 Recently, we and others have shown that combined signaling of G12/13 and Gi pathways also is sufficient to cause platelet aggregation.35,36
Mice deficient in Gq, G12, or G13 proteins confirm that signaling through multiple G-protein pathways are necessary for normal platelet aggregation.37,38 Gq-deficient mice have platelets that undergo shape change but no aggregation in response to thromboxane A2 or thrombin, while ADP causes neither shape change nor aggregation.37 Consistent with observations that G12/13 signaling is pro-aggregatory when combined with Gi signaling, mice deficient in alpha subunit of G13 have impaired platelet aggregation and granule secretion responses.38 Mice deficient in the alpha subunit of Gi2 have both decreased ADP-induced platelet aggregation and decreased inhibition of adenylyl cyclase, suggesting that the P2Y12 receptor primarily couples to Gi2.14,17 Mice that are deficient in the alpha subunit of Gz have an impaired response to epinephrine-mediated effects, suggesting that the α2A adrenergic receptor couples primarily to Gz.39 Both Gi2 and Gz belong to the Gi class of G proteins.33 Because the interactions between the multiple G-protein pathways have been shown to elicit different effects than either pathway alone, we characterized the functional effects of the Gi and Gz pathways on platelet activation. We then compared the signaling requirements for Gi and Gz signaling with those of Gq and Gi signaling, 2 pathways simultaneously activated by ADP.
The platelet contains many serine/threonine and tyrosine kinases that are responsible for transducing intracellular signals upon agonist stimulation. Serine/threonine kinases such as protein kinase C (PKC) and Akt are activated by thrombin, collagen, and thromboxane A2 and are important for platelet responses such as dense granule release and GPIIb/IIIa activation.23,24,40 ADP, though not dependent on PKC, does contribute to Akt activation through P2Y12 receptor signaling.23 Tyrosine kinases such as Syk and members of the Src family of tyrosine kinases are activated by agonists such as thrombin and collagen and also play a role in platelet aggregation.41-43 Syk-deficient platelets have impaired ability to aggregate in response to ADP and epinephrine, while Src family tyrosine kinase inhibitors decrease ADP-mediated effects41,44 as well as PAR1-mediated PAC-1 Ab binding.43 While these kinases are important to platelet function downstream of many agonists, the implications of their activity have not yet been elucidated.
Here, we demonstrate that combined stimulation of Gi and Gz pathways leads to fibrinogen receptor activation and thromboxane generation in platelets and depends on signaling pathways distinct from those required for Gq and Gi-mediated platelet functional responses. PI-3 kinases and Src family kinases are important mediators of Gi and Gz-mediated functional responses, whereas PI-3 kinase and calcium are important for Gq and Gi-mediated platelet responses.
Materials and methods
Approval for this study was obtained from the Institutional Review Board of Temple University (Philadelphia, PA).
Materials
ADP and epinephrine were purchased from Chrono-Log (Havertown, PA). Fluorescein isothiocyanate (FITC)–conjugated monoclonal antibody PAC-1 was purchased from BD Pharmingen (San Diego, CA). Fura-2, am was purchased from Molecular Probes (Eugene, OR). The acetoxymethyl ester of 5,5-dimethyl-bis-(o-aminophenoxy)ethane-N′, N′, N′, N′ tetra-acetic acid (dimethyl BAPTA-am), LY294002, PP1, PP2, PP3, and Ro 31-8220 were purchased from Biomol (Plymouth Meeting, PA). AR-C 69931MX was a gift from Astra-Zeneca Research Laboratories-Charnwood (Loughborough, United Kingdom). MRS 2179, 2-MeSADP, apyrase grade VII, human fibrinogen, and acetylsalicylic acid were purchased from Sigma (St Louis, MO). The phospho-Src (416) antibody was purchased from Cell Signaling (Beverly, MA).
Animals
Platelet preparation
Whole blood was drawn from healthy, consenting human volunteers into tubes containing one-sixth volume of ACD (2.5 g sodium citrate, 1.5 g citric acid, and 2 g glucose in 100 mL deionized water). Blood was centrifuged (Eppendorf 5810R centrifuge, Hamburg, Germany) at 230 rcf for 20 minutes at room temperature to obtain platelet-rich plasma (PRP). PRP was incubated with 1 mM acetylsalicylic acid for 30 minutes at 37°C. The PRP was then centrifuged for 10 minutes at 980 rcf (room temperature) to pellet the platelets. Platelets were resuspended in Tyrode buffer (138 mM NaCl, 2.7 mM KCl, 1 mM MgCl2, 3 mM NaH2PO4, 5 mM glucose, 10 mM HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid) pH 7.4, 0.2% bovine serum albumin) containing 0.01 U/mL apyrase. Cells were counted using a Z1 Coulter Particle Counter. For flow cytometry studies, cells were adjusted to a concentration of 1.875 × 107 platelets/mL. Platelets were left at room temperature for 30 minutes after resuspension to obtain a quiescent state.
Aggregometry and ATP release
Aggregation of 0.5 mL of washed platelets was analyzed using a PICA lumiaggregometer (Chrono-log, Havertown, PA). Aggregation was measured using light transmission under stirring conditions (900 rpm) at 37°C. Agonists were added simultaneously for platelet stimulation; however, platelets were preincubated with each inhibitor (where noted) as follows: 10 μM Ro 31-8220 for 3 minutes at 37°C and 25 μM LY294002, 10 μM PP1, 10 μM PP2, 10 μM PP3, and 1 μM dimethyl BAPTA-am for 10 minutes at 37°C. Each sample was allowed to aggregate for at least 3 minutes. The chart recorder (Kipp and Zonen, Bohemia, NY) was set for 0.2 mm per second. All samples contained exogenously added human fibrinogen (1 mg/mL). For adenosine triphosphate (ATP) release, 5 μL 2 μM luciferin/luciferase was added to the washed platelets 1 minute prior to initiation of secretion. Aggregation and ATP release were measured simultaneously. Aggregation tracings are representative of results obtained from 3 separate experiments on 3 different donors.
Intracellular calcium mobilization
Calcium mobilization was measured in platelets that were loaded with 2 μM fura-2, am in PRP for 45 minutes at 37°C, and washed platelets were isolated as noted above in “Platelet preparation,” and brought to a final concentration of 2 × 108 platelets/mL in Tyrode buffer. Samples of Fura-2, am-loaded platelets (0.5 mL) were placed in a quartz cuvette with a magnetic stir bar and incubated for 1 minute at 37°C in a temperature-controlled chamber. An Aminco Bowman Series 2 luminescence spectrometer (Aminco Bowman, Rochester, NY) was used for measurement of intracellular calcium mobilization. Two wavelengths (340 and 380 nm) were used for excitation, and the emitted light was measured at 510 nm. Samples were stimulated after 1 minute of incubation at 37°C. Fmin was obtained by addition of 20 mM Tris (tris(hydroxymethyl)aminomethane) and 4 mM EGTA (ethylene glycol tetraacetic acid), and Fmax was determined by adding 0.25% Triton and saturating levels of CaCl2. Calculation of the calcium mobilization was performed as outlined previously.45
Sample preparation and Western blotting
Platelets were resuspended in Tyrode buffer at a concentration of 2 × 108 platelets/mL as noted above in “Platelet preparation.” Platelets were stimulated in the presence of 10 μM SC57101A, a GPIIb/IIIa antagonist, to eliminate outside-in signaling. Platelets were stimulated with 10 μM ADP + 10 μM MRS2179 for selective Gi signaling, 10 μM epinephrine for Gz signaling, or a combination of both for Gi and Gz signaling. The reaction was stopped with 3X Sample Buffer, boiled for 10 minutes, and platelet lysates were subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and Western blotting according to a procedure that has been described previously.23 Membranes were probed with a primary antibody that recognizes Src Tyr416 (1:1000 titer) (Cell Signaling) as an indication of Src activity, then probed with an antimouse secondary antibody (Ab) (Kirkegaard and Perry Laboratories, Gaithersburg, MD) that is conjugated to alkaline phosphatase. Chemiluminescence was measured using a Fujifilm LAS-3000 luminescent image analyzer (Fuji, Tokyo, Japan).
Analysis of PAC-1 binding
Activation of GPIIb/IIIa was measured by PAC-1 monoclonal Ab (mAb) binding to washed platelets and subsequent analysis by flow cytometry. Aspirin-treated platelets were isolated by centrifugation as noted, then counted and brought to a concentration of 1.7 × 107 platelets/mL. PAC-1-FITC mAb (5 μL) was present in each sample. The samples were stimulated for 10 minutes in the dark and then diluted with 450 μL Tyrode buffer. Each sample was transferred to a 12-mm × 75-mm cuvette (Fisher Scientific, Pittsburgh, PA) and analyzed by flow cytometry using FACS-CAN (BD Biosciences, San Jose, CA) to measure an increase in fluorescence that indicates an increase in GPIIb/IIIa receptor activation. The experiment was performed 3 times, and data are presented as mean ± SE.
Mouse platelet preparation
C57BL mice were sedated and anesthetized using 0.05 cc ketamine injected intramuscularly into the hind quarter. After induction, blood was drawn from the right ventricle into a 1-cc syringe containing 0.1 cc of 3.8% sodium citrate. After drawing the blood, the needle of the syringe was removed (to eliminate shearing of the cells), and the blood was ejected into an Eppendorf tube containing 200 μL 3.8% sodium citrate. Blood was centrifuged at 100 rcf for 10 minutes to obtain platelet-rich plasma. 3.8% sodium citrate (200 μL) was added to the remaining cells to extract more PRP and spun at 100 rcf for 10 minutes. PGE1 (1 μM) was added to the PRP and was centrifuged at 500 rcf for 10 minutes at room temperature to pellet platelets. Platelets were resuspended in 1 mL of Tyrode containing 0.01 U/mL apyrase.
Measurement of thromboxane A2 generation
Washed, aspirin-treated human platelets were prepared as noted above and brought to a concentration of 2 × 108 platelets/mL. Stimulations were performed in a platelet aggregometer under stirring conditions (900 rpm) at 37°C. All antagonists were added 1 minute prior to the stimulation, and all agonists were added simultaneously for stimulation. All samples contained exogenously added human fibrinogen (1 mg/mL). Stimulations occurred for 3.5 minutes and were stopped by snap freezing. The samples were stored at –80°C until thromboxane analysis was performed. Levels of thromboxane generation were determined in duplicate using a Correlate-EIA Thromboxane B2 Enzyme Immunoassay Kit (Assay Designs, Ann Arbor, MI), according to manufacturer's instructions. Data represent the average of 3 days' data ± standard error.
Results
Costimulation of Gi and Gz signaling causes platelet aggregation
Our laboratory is interested in the G protein signaling pathways that lead to the activation of GPIIb/IIIa and platelet aggregation.34,35 We began by measuring the aggregation of washed human platelets to determine if a combination of Gi signaling through the P2Y12 receptor and Gz signaling through the α2A adrenergic receptor could cause platelet aggregation. We used a washed aspirin-treated platelet preparation devoid of any plasma proteins to eliminate the positive feedback from generated thromboxane A2 and thrombin.
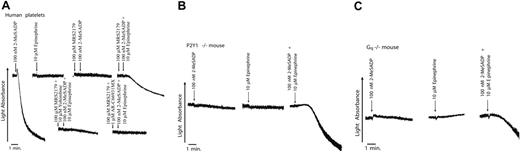
The agonist 2-MeSADP, which stimulates both the P2Y1 and P2Y12 platelet receptors, caused the aggregation of washed, aspirin-treated human platelets. Using 2-MeSADP in the presence of a P2Y1 receptor antagonist causes signaling selectively through the Gi-coupled P2Y12 receptor. The platelet aggregation was blocked with the P2Y1 receptor antagonist MRS2179, confirming that 2-MeSADP requires signaling through both P2Y1 and P2Y12 receptors to cause platelet aggregation.34 Epinephrine-mediated signaling through the Gz pathway also did not cause aggregation, however, the combination of Gi stimulation (2-MeSADP plus MRS2179) with Gz signaling (epinephrine) caused aggregation in human platelets (Figure 1A). The aggregation was slower than 2-MeSADP–induced aggregation and was irreversible. This platelet aggregation was inhibited by antagonism of either the P2Y12 receptor with AR-C69931MX or the α2A adrenergic receptor using yohimbine, confirming that the aggregation is mediated by the combination of these 2 receptors (Figure 1A). Platelet aggregation caused by P2Y12 selective stimulation plus epinephrine also occurred in P2Y1-deficient or Gq-deficient mouse platelets (Figure 1B-C); therefore, signaling through the Gi and Gz pathways is sufficient to cause aggregation in both human and mouse platelets.
Platelet aggregation induced by simultaneous stimulation of the Gi and Gz pathways. (A) Aspirin-treated, washed human platelets were stimulated with 100 nM 2-MeSADP, 10 μM epinephrine, or a combination of both agonists, as noted. MRS2179 (100 μM), 100 nM AR-C69931MX, or 10 μM yohimbine, antagonizing P2Y1, P2Y12, and α2A adrenergic receptor antagonists, respectively, were added 1 minute prior to platelet stimulation. All aggregation tracings were performed in the presence of 1 mg/mL fibrinogen. Tracings are representative of results obtained from 3 donors and 3 separate experiments. (B, C) Aspirin-treated, washed platelets were isolated from P2Y1- or Gq-deficient mice as noted in “Materials and methods.” Samples were stimulated with either 3 μM ADP or 10 μM epinephrine at 37°C under stirring conditions (900 rpm). In the case where both ADP and epinephrine were added, the additions of the agonists were made simultaneously. All tracings are representative of data obtained from 3 separate experiments.
Platelet aggregation induced by simultaneous stimulation of the Gi and Gz pathways. (A) Aspirin-treated, washed human platelets were stimulated with 100 nM 2-MeSADP, 10 μM epinephrine, or a combination of both agonists, as noted. MRS2179 (100 μM), 100 nM AR-C69931MX, or 10 μM yohimbine, antagonizing P2Y1, P2Y12, and α2A adrenergic receptor antagonists, respectively, were added 1 minute prior to platelet stimulation. All aggregation tracings were performed in the presence of 1 mg/mL fibrinogen. Tracings are representative of results obtained from 3 donors and 3 separate experiments. (B, C) Aspirin-treated, washed platelets were isolated from P2Y1- or Gq-deficient mice as noted in “Materials and methods.” Samples were stimulated with either 3 μM ADP or 10 μM epinephrine at 37°C under stirring conditions (900 rpm). In the case where both ADP and epinephrine were added, the additions of the agonists were made simultaneously. All tracings are representative of data obtained from 3 separate experiments.
Intracellular signaling events resulting from combined Gi and Gz signaling
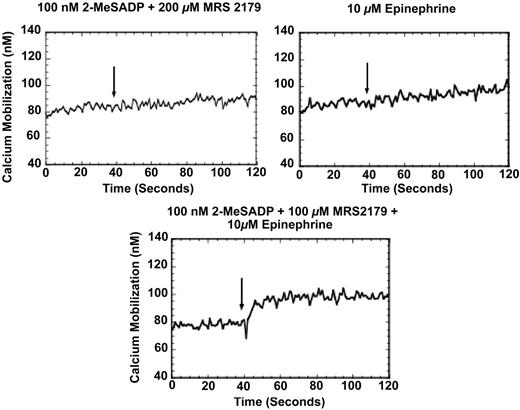
Because our previous work showed that combined G12/13 and Gi signaling mobilizes small amounts of intracellular calcium,35 we tested the effects of Gi and Gz signaling on intracellular calcium mobilization. Washed, aspirin-treated platelets were loaded with 2 μM Fura-2 and stimulated through either Gi or Gz signaling alone, then in combination, to measure whether increases in intracellular calcium occurred with the combination of Gi and Gz signaling pathways. Interestingly, though Gz or Gi signaling alone did not cause any calcium mobilization, the combination of the 2 signaling pathways produced a small increase in fluorescence, indicating that mobilization of intracellular calcium occurs upon simultaneous stimulation of Gi and Gz pathways (Figure 2).
Intracellular calcium mobilization in response to simultaneous stimulation of the Gi and Gz signaling. Fura-loaded, aspirinated, washed human platelets were stimulated as noted under stirring conditions at 37°C. Where noted, the antagonist 200 μM MRS2179 was added 1 minute prior to the addition of other agonists. Arrows indicate addition of agonist. Tracings are representative of results obtained from 3 different donors.
Intracellular calcium mobilization in response to simultaneous stimulation of the Gi and Gz signaling. Fura-loaded, aspirinated, washed human platelets were stimulated as noted under stirring conditions at 37°C. Where noted, the antagonist 200 μM MRS2179 was added 1 minute prior to the addition of other agonists. Arrows indicate addition of agonist. Tracings are representative of results obtained from 3 different donors.
G-protein–mediated activation of GPIIb/IIIa involves many intracellular signaling mediators, including calcium, protein kinase C, and PI-3 kinase.10,32 We tested the effects of inhibiting these molecules on the aggregation caused by combined Gi plus Gz-mediated or Gq plus Gi-mediated signaling in aspirin-treated platelets by using the PKC inhibitor Ro 31-8220, the intracellular calcium chelator dimethyl BAPTA-am, and the PI-3 kinase inhibitor LY 294002. PKC inhibition, using 10 μM Ro 31-8220, had no effect on the aggregation caused by either costimulation of Gi and Gz signaling pathways or on Gq- and Gi-mediated platelet aggregation (Figure 3). Preincubation with 1 μM dimethyl BAPTA-am slowed and decreased the extent of platelet aggregation for Gi and Gz signaling by 35% (SD ± 5.29) and completely inhibited the aggregation caused by Gq and Gi signaling (Figure 3). PI-3 kinase inhibition with 25 μM LY294002 completely blocked platelet aggregation that was caused by Gi and Gz and also dramatically blocked the aggregation that was caused by Gq and Gi signaling (Figure 3).
The effect of kinase inhibitors on platelet aggregation caused by the simultaneous stimulation of Gi and Gz or Gq and Gi pathways. Platelets were preincubated with kinase inhibitors as follows: 10 minutes at 37°C with dimethyl sulfoxide (DMSO) (vehicle), 3 minutes at 37°C with 10 μM Ro 31-8220, 10 minutes of preincubation at 37°C with 1 μM dimethyl BAPTA-AM, or 10 minutes of preincubation with 25 μM LY294002. Platelets were stimulated with 100 nM 2-MeSADP, 10 μM epinephrine, or a combination of both agonists, as noted, for Gi and Gz stimulation. MRS2179 (100 μM) was added 1 minute prior to platelet stimulation. ADP (10 μM) was used as an agonist for stimulation of Gq and Gi pathways. All aggregation tracings were performed in the presence of 1 mg/mL fibrinogen. Tracings are representative of results obtained from 3 donors and 3 separate experiments.
The effect of kinase inhibitors on platelet aggregation caused by the simultaneous stimulation of Gi and Gz or Gq and Gi pathways. Platelets were preincubated with kinase inhibitors as follows: 10 minutes at 37°C with dimethyl sulfoxide (DMSO) (vehicle), 3 minutes at 37°C with 10 μM Ro 31-8220, 10 minutes of preincubation at 37°C with 1 μM dimethyl BAPTA-AM, or 10 minutes of preincubation with 25 μM LY294002. Platelets were stimulated with 100 nM 2-MeSADP, 10 μM epinephrine, or a combination of both agonists, as noted, for Gi and Gz stimulation. MRS2179 (100 μM) was added 1 minute prior to platelet stimulation. ADP (10 μM) was used as an agonist for stimulation of Gq and Gi pathways. All aggregation tracings were performed in the presence of 1 mg/mL fibrinogen. Tracings are representative of results obtained from 3 donors and 3 separate experiments.
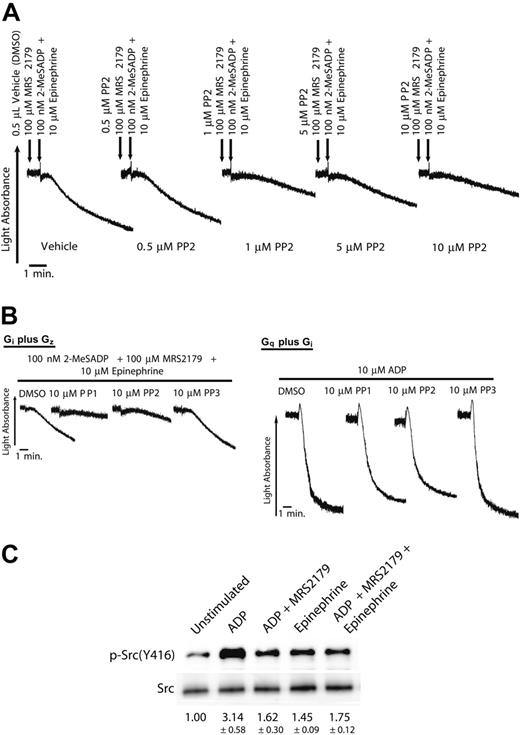
The Src family tyrosine kinase inhibitor PP2 dose-dependently decreased platelet aggregation caused by combined Gi and Gz stimulation (Figure 4A). Platelet aggregation was drastically decreased at the maximal concentration of 10 μM PP2. To confirm the inhibitory effect of Src family tyrosine kinase inhibitors on platelet aggregation, we also tested PP1, an Src family tyrosine kinase inhibitor, and PP3, a control compound that does not inhibit tyrosine kinase activity. Whereas PP1 decreased the extent of aggregation caused by combined Gi and Gz signaling, the control compound PP3 had no effect (Figure 4B). On the other hand, PP1, PP2, or PP3 did not have a significant inhibitory effect on the aggregation caused by Gq plus Gi-induced aggregation (Figure 4B). We next measured if the agonist-induced inside-out signaling causes Src activity. After stimulating the platelets, we subjected the platelet lysates to SDS-PAGE and Western blotting in the presence of 10 μM SC57101A, a GPIIb/IIIa antagonist. We probed the membrane for the presence of the phosphorylation of Src tyrosine 416, which is an indicator of Src activity. Compared with control, both Gi and Gz signaling were able to cause an increase in Src416 phosphorylation (Figure 4C).
The effect of Src family tyrosine kinase inhibition on platelet aggregation caused by the simultaneous stimulation of Gi and Gz pathways. (A) Platelets were preincubated at 37°C with DMSO (vehicle) or varying doses of PP2 for10 minutes prior to the stimulation of the platelets. Platelets were stimulated with 100 nM 2-MeSADP plus 100 μM MRS 2179 (Gi signaling), 10 μM epinephrine (Gz signaling), or a combination of both agonists, as noted. MRS2179 (100 μM) was added 1 minute prior to platelet stimulation. (B) Washed human platelets were preincubated at 37°C with DMSO (vehicle) or with 10 μM PP1, 10 μM PP2, or 10 μM PP3 for 10 minutes prior to the stimulation of the platelets. Platelets were stimulated with 100 nM 2-MeSADP, 10 μM epinephrine, or a combination of both agonists, as noted. MRS2179 (100 μM) was added 1 minute prior to platelet stimulation. ADP (10 μM) was added for simultaneous stimulation of Gq and Gi pathways. All aggregation tracings were performed in the presence of 1 mg/mL fibrinogen. Tracings are representative of results obtained from 3 donors and 3 separate experiments. (C) Washed, aspirin-treated platelets (2 × 108 platelets/mL) were stimulated as noted in “Materials and methods.” Chemiluminescence was measured on membranes that were probed with an anti–phospho-Src416 antibody for measurement of Src kinase activity. The intensity of the bands was first calculated as a ratio of phosphorylated-to-total Src in each lane. Then the ratios from 3 independent experiments were normalized to the control, taken as 1, and expressed as fold increase in other lanes (mean ± SEM). The blot shown is representative of 3 independent experiments.
The effect of Src family tyrosine kinase inhibition on platelet aggregation caused by the simultaneous stimulation of Gi and Gz pathways. (A) Platelets were preincubated at 37°C with DMSO (vehicle) or varying doses of PP2 for10 minutes prior to the stimulation of the platelets. Platelets were stimulated with 100 nM 2-MeSADP plus 100 μM MRS 2179 (Gi signaling), 10 μM epinephrine (Gz signaling), or a combination of both agonists, as noted. MRS2179 (100 μM) was added 1 minute prior to platelet stimulation. (B) Washed human platelets were preincubated at 37°C with DMSO (vehicle) or with 10 μM PP1, 10 μM PP2, or 10 μM PP3 for 10 minutes prior to the stimulation of the platelets. Platelets were stimulated with 100 nM 2-MeSADP, 10 μM epinephrine, or a combination of both agonists, as noted. MRS2179 (100 μM) was added 1 minute prior to platelet stimulation. ADP (10 μM) was added for simultaneous stimulation of Gq and Gi pathways. All aggregation tracings were performed in the presence of 1 mg/mL fibrinogen. Tracings are representative of results obtained from 3 donors and 3 separate experiments. (C) Washed, aspirin-treated platelets (2 × 108 platelets/mL) were stimulated as noted in “Materials and methods.” Chemiluminescence was measured on membranes that were probed with an anti–phospho-Src416 antibody for measurement of Src kinase activity. The intensity of the bands was first calculated as a ratio of phosphorylated-to-total Src in each lane. Then the ratios from 3 independent experiments were normalized to the control, taken as 1, and expressed as fold increase in other lanes (mean ± SEM). The blot shown is representative of 3 independent experiments.
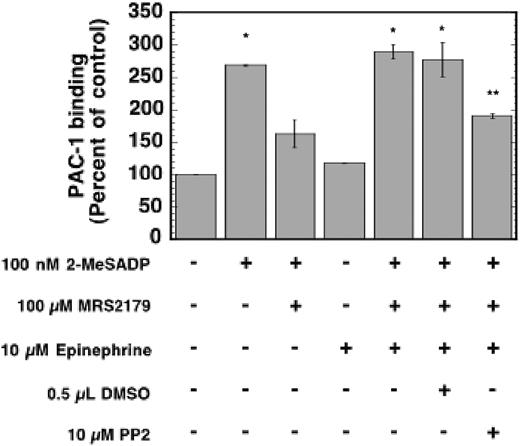
We used the FITC-labeled PAC-1 monoclonal antibody, which recognizes the active conformation of GPIIb/IIIa, to measure if the combination of Gi and Gz signaling pathways is mediating platelet aggregation through activation of GPIIb/IIIa. While there was no increase in the fluorescence of samples stimulated with either Gi or Gz signaling alone, the combination of both agonists produced an increase in fluorescence, indicating that the aggregation is mediated by GPIIb/IIIa activation (Figure 5). Pretreating the aspirin-treated platelets with the Src family tyrosine kinase inhibitor PP2 caused a decrease in the mean fluorescence of platelets that were stimulated with Gi and Gz signaling pathways (Figure 5).
The effect of Src family tyrosine kinase inhibition on PAC-1 binding that is induced by combined Gi and Gz stimulation. Aspirin-treated washed human platelets were stimulated for 10 minutes with the noted agonists in the presence of FITC-labeled PAC-1 mAb. Samples were diluted with Tyrode buffer and analyzed on a flow cytometer for increases in fluorescence that are correlated with GPIIb/IIIa activation. Data were calculated as mean fluorescence of the total platelet population. Each bar is the average of 3 experiments ± SE from 3 donors. Single asterisk (*) denotes P < .05 compared to unstimulated. Double asterisk (**) denotes P < .05 when compared to single asterisk samples.
The effect of Src family tyrosine kinase inhibition on PAC-1 binding that is induced by combined Gi and Gz stimulation. Aspirin-treated washed human platelets were stimulated for 10 minutes with the noted agonists in the presence of FITC-labeled PAC-1 mAb. Samples were diluted with Tyrode buffer and analyzed on a flow cytometer for increases in fluorescence that are correlated with GPIIb/IIIa activation. Data were calculated as mean fluorescence of the total platelet population. Each bar is the average of 3 experiments ± SE from 3 donors. Single asterisk (*) denotes P < .05 compared to unstimulated. Double asterisk (**) denotes P < .05 when compared to single asterisk samples.
Effect of costimulation of Gi and Gz signaling pathways on thromboxane generation
There was a marked difference in the extent of platelet aggregation of non–aspirin-treated platelets versus aspirin-treated platelets when treated with combined Gi and Gz signaling (Figure 6). ADP alone caused platelet aggregation in both aspirin-treated and non–aspirin-treated platelets, though selective Gi or Gz stimulation alone failed to cause platelet aggregation. The aggregation stimulated via Gi and Gz signaling was significantly more robust in non–aspirin-treated human platelets compared to aspirin-treated platelets (Figure 6). Whereas ADP caused dense granule release in non–aspirin-treated human platelets, either Gi or Gz stimulation alone did not lead to dense granule release. Thromboxane from combined Gi and Gz stimulation resulted in dense granule release in the non–aspirin-treated platelets but not in the aspirin-treated human platelets (data not shown).
The effect of aspirin on platelet aggregation caused by simultaneous stimulation of the Gi and Gz pathways. Top panel contains aggregation tracings of non–aspirin-treated washed human platelets, while the bottom panel contains aspirin-treated washed human platelets. Where noted, the antagonist 100 μM MRS2179 was added 1 minute prior to the addition of other agonists. The agonists 100 nM 2-MeSADP and 10 μM epinephrine were added simultaneously where noted. The arrows indicate addition of agonists. All samples are performed in the presence of 1 mg/mL fibrinogen. Tracings are representative of data obtained from 3 different donors.
The effect of aspirin on platelet aggregation caused by simultaneous stimulation of the Gi and Gz pathways. Top panel contains aggregation tracings of non–aspirin-treated washed human platelets, while the bottom panel contains aspirin-treated washed human platelets. Where noted, the antagonist 100 μM MRS2179 was added 1 minute prior to the addition of other agonists. The agonists 100 nM 2-MeSADP and 10 μM epinephrine were added simultaneously where noted. The arrows indicate addition of agonists. All samples are performed in the presence of 1 mg/mL fibrinogen. Tracings are representative of data obtained from 3 different donors.
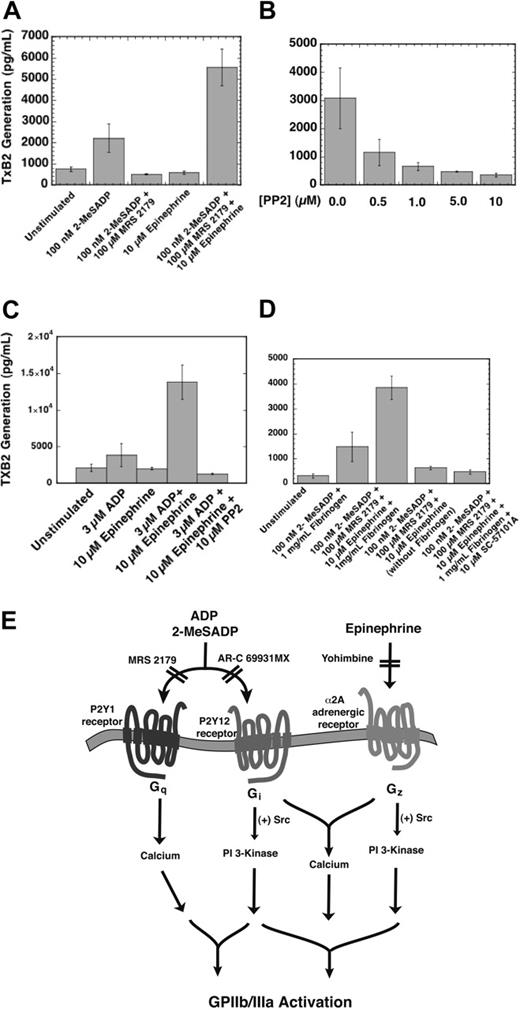
Because the difference in platelet aggregation was dependent on aspirin, we continued by measuring thromboxane generation after combined Gi and Gz signaling. A competitive ELISA (enzyme-linked immunosorbent assay) was used to quantitate the amount of thromboxane A2 generation that occurred after 3.5 minutes of stimulation. Because of the short half-life of generated thromboxane A2, samples were probed for their thromboxane B2 content, a more stable product that reflects thromboxane A2 production. Either Gi or Gz stimulation alone generated similar levels of thromboxane compared with unstimulated human platelets (Figure 7A). Combined Gi and Gz stimulation caused a sizeable increase in thromboxane A2 generation compared with the thromboxane levels generated by 100 nM 2-MeSADP (Figure 7A).
The effect of combined Gi and Gz signaling on thromboxane generation in human and mouse platelets. (A) Thromboxane B2 (TxB2) was generated from washed human platelets in the presence of 1 mg/mL fibrinogen and stopped after 3.5 minutes of stimulation. Levels of TxB2 were obtained using a 96-well competitive ELISA kit as noted in the “Materials and methods” section. (B) Samples were preincubated with varying doses of PP2 for 10 minutes at 37°C and then stimulated with combined Gi and Gz signaling. (C) The effect of combined Gi and Gz stimulation on thromboxane generation in P2Y1-deficient mouse platelets. Where noted, samples were preincubated with 10 μM PP2 for 10 minutes at 37°C. (D) The effect of GPIIb/IIIa antagonism on thromboxane generation induced by combined P2Y12 and α2A adrenergic stimulation. The GPIIb/IIIa antagonist, 10 μM SC57101A, was added 1 minute prior to the addition of agonists (where noted). Similarly, 1 mg/mL fibrinogen was added to samples 1 minute prior to stimulation (where noted). In A-D, the bars are representative of the average thromboxane A2 generated (± standard error) from 3 different donors on 3 days of experiments. (E) Final model depicting Gi- and Gz-induced platelet aggregation. The agonists 2-MeSADP and ADP both activate the P2Y1 and P2Y12 receptors. MRS2179 and AR-C 69931MX antagonize the P2Y1 and P2Y12 receptors, respectively (noted by hatch marks). Epinephrine is an agonist for the α2A adrenergic receptor and is blocked by the antagonist yohimbine. The combined signaling through the P2Y12 and α2A adrenergic receptors mediates Src family tyrosine kinase activity, which modulates integrin activation.
The effect of combined Gi and Gz signaling on thromboxane generation in human and mouse platelets. (A) Thromboxane B2 (TxB2) was generated from washed human platelets in the presence of 1 mg/mL fibrinogen and stopped after 3.5 minutes of stimulation. Levels of TxB2 were obtained using a 96-well competitive ELISA kit as noted in the “Materials and methods” section. (B) Samples were preincubated with varying doses of PP2 for 10 minutes at 37°C and then stimulated with combined Gi and Gz signaling. (C) The effect of combined Gi and Gz stimulation on thromboxane generation in P2Y1-deficient mouse platelets. Where noted, samples were preincubated with 10 μM PP2 for 10 minutes at 37°C. (D) The effect of GPIIb/IIIa antagonism on thromboxane generation induced by combined P2Y12 and α2A adrenergic stimulation. The GPIIb/IIIa antagonist, 10 μM SC57101A, was added 1 minute prior to the addition of agonists (where noted). Similarly, 1 mg/mL fibrinogen was added to samples 1 minute prior to stimulation (where noted). In A-D, the bars are representative of the average thromboxane A2 generated (± standard error) from 3 different donors on 3 days of experiments. (E) Final model depicting Gi- and Gz-induced platelet aggregation. The agonists 2-MeSADP and ADP both activate the P2Y1 and P2Y12 receptors. MRS2179 and AR-C 69931MX antagonize the P2Y1 and P2Y12 receptors, respectively (noted by hatch marks). Epinephrine is an agonist for the α2A adrenergic receptor and is blocked by the antagonist yohimbine. The combined signaling through the P2Y12 and α2A adrenergic receptors mediates Src family tyrosine kinase activity, which modulates integrin activation.
Src family tyrosine kinase inhibition decreased the aggregation caused by combined Gi and Gz stimulation (Figure 4). We therefore tested the effect of PP2 on thromboxane generation from combined stimulation of these pathways. Src family tyrosine kinase inhibition completely inhibited thromboxane production in human platelets from combined Gi and Gz signaling (Figure 7B). To confirm that there was no contribution from P2Y1 receptor signaling, we performed the same experiment in P2Y1–/– mice. Similar to human platelets, thromboxane was generated in P2Y1-deficient mouse platelets only when Gi and Gz signaling were combined (Figure 7C). This confirms that costimulation of the P2Y12 and α2A adrenergic receptors is also sufficient to cause thromboxane generation in mouse platelets. Similar to human platelets, pretreating the mouse platelets with 10 μM PP2 inhibited the thromboxane production that resulted from combined Gi and Gz stimulation (Figure 7C).
It has previously been reported that fibrinogen must be present for ADP to cause thromboxane generation in platelets.46 We noted that fibrinogen also was necessary for thromboxane production via combined Gi and Gz signaling (Figure 7D). In the absence of fibrinogen, thromboxane levels were comparable to unstimulated samples. We also used a GPIIb/IIIa antagonist, SC57101, in the presence of fibrinogen, to test if the effect of fibrinogen was GPIIb/IIIa-specific. The GPIIb/IIIa antagonist blocked thromboxane production when fibrinogen was present, suggesting that fibrinogen interactions with GPIIb/IIIa are necessary for thromboxane generation via combined Gi and Gz signaling (Figure 7D).
Discussion
The Gi signaling pathway is central to platelet activation, primarily because the Gi pathway potentiates alpha- and dense granule release, GPIIb/IIIa activation, and platelet procoagulant activity. Whereas the Gi pathway alone does not cause aggregation, Gi signaling can interact with other G-protein signaling pathways to cause platelet aggregation. Previous work has shown that Gi signaling causes platelet aggregation when it is combined with either Gq or G12/13 signaling.9,34-36 Hence, we examined the functional responses that occur when Gi is combined with Gz signaling, another member of the Gi family of G proteins, to further characterize the complexities of the interactions between the Gi and other G-protein pathways.
Platelet aggregation triggered by costimulation of the P2Y12 and α2A adrenergic receptor results in signaling through multiple members of the Gi family because each receptor signals primarily through different members of the Gi family.17 Although the Gi and Gz pathways couple to many of the same effectors, there still remains the possibility that these pathways signal through distinct signaling pathways. However, there is no published evidence for this.
Our work and work by others has shown that partial irreversible platelet aggregation occurs with the co-activation of the G12/13 and Gi pathways.35,36 Although 100 μM ADP has been reported to cause platelet aggregation in the absence of any calcium mobilization in mouse platelets,47 we have found that a combination of low-level P2Y12 receptor and α2A adrenergic receptor stimulation results in a small increase in calcium in human platelets, which is similar to our previous work with combined G12/13 and Gi stimulation.35 The mechanism of this increase of intracellular calcium is presently unclear, however, βγ-mediated activation of PLC or calcium leakage resulting from inhibition of adenylyl cyclase are 2 possibilities. Chelation of intracellular calcium moderately decreased the extent of aggregation for Gi plus Gz signaling, while ADP-mediated signaling through Gq and Gi was completely inhibited by intracellular calcium chelation.
Calcium is known to play a significant role in thromboxane A2 generation in platelets.48 The amount of thromboxane A2 generated by 2-MeSADP was consistently less than the levels generated with combined Gi and Gz stimulation in human platelets. This is unexpected, primarily because ADP mobilizes higher levels of intracellular calcium through the Gq-coupled P2Y1 receptor than the combined signaling through Gi and Gz pathways. Thus, combined Gi- and Gz-mediated thromboxane generation may activate phospholipase A2 (PLA2) more readily than ADP-mediated signaling, leading us to speculate that this robust activation of PLA2 is not completely dependent on intracellular calcium mobilization.
An Src family tyrosine kinase inhibitor, PP2, decreased the platelet aggregation caused by P2Y12 receptor and α2A adrenergic receptor stimulation. Others have used pharmacologic approaches to suggest that Src family tyrosine kinase activity affects platelet aggregation caused by other agonists. Two Src family tyrosine kinase inhibitors, PP2 and PD173956, inhibited PAR1-stimulated PAC-1 binding by 35%.43 ADP and epinephrine-induced fibrinogen binding was decreased by piceatannol, an inhibitor of Syk and Src.41 This suggests that multiple agonists stimulate GPIIb/IIIa activity that is partially regulated by tyrosine kinase activity. The Src family tyrosine kinase inhibitor PP1 also inhibited the aggregation caused by combined Gi and Gz signaling, while the control compound PP3 had no effect on this aggregation (Figure 4B). PAC-1 binding, which is indicative of GPIIb/IIIa activation,49 resulted from combined stimulation of Gi and Gz signaling. Src tyrosine kinase inhibition led to a decrease in PAC-1 binding, suggesting that tyrosine kinases have a role in the modulation of GPIIb/IIIa activity downstream of these 2 pathways. Notably, PP2 did not completely inhibit the platelet aggregation or GPIIb/IIIa activation that is caused by costimulation of Gi and Gz signaling. Therefore, while Src family tyrosine kinases may increase the extent of GPIIb/IIIa activation, they are not absolutely necessary for GPIIb/IIIa activation.
Recent work has measured Src activity that occurs downstream of ADP-mediated stimulation.50 This work showed that ADP-mediated Src activation is activated primarily by the P2Y1 receptor but did not consider that ADP-mediated Src activation is still increased above baseline when ADP is signaling solely through the P2Y12 receptor.50 Thus, it appears that both the P2Y1 and P2Y12 receptors are able to cause Src activation through Gq and Gi signaling, respectively, albeit to different levels. Our data illustrate that Src is activated downstream of the P2Y12 receptor and the α2A adrenergic receptor. Thus, either Gi or Gz signaling is sufficient to activate Src, and this Src activity plays a role in the level of GPIIb/IIIa activation.
Multiple enzymes have been implicated in the regulation of PLA2, including protein kinase C,51 mitogen-activated protein (MAP) kinases,52 and calcium,53 though the candidates that phosphorylate and activate PLA2 remain the subject of controversy. Lyn-deficient mice have impaired thromboxane generation in response to γ-thrombin when compared to wild-type mice.42 Thus, Src family tyrosine kinases play a role in the generation of thromboxane A2 in platelets that are activated by Gi and Gz signaling pathways, although it is presently unclear how proximal this family of kinases is to PLA2 activation.
ADP requires signaling through the P2Y1 receptor, P2Y12 receptor, and outside-in signaling through GPIIb/IIIa to cause thromboxane A2 generation.46 Similarly, we have found that combined P2Y12 receptor and α2A adrenergic receptor stimulation causes thromboxane A2 generation with a requirement for outside-in signaling through GPIIb/IIIa. An alternative explanation is that postaggregatory events may be necessary for thromboxane generation and that fibrinogen is facilitating platelet-to-platelet contact for the postaggregatory response. We cannot presently differentiate between the 2 because of limited knowledge regarding postaggregatory events.
While G12/13, Gq, or Gz can interact with P2Y12-mediated Gi signaling to cause platelet aggregation, the signaling intermediates for these pathways differ (Figure 7E). PI-3 kinases and Src family kinases are pro-aggregatory signals for Gi plus Gz-mediated platelet aggregation, while calcium and PI-3 kinase are important for Gq plus Gi-mediated platelet aggregation. Although calcium chelation decreased the extent of platelet aggregation caused by G12/13 plus Gi,35 the pro-aggregatory signals downstream of the G12/13 pathway have not yet been identified. Based on the recent findings of Hardy et al,50 we speculate that Src family kinases are upstream of the PI-3 kinases. The diversity in the signaling of these somewhat nonredundant pathways may serve as a protection from deficiencies but also allows multiple pro-aggregatory signals to contribute to this highly complex, fine-tuned system.
Prepublished online as Blood First Edition Paper, November 16, 2004; DOI 10.1182/blood-2004-07-2821.
Supported by research grants HL60683 and HL64943 from the National Institutes of Health (S.P.K.) and by training grant T32 HL07777 (R.T.D.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal