Abstract
Overwhelming evidence from leukemia research has shown that the clonal population of neoplastic cells exhibits marked heterogeneity with respect to proliferation and differentiation. There are rare stem cells within the leukemic population that possess extensive proliferation and self-renewal capacity not found in the majority of the leukemic cells. These leukemic stem cells are necessary and sufficient to maintain the leukemia. Interestingly, the BCR/ABL fusion gene, which is present in chronic myelogenous leukemia (CML), was also detected in the endothelial cells of patients with CML, suggesting that CML might originate from hemangioblastic progenitor cells that can give rise to both blood cells and endothelial cells. Here we isolated fetal liver kinase-1–positive (Flk1+) cells carrying the BCR/ABL fusion gene from the bone marrow of 17 Philadelphia chromosome–positive (Ph+) patients with CML and found that these cells could differentiate into malignant blood cells and phenotypically defined endothelial cells at the single-cell level. These findings provide direct evidence for the first time that rearrangement of the BCR/ABL gene might happen at or even before the level of hemangioblastic progenitor cells, thus resulting in detection of the BCR/ABL fusion gene in both blood and endothelial cells.
Introduction
Stem cells are loosely defined as self-renewing progenitor cells that can generate one or more specialized cell types of a particular tissue through differentiation. Emerging evidence suggests that stem cell biology could provide new insights into cancer biology. Firstly, both tumors and normal tissues are composed of heterogeneous combinations of cells with different phenotypic characteristics and different proliferative potentials. Thus clonal tumorigenic cancer cells will give rise to phenotypically diverse progeny, including cancer cells with indefinite proliferative potential, as well as cancer cells with limited or no proliferative potential.1,2 This suggests that tumorigenic cancer cells undergo processes that are analogous to the self-renewal and differentiation of normal stem cells.3 In malignancies such as leukemia,4 multiple myeloma,5 and most recently breast cancer,6 rare cells with a remarkable potential for self-renewal were isolated and found to be responsible for tumorigenesis. Thus cancer can be contextualized as a disease of unregulated self-renewal of stem cells.3 Secondly, by self-renewing, stem cells often persist for long periods of time, instead of dying after short periods of time like many mature cells in highly proliferative tissues. This means that there is a much greater opportunity for mutations to accumulate in stem cells than in mature cells.7 Stem cells may be the only cells that live long enough to accumulate the several mutations needed to produce cancers.
Leukemia provides the best evidence that normal stem cells are the targets of transforming mutations, as certain types of leukemia are shown to arise from mutations in hematopoietic stem cells (HSCs). The cells capable of initiating human acute myeloid leukemia (AML) in NOD/SCID (nonobese diabetic/severe combined immunodeficiency) mice have a CD34+CD38– phenotype (a population enriched for HSCs) in most AML subtypes and thus have a phenotype similar to normal HSCs.4 Conversely, CD34+CD38+ leukemia cells cannot transfer disease to mice in the vast majority of cases, despite the fact that they exhibit a leukemic blast phenotype. This suggests that HSCs rather than committed progenitors are the target for leukemic transformation. In chronic myelogenous leukemia (CML),8 leukemia-associated chromosomal rearrangements have also been found in CD34+CD38– cells. Further, work by Gunsilius et al9 suggested that Philadelphia chromosome (Ph) gene rearrangement might happen in stem cells more primitive than CD34+CD38– cells (ie, it may occur at the level of putative hemangioblasts).9 They identified the BCR/ABL fusion gene in various proportions of endothelial cells generated in vitro from the bone marrow (BM) cells of patients with CML. Also, endothelial cells expressing the fusion gene were found in the vascular endothelium of a patient. Based on these findings, they hypothesized that CML might originate from bone marrow–derived hemangioblastic precursor cells. But they did not provide evidence that a single cell from the CML patient could give rise to Ph+ blood cells and Ph+ endothelial cells.
Recently, we identified a rare cell population (adherent fetal liver kinase-1–positive [Flk1+]CD31–CD34– cells) within human fetal bone marrow mesenchymal stem cell culture that could differentiate into endothelial and hematopoietic cells in vitro and in vivo at the single-cell level.10,11 The results of Gunsilius et al9 prompted us to investigate whether these cells may be the target of fusion gene rearrangement during the development of CML. Our results showed that the Flk1+CD31–CD34– cell population carrying the BCR/ABL fusion gene did exist in the bone marrows of all 17 patients with CML as expected. In vitro study showed they could give rise to both malignant blood cells and phenotypically defined endothelial cells at the single-cell level; when transplanted into SCID mice, these cells could reproductively transfer disease to mice. These cells also have extensive self-renewal capacity, as shown by the ability to reinitiate the disease in secondary SCID recipients. Thus our results provided direct evidence that cells capable of initiating human CML have a differentiation potential similar to hemangioblasts. This suggests that hemangioblasts rather than hematopoietic stem/progenitor cells are the target for leukemic transformation in CML.
Patients, materials, and methods
Patient characteristics and specimens
Seventeen patients with newly diagnosed CML (all male, aged 17-63 years) were recruited in this study. All were Ph+ patients with CML in chronic phase as revealed by bone marrow histology and cytogenetic analysis. None were treated with hydroxyurea or interferon before. Fifteen patients had exclusively 100% Ph+ metaphases in BM at diagnosis, with 1 patient having 86% Ph+ metaphases and the other having 78%. The white blood cell counts at presentation ranged between 29 × 109/L and 268 × 109/L (Table 1). The immunophenotype of thawed cells was quite variable. CD34 levels ranged between 5% and 15%. Myeloid cells, as assessed by CD33 expression, ranged between 19% and 57%, and T lymphocytes, as assessed by CD3, ranged between 7% and 37%. The levels of B cells were low, less than 10%, in all cases. Analysis of thawed material by fluorescence in situ hybridization (FISH) showed 39% to 87% BCR/ABL+ cells in 6 samples. BM samples were collected after obtaining informed consent according to procedures approved by the Ethics Committee at the Institute of Hematology and Blood Diseases Hospital, Chinese Academy of Medical Sciences.
Presenting features of patients at diagnosis
. | . | . | . | Immunophenotype . | . | . | . | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Patient . | WCC, × 109/L . | Ph+ metaphases, % . | FISH*BCR/ABL, % . | CD34 . | CD33 . | CD3 . | CD19 . | |||
| 1 | 135 | 100 | 49 | 8 | 56 | 11 | 3 | |||
| 2 | 86 | 100 | ND | 11 | 31 | 17 | 5 | |||
| 3 | 219 | 100 | 39 | 5 | 46 | 26 | 2 | |||
| 4 | 29 | 100 | ND | 7 | 19 | 37 | 4 | |||
| 5 | 157 | 78 | 55 | 15 | 29 | 15 | 6 | |||
| 6 | 163 | 100 | ND | 9 | 57 | 13 | 4 | |||
| 7 | 72 | 100 | 87 | 6 | 39 | 16 | 3 | |||
| 8 | 117 | 100 | ND | 13 | 41 | 9 | 4 | |||
| 9 | 243 | 100 | ND | 9 | 38 | 24 | 5 | |||
| 10 | 213 | 100 | ND | 10 | 32 | 16 | 3 | |||
| 11 | 268 | 100 | ND | 13 | 43 | 7 | 5 | |||
| 12 | 135 | 100 | ND | 8 | 34 | 14 | 4 | |||
| 13 | 97 | 100 | 69 | 9 | 24 | 13 | 2 | |||
| 14 | 103 | 86 | ND | 12 | 46 | 19 | 3 | |||
| 15 | 167 | 100 | ND | 8 | 37 | 21 | 5 | |||
| 16 | 140 | 100 | 74 | 6 | 43 | 17 | 5 | |||
| 17 | 121 | 100 | ND | 7 | 49 | 13 | 3 | |||
. | . | . | . | Immunophenotype . | . | . | . | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Patient . | WCC, × 109/L . | Ph+ metaphases, % . | FISH*BCR/ABL, % . | CD34 . | CD33 . | CD3 . | CD19 . | |||
| 1 | 135 | 100 | 49 | 8 | 56 | 11 | 3 | |||
| 2 | 86 | 100 | ND | 11 | 31 | 17 | 5 | |||
| 3 | 219 | 100 | 39 | 5 | 46 | 26 | 2 | |||
| 4 | 29 | 100 | ND | 7 | 19 | 37 | 4 | |||
| 5 | 157 | 78 | 55 | 15 | 29 | 15 | 6 | |||
| 6 | 163 | 100 | ND | 9 | 57 | 13 | 4 | |||
| 7 | 72 | 100 | 87 | 6 | 39 | 16 | 3 | |||
| 8 | 117 | 100 | ND | 13 | 41 | 9 | 4 | |||
| 9 | 243 | 100 | ND | 9 | 38 | 24 | 5 | |||
| 10 | 213 | 100 | ND | 10 | 32 | 16 | 3 | |||
| 11 | 268 | 100 | ND | 13 | 43 | 7 | 5 | |||
| 12 | 135 | 100 | ND | 8 | 34 | 14 | 4 | |||
| 13 | 97 | 100 | 69 | 9 | 24 | 13 | 2 | |||
| 14 | 103 | 86 | ND | 12 | 46 | 19 | 3 | |||
| 15 | 167 | 100 | ND | 8 | 37 | 21 | 5 | |||
| 16 | 140 | 100 | 74 | 6 | 43 | 17 | 5 | |||
| 17 | 121 | 100 | ND | 7 | 49 | 13 | 3 | |||
WCC indicates white blood cell count; ND, not determined.
At diagnosis.
Cell culture
Isolation and culture of BM-derived Flk1+CD31–CD34– cells from patients with CML were performed as described previously with some modifications.10,12 Briefly, mononuclear cells were separated by a Ficoll-Paque gradient centrifugation (specific gravity 1.077 g/mL; Nycomed Pharma AS, Oslo, Norway) and depleted of hematopoietic cells using magnetic-activated cell separation (MACS) CD45, GlyA, and CD34 micromagnetic beads (Miltenyi Biotec, Auburn, CA). To ensure single-cell originality of each cell colony, sorted cells were plated at concentrations of 1 cell/well by limiting dilution in a total of 96 × 10 wells coated with fibronectin (Sigma, St Louis, MO) and collagen (Sigma) for each patient. Culture medium was Dulbecco modified Eagle medium and Ham F12 medium (DF12) containing 40% MCDB-201 medium complete with trace elements (MCDB) (Sigma), 2% fetal calf serum (FCS; Gibco Life Technologies, Paisley, United Kingdom), 1 × insulin transferrin selenium (Gibco Life Technologies), 10–9 M dexamethasone (Sigma), 10–4 M ascorbic acid 2-phosphate (Sigma), 20 ng/mL interleukin-6 (Sigma), 10 ng/mL epidermal growth factor (Sigma), 10 ng/mL platelet-derived growth factor BB (Sigma), 50 ng/mL fetal liver tyrosine kinase 3 (Flt-3) ligand (Sigma), 30 ng/mL bone morphogenetic protein-4 (Sigma), and 100 U/mL penicillin and 100 μg/mL streptomycin (Gibco Life Technologies) at 37°C anda5%CO2 humidified atmosphere. Culture media were changed every 4 to 6 days. Wells with a single adherent cell were identified during the first 24 hours. The appearance of cell colonies was checked daily. Single colonies were harvested by trypsinization and culture expanded.
Cell growth pattern and fluorescence-activated cell sorter (FACS) analysis
To study the growth pattern of the expanded clonal cells, cells were seeded at a concentration of 5000 cells/well in a 24-well plate. The adherent cells were trypsinized, harvested, and counted by 0.4% trypan blue exclusion method (dead cells would be stained by trypan blue and thus excluded) at different time points.
For immunophenotype analysis, expanded clonal cells were stained with antibodies against Flk1, von Willebrand factor (VWF), GlyA (all from Santa Cruz Biotechnology, Santa Cruz, CA); CD13, CD29, CD31, CD34, CD36, CD44, CD45, HLA class I, HLA-DR, c-Kit (all from Becton Dickinson Immunocytometry Systems, Mountain View, CA); CD62E, CD62L, CD62P (all from Chemicon International USA, Temecula, CA). For intracellular antigen detection, cells were first fixed in 2% paraformaldehyde (Sigma) for 15 minutes at 4°C and permeabilized with 0.1% saponin (Sigma) for 1 hour at room temperature. Cells were washed and labeled with fluorescein isothiocyanate (FITC)–conjugated secondary goat antimouse, goat antirabbit, or sheep antigoat antibodies (Sigma), then washed and analyzed using a FACSCalibur flow cytometer (Becton Dickinson, San Jose, CA).
In vitro differentiation of cultured cells
The expanded clonal cells were harvested onto clean glass coverslips that were precoated with a thin layer of matrigel (Becton Dickinson) and cultured in 35-mm Petri dishes in endothelial (End) differentiation medium containing 10% FCS (Gibco Life Technologies), 10% horse serum (Gibco Life Technologies), 5 ng/mL vascular endothelial growth factor (VEGF; Sigma), 10 ng/mL insulin-like growth factor-1 (Sigma), 2 U/mL erythropoietin (Epo; Sigma), 10 ng/mL basic fibroblast growth factor (bFGF; Gibco Life Technologies), 50 ng/mL interleukin-11 (Sigma), 100 mg/mL endothelial cell growth supplement (Collaborative Research, Waltham, United Kingdom), 2 mM l-glutamine (Gibco Life Technologies), and 4.5 × 10–4 M monothioglycerol (Sigma). For fluorescence analysis, adherent cells were first cultured in the presence of 10 μg/mL DiI-acetylated low density lipoprotein (DiI-Ac-LDL) (Biomedical Technologies, Stoughton, MA) for 2 hours. Then the cells were washed 3 times and fixed for 10 minutes in phosphate-buffered saline (PBS) containing 3% paraformaldehyde and 3% sucrose. The fixed cells were washed 3 times, incubated initially with Biotin-α-mouse CD31 (Becton Dickinson) or Biotin-α-mouse VWF (Becton Dickinson) for 1 hour, washed 5 times, and then incubated with Streptavidin-FITC for 1 hour. The images were acquired using the Slidebook software program (Intelligent Imaging Innovations, Denver, CO). Images were visualized using an Olympus BX51 microscope equipped with objective lenses (see figure legends for specific numerical apertures and magnifications) and a CAMedia C70 digital camera (Olympus, Tokyo, Japan). Images were processed using Adobe Photoshop 7.0 software (Adobe, San Jose, CA). Cells were also cultured on matrigel (Sigma) supplemented with 50 ng/mL VEGF (Sigma), 10 ng/mL bFGF (Gibco), 3% FCS (Peprotech, Rocky Hill, NJ) for vascular formation as we previously described.10
For hematopoietic differentiation, cells were cultured in Methocult GF+ media (StemCell Technologies, Vancouver, BC, Canada) consisting of 1% methylcellulose, 30% FCS (Gibco Life Technologies), 1% albumin (Sigma), 50 ng/mL stem cell factor (SCF; Sigma), 20 ng/mL granulocyte macrophage–colony-stimulating factor (GM-CSF; Sigma), 20 ng/mL interleukin-3, 20 ng/mL interleukin-6, 20 ng/mL granulocyte–colony-stimulating factor (Sigma), and 3 U/mL Epo for colony-forming units (CFUs).
Reverse transcriptase–polymerase chain reaction (RT-PCR) analysis
RNA isolation and reverse transcription were performed as previously described.10,11 Oligonucleotide primer sequences were as follows: CD45 (418 bp), forward: 5′-CAG GCA TGG TTT CCA CAT TC-3′, reverse: 5′-CTA CAA ATA TTG GTT CGC TGC-3′; CD34 (647 bp), forward: 5′-GTC TTG ACAACAACG GTA CTG C-3′, reverse: 5′-CAA GAC CAG CAG TAG ACA CTG A-3′; stem cell leukemia (SCL; 331bp), forward: 5′-ATG GTG CAG CTG AGT CCT CC-3′, reverse: 5′-TCT CAT TTC TTG CTG AGC TTC-3′; glutamate acetyltransferase 1 (GATA-1; 522 bp), forward: 5′-CTC CCT GTC CCC AAT AGT GC-3′, reverse: 5′-GTC CTT CGG CTG CTC CTG TG-3′; GATA-2 (242 bp), forward: 5′-AGC CGG CAC CTG TTG TGC AA-3′, reverse: 5′-TGA CTT CTC CTG CAT GCA CT-3′. For CD45 and CD34, amplification was performed under the same cycling conditions (1 minute at 94°C, 50 seconds at 57°C, 1 minute at 72°C), except the number of cycles that were specified for each gene (31 for CD45, 32 for CD34); for SCL, GATA-1, and GATA-2, amplification was performed under the same cycling conditions (1 minute at 94°C, 50 seconds at 56°C, 1 minute at 72°C), except the number of cycles that were specified for each gene (31 for GATA-2, 32 for GATA-1, 35 for SCL).
The expression of the BCR/ABL fusion gene was assayed by nested RT-PCR.13 Ten microliters of cDNA was amplified for 30 cycles in a volume of 50 μL containing 10 × PCR buffer, 0.05 mM deoxynucleotide triphosphates, 0.4 μM of each primer (BCR-1A and ABL-2C), and 1 U Ampli Taq Polymerase. One microliter PCR product was reamplified for 30 cycles in a second PCR with 10 × PCR buffer, 0.1 mM deoxynucleotide triphosphates, 0.4 μM of each internal primer (BCR-1B and ABL-2D), and 1 U Ampli Taq polymerase.14 The first primer set was BCR1A (5′-AGT TAC ACG TTC CTG ATC TC-3′) and ABL2C (5′-TTA TCT CCA CTG GCC ACA AA-3′). The “nested” set of primers was BCR1B (5′-TCT GAC TAT GTG CGT GCA GA-3′) and ABL2D (5′-AGT GCA ACG AAA AGG TTG GG-3′). The expected product size is either 363 bp (b3a2 transcript) or 288 bp (b2a2 transcript). The PCR products were separated on a 2% agarose gel and stained with ethidium bromide.
Transplantation of cultured cells into NOD/SCID mice
Six- to 8-week-old female NOD/SCID mice were bred and maintained under defined flora conditions in individually ventilated (high-efficiency particle-arresting filtered air) sterile microisolator cages (Techniplast, Milan, Italy). All animal handle and experiment procedures were approved by the Animal Care and Use Committee of the Chinese Academy of Medical Sciences. Mice were sublethally irradiated (300 cGy) with a cesium source (MDS Nordion; Gammacell, Ottawa, QC, Canada) prior to transplantation. BM-derived Flk1+CD31–CD34– cells from patients with CML (1 × 105 cells per mouse) were injected via tail vain into the irradiated mice in 0.4-mL Roswell Park Memorial Institute medium (RPMI 1640; Gibco Life Technologies). The peripheral white blood cell count was done once a week. Mice were killed 2 months later by cervical dislocation because all developed very obvious weight loss and lethargy. The BM from both femora and tibiae was collected. Cell smears were prepared from BM. They were air dried and then stained with Jenner-Giemsa (BDH Ltd, Poole, United Kingdom). Conventional 4-μm histologic sections of spleen and decalcified tibia were cut from formalin-fixed, paraffin-embedded material and stained.
BM of mice that received a transplant was assessed for presence of human cells by FACS. Mononuclear cells were harvested as described in the previous paragraph and red blood cells were lysed by adding 8.3% ammonium chloride. Single-cell suspensions were prepared. After blocking of Fc receptors with human serum, cells were determined by dual-color labeling with anti-CD45 FITC and anti-CD34, -CD33, -CD3, and -CD19 phycoerythrin (PE; Becton Dickinson). Cells labeled with anti–immunoglobulin G (anti-IgG) monoclonal antibody (mAb) were used as control. We gated cells to include both lymphoid and myeloid fractions.15
FISH analysis
We also detected human cells from the bone marrow of mice that received a transplant when the patients were male (n = 6). Human Y chromosome was detected using a probe (CEP Y Spectrum Red; Vysis, Downers Grove, IL) according to the manufacturer's instructions. BM cells from these mice were also detected for the presence of the BCR/ABL gene. LSI bcr SpectrumGreen/abl SpectrumOrange dual-color DNA probe mixture (Vysis) was prepared according to the manufacturer's instructions. Dual-labeled cells were examined using a dual band–pass filter for both FITC and Texas red. Whenever possible, 300 cells were examined. Normal cells showed 2 red abl signals and 2 green bcr signals. Leukemic cells show a single red and a single green signal representing normal abl and bcr genes and the yellow signal representing fusion of abl and bcr genes.
Granulocyte-macrophage colony-forming unit (CFU-GM) assay of transplanted cells
Triplicate 1-mL cultures with 5 × 105 BM cells were established in 35-mm plates in 0.9% methylcellulose (Methocel; Dow Chemical, Midland, MI) in Iscoves Modified Dulbecco Medium (Gibco Life Technologies) supplemented with 30% FCS and 3 mM l-glutamine. Cultures were stimulated by 10 ng each of recombinant human interleukin-3 (Sigma), GM-CSF, and SCF, a combination that does not stimulate murine progenitors. After a 14-day incubation at 37°C in 5% CO2, CFU-GMs were scored as aggregated of more than 50 cells.16
Neoangiogenesis analysis in NOD/SCID mice after transplantation
We used a wound-healing model to investigate the potential of Flk1+CD31–CD34– cells to differentiate into endothelial cells in neoangiogenesis.11 Recipient mice were killed by cervical dislocation 2 months after transplantation; tissue samples from small intestine were embedded in paraffin wax and cut into 4-μm serial sections. After deparaffinizing with xylene, tissue sections were rehydrated. These sections were first incubated with 5% normal donkey serum in PBS (blocking solution), then incubated with rabbit antimouse VWF polyclonal antibodies (1:400; DAKO, Glostrup, Denmark), followed by cyanin 5 (Cy5)–conjugated F(ab)2 fragment of donkey antirabbit IgG (1:600; Jackson Immuno Research, West Grove, PA). After being washed with PBS, slides were blocked again with 5% normal sheep serum for 10 minutes and then localized with diluted sheep antihuman VWF antibody conjugated with FITC (Novus Biologicals, Littleton, CO) for 1 hour, rinsed with PBS for 5 minutes (2 times), blocked again with 5% normal rabbit serum for 10 minutes, and finally incubated with diluted monoclonal PE-conjugated antihuman CD45 IgG1 antibody (Becton Dickinson) for 30 minutes.
Statistical analysis
Results are expressed as the mean ± 1 standard error of the mean (SEM) percentages, unless otherwise stated. Analysis of engraftment used regression analysis and analysis of variance.
Results
The biologic characteristics of the culture-expanded clonal cells
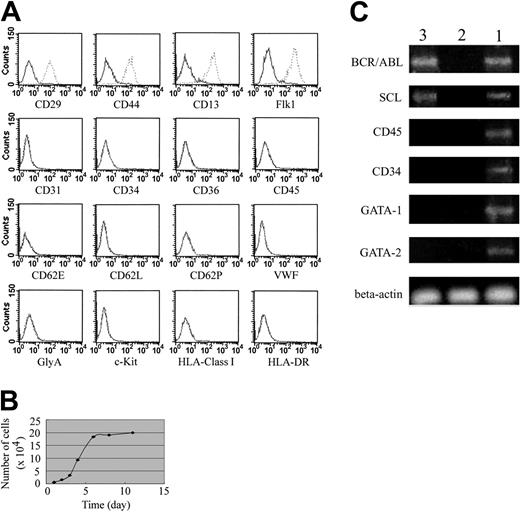
After sorted mononuclear cells were cultured at 1 cell per well for 2 weeks, 112 clones were observed in a total of 96 × 10 × 17 wells. At least 2 clones were harvested for each patient's BM. These undifferentiated expanded clonal cells were persistently negative for CD31, CD34, CD36, CD45, CD62E, CD62L, CD62P, VWF, GlyA, c-Kit, HLA class I, and HLA-DR but expressed high levels of Flk1 (more than 99%), CD29, CD44, and CD13 (more than 95%) even after they were expanded for more than 40 passages (Figure 1A; data not shown). Furthermore, these cells persistently displayed fibroblast-like morphology and proliferated with a population doubling time of about 32 hours during the log phase of growth (Figure 1B). In addition, these expanded clonal cells did not express GATA-1 or GATA-2 genes but expressed the SCL gene and the BCR/ABL fusion gene (Figure 1C). Expression of the BCR/ABL fusion gene was confirmed by FISH analysis (data not shown).
Cell growth pattern and FACS analysis. (A) Immunophenotype of the expanded clonal cells. The solid line outlines the region of fluorescent intensity for cells labeled with control antibodies. The dotted lines indicate the region of fluorescent intensity for cells labeled with specific antibodies. (B) The expanded clonal cells proliferated with a population doubling time of about 32 hours during the log phase of growth. (C) These expanded clonal cells did not express GATA-1 or GATA-2 genes but expressed SCL gene and BCR/ABL fusion gene. Lanes 1, 2, and 3 represent positive control, negative control, and experimental samples, respectively.
Cell growth pattern and FACS analysis. (A) Immunophenotype of the expanded clonal cells. The solid line outlines the region of fluorescent intensity for cells labeled with control antibodies. The dotted lines indicate the region of fluorescent intensity for cells labeled with specific antibodies. (B) The expanded clonal cells proliferated with a population doubling time of about 32 hours during the log phase of growth. (C) These expanded clonal cells did not express GATA-1 or GATA-2 genes but expressed SCL gene and BCR/ABL fusion gene. Lanes 1, 2, and 3 represent positive control, negative control, and experimental samples, respectively.
Sibling cells display endothelial and hematopoietic characteristics
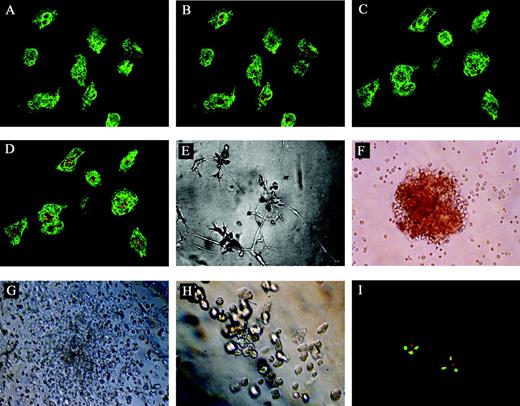
The clonal Flk1+CD31–CD34– cells were cultured in endothelial differentiation medium for 10 days then analyzed by immunofluorescence for the presence of CD31 and VWF and for their ability to take up fluorescenated DiI-Ac-LDL. Figure 2 showed the morphology of the cells in the endothelial differentiation culture and their immunofluorescence staining patterns. The induced cells expressed high levels of CD31 (green) and VWF (green) and took up DiI-Ac-LDL, showing a punctate pattern of fluorescence (orange) characteristic of endothelial cells (Figure 2A-D). However, the expanded clonal cells did not express any CD31 or VWF and were unable to take up DiI-Ac-LDL before they were cultured in End medium (data not shown). These cells were also BCR/ABL+ in FISH analysis (Figure 2I). Besides, as shown in Figure 2E, culture of the induced sibling cells from single-cell–derived clones on matrigel resulted in vascular tube formation on day 10. Taken together, these findings suggest that single-cell–derived clones were progenitors of endothelial cells.
Morphology of the cells in the End or Methocult GF+ media and their immunofluorescence staining patterns. (A) The induced cells expressed high levels of CD31 (green; original magnification × 400). (B) The cells expressing high levels of CD31 could take up DiI-Ac-LDL, showing a punctate pattern of fluorescence (orange) characteristic of endothelial cells (original magnification × 400). (C) The induced cells expressed high levels of VWF (green; original magnification × 400). (D) The cells expressing high levels of VWF could take up DiI-Ac-LDL, showing a punctate pattern of fluorescence (orange) characteristic of endothelial cells (original magnification × 400). (E) The induced sibling cells from single-cell–derived clones on matrigel extended gradually to form a capillary-like structure on day 10 (original magnification × 100). The induced sibling cells formed BFU-Es (F, original magnification × 100), CFU-GMs (G, original magnification × 100), and CFU-Mk's (H, original magnification × 100). (I) FISH analysis of the induced cells, single red and green signal representing normal abl and bcr genes respectively, and the yellow signal representing fusion of abl and bcr genes (original magnification, × 1000). For panels A-D, a 40×/0.65 objective lens was used; for panels E-H, a 10×/0.25 objective lens was used; and for panel I, a 100×/1.25 objective lens was used.
Morphology of the cells in the End or Methocult GF+ media and their immunofluorescence staining patterns. (A) The induced cells expressed high levels of CD31 (green; original magnification × 400). (B) The cells expressing high levels of CD31 could take up DiI-Ac-LDL, showing a punctate pattern of fluorescence (orange) characteristic of endothelial cells (original magnification × 400). (C) The induced cells expressed high levels of VWF (green; original magnification × 400). (D) The cells expressing high levels of VWF could take up DiI-Ac-LDL, showing a punctate pattern of fluorescence (orange) characteristic of endothelial cells (original magnification × 400). (E) The induced sibling cells from single-cell–derived clones on matrigel extended gradually to form a capillary-like structure on day 10 (original magnification × 100). The induced sibling cells formed BFU-Es (F, original magnification × 100), CFU-GMs (G, original magnification × 100), and CFU-Mk's (H, original magnification × 100). (I) FISH analysis of the induced cells, single red and green signal representing normal abl and bcr genes respectively, and the yellow signal representing fusion of abl and bcr genes (original magnification, × 1000). For panels A-D, a 40×/0.65 objective lens was used; for panels E-H, a 10×/0.25 objective lens was used; and for panel I, a 100×/1.25 objective lens was used.
To evaluate the hematopoietic differentiation potential of the single-cell–derived clones, some of the single-cell–derived cells were cultured in Methocult GF+ media for 14 days using CFU assay.17-20 These cells were able to give rise to multiple hematopoietic progenitors of erythroid (erythroid burst-forming units [BFU-Es]; Figure 2F), granulocyte and macrophage (CFU-GMs; Figure 2G), and megakaryocyte (CFU-Mk's; Figure 2H). Furthermore, these hematopoietic progenitors were also BCR/ABL+ in FISH analysis (data not shown).
Flk1+CD31–CD34– cells from patients with CML give rise to hematopoietic cells in NOD/SCID mice
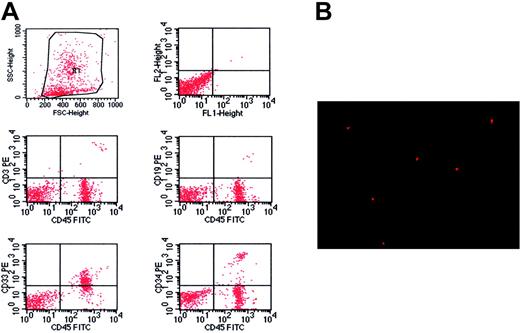
Transplantations of culture-expanded clonal cells from 10 patients with CML resulted in high levels of human hematopoietic cells in 86% (48/56) of NOD/SCID mice after 2 months. The peripheral white blood cell count of the recipient mice was drastically elevated, ranging between 20 × 109/L and 60 × 109/L, which was in sharp contrast to about 3 × 109/L in normal NOD/SCID mice. Flow cytometry analysis showed that the engrafted cells were predominantly myeloid in the BM of recipient mice. There were up to 74.1% of human CD33+ cells in the BM of the recipient mice (range, 27.9% to 74.1%), but CD19+ cells were rare (Figure 3A; Table 2). Besides, primitive human cells, defined by expression of CD34, were detected in the BM of the recipient mice, ranging from 1.6% to 5.3%. Furthermore, there were human CD33+, CD3+, and CD45+ cells in the spleens of recipient mice, but the predominant ones were CD3+ human cells (Table 2). These results were further confirmed by analysis of human Y chromosome (Figure 3B).
Flk1+CD31–CD34– cells from patients with CML give rise to hematopoietic cells in NOD/SCID mice. (A) Immunophenotype of BM specimen at day 62 showing predominant engraftment of myeloid cells with lower level of CD3 and CD19 cells. Besides, the engraftment of primitive human cells was confirmed in the BM of recipient mice by detection of CD34. (B) Human Y chromosome was detected by FISH (red signal) in the cells of bone marrow from mice that received a transplant (original magnification × 400). A 40×/0.65 objective lens was used.
Flk1+CD31–CD34– cells from patients with CML give rise to hematopoietic cells in NOD/SCID mice. (A) Immunophenotype of BM specimen at day 62 showing predominant engraftment of myeloid cells with lower level of CD3 and CD19 cells. Besides, the engraftment of primitive human cells was confirmed in the BM of recipient mice by detection of CD34. (B) Human Y chromosome was detected by FISH (red signal) in the cells of bone marrow from mice that received a transplant (original magnification × 400). A 40×/0.65 objective lens was used.
Phenotype of engrafted cells in mouse BM and spleen
. | Phenotype of engrafted cells in BM* . | . | . | . | Phenotype of engrafted cells in spleen* . | . | . | . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient . | CD34 . | CD33 . | CD3 . | CD19 . | CD34 . | CD33 . | CD3 . | CD19 . | ||||||
| 1 | 2.5 ± 0.3 | 37.5 ± 6.2 | 7.3 ± 3.4 | ND | 0 | 1.7 ± 0.9 | 56.7 ± 6.9 | ND | ||||||
| 2 | 2.7 ± 0.4 | 29.7 ± 3.4 | 5.4 ± 0.5 | 0.7 ± 0.3 | 0 | 1.2 ± 0.6 | 74.5 ± 8.7 | 1.4 ± 0.7 | ||||||
| 3 | 3.5 ± 0.5 | 30.1 ± 5.1 | ND | 1.3 ± 0.6 | 0.5 ± 0.4 | 3 | 48.9 ± 5.3 | ND | ||||||
| 5 | 3.1 ± 0.6 | 52.4 ± 6.5 | 0.9 ± 0.3 | 0.6 ± 0.4 | ND | ND | 47.4 ± 6.2 | 0.7 ± 0.3 | ||||||
| 6 | 5.3 ± 1.5 | 46.3 ± 3.8 | 6.1 ± 0.7 | 2.5 ± 0.3 | ND | 2.1 ± 1.2 | 57.2 ± 9.6 | 1.1 ± 0.7 | ||||||
| 7 | 4.7 ± 1.2 | 74.1 ± 4.9 | ND | 3.1 ± 0.3 | 0 | 0 | 49.1 ± 3.8 | 2.4 ± 1.1 | ||||||
| 8 | 3.3 ± 0.9 | 42.9 ± 7.4 | 4.2 ± 1.6 | 1.4 ± 0.6 | ND | 1.3 ± 0.7 | ND | ND | ||||||
| 10 | 2.3 ± 1.4 | 35.6 ± 3.8 | 3.6 ± 1.2 | 2.1 ± 1.1 | ND | 6.7 ± 1.9 | ND | ND | ||||||
| 11 | 1.6 ± 0.7 | 27.9 ± 2.9 | ND | 1.7 ± 0.8 | ND | ND | 28.6 ± 5.7 | 0.8 ± 0.4 | ||||||
| 13 | 2.6 ± 1.1 | 57.6 ± 7.1 | 1.7 ± 0.4 | ND | 0 | ND | 48.1 ± 4.8 | ND | ||||||
. | Phenotype of engrafted cells in BM* . | . | . | . | Phenotype of engrafted cells in spleen* . | . | . | . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient . | CD34 . | CD33 . | CD3 . | CD19 . | CD34 . | CD33 . | CD3 . | CD19 . | ||||||
| 1 | 2.5 ± 0.3 | 37.5 ± 6.2 | 7.3 ± 3.4 | ND | 0 | 1.7 ± 0.9 | 56.7 ± 6.9 | ND | ||||||
| 2 | 2.7 ± 0.4 | 29.7 ± 3.4 | 5.4 ± 0.5 | 0.7 ± 0.3 | 0 | 1.2 ± 0.6 | 74.5 ± 8.7 | 1.4 ± 0.7 | ||||||
| 3 | 3.5 ± 0.5 | 30.1 ± 5.1 | ND | 1.3 ± 0.6 | 0.5 ± 0.4 | 3 | 48.9 ± 5.3 | ND | ||||||
| 5 | 3.1 ± 0.6 | 52.4 ± 6.5 | 0.9 ± 0.3 | 0.6 ± 0.4 | ND | ND | 47.4 ± 6.2 | 0.7 ± 0.3 | ||||||
| 6 | 5.3 ± 1.5 | 46.3 ± 3.8 | 6.1 ± 0.7 | 2.5 ± 0.3 | ND | 2.1 ± 1.2 | 57.2 ± 9.6 | 1.1 ± 0.7 | ||||||
| 7 | 4.7 ± 1.2 | 74.1 ± 4.9 | ND | 3.1 ± 0.3 | 0 | 0 | 49.1 ± 3.8 | 2.4 ± 1.1 | ||||||
| 8 | 3.3 ± 0.9 | 42.9 ± 7.4 | 4.2 ± 1.6 | 1.4 ± 0.6 | ND | 1.3 ± 0.7 | ND | ND | ||||||
| 10 | 2.3 ± 1.4 | 35.6 ± 3.8 | 3.6 ± 1.2 | 2.1 ± 1.1 | ND | 6.7 ± 1.9 | ND | ND | ||||||
| 11 | 1.6 ± 0.7 | 27.9 ± 2.9 | ND | 1.7 ± 0.8 | ND | ND | 28.6 ± 5.7 | 0.8 ± 0.4 | ||||||
| 13 | 2.6 ± 1.1 | 57.6 ± 7.1 | 1.7 ± 0.4 | ND | 0 | ND | 48.1 ± 4.8 | ND | ||||||
ND indicates not determined.
Results are percentage of CD45+ cells expressing the denoted antigen (mean ± SEM).
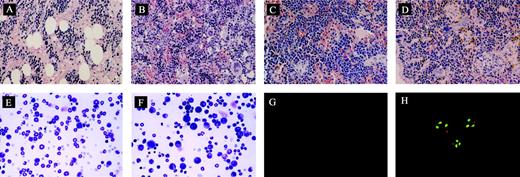
The ability of engrafted cells to produce CFU-GMs was assessed in 30 mice. CFU-GMs were detected in 27 of 30 cases, with a level of 31 ± 5 (range, 4 to 81) colonies per 105 BM cells plated. The total numbers of human CFU-GMs present in 2 femora and 2 tibiae ranged from 130 to 1826 (mean, 723). Morphologic assessment showed hypercellularity of BM with increased numbers of myeloid cells in highly engrafted animals, but no fibrosis was observed (Figure 4A-B). But histologic examination of the spleen showed that fibrosis was evident as well as infiltration by megakaryocytes, similar to the pathologic characteristics seen in patients with CML (Figure 4C-D). In addition, examination of the bone marrow smears showed that the majority of white blood cells were granulocytes but with some myeloblasts (Figure 4E-F). Furthermore, cells in the peripheral blood were composed of 86% ± 1.9% granulocytes, 7.6% ± 5.5% lymphocytes, and 5.4% ± 4.3% myeloblasts. BM cells from these mice were also examined for the presence of the BCR/ABL gene by FISH. Leukemic cells show a single red and a single green signal representing normal abl and bcr genes and the yellow signal representing fusion of abl and bcr genes (Figure 4G-H). The mean level of BCR/ABL+ cells detected was about 81% (Table 3) for the human cells. We also analyzed spleen specimens that had successful human BM cell engraftment. A similar level of BCR/ABL+ cells was detected compared with BM (data not shown).
Morphologic analysis of murine BM and spleen engrafted with CML cells. (A) Histologic section of tibia of NOD/SCID mouse irradiated but not infused with human cells at day 62 (original magnification × 400). (B) Histologic section of tibia of mouse with human cells detectable at day 62. Hypercellular marrow with proliferation of megakaryocytes is shown (original magnification × 400). (C) Histologic section of spleen from control mouse showing monomorphic lymphoid population (original magnification × 400). (D) Histologic section of spleen of mouse with human cells detectable at day 62. Infiltration with megakaryocytes is shown (original magnification, ×400). (E) Cytospin preparation of bone marrow from control mouse (original magnification × 400). (F) Cytospin preparation of bone marrow of mouse with human cells detected at day 62 showing prominent myeloid cells (original magnification × 400). (G) Cells detected by FISH with dual probes for bcr and abl showing no signal in cells from the bone marrow of control mouse (original magnification × 1000). (H) Differential engraftment of normal and leukemic CML cells in NOD/SCID mice detected by dual probes for bcr and abl genes. Normal cells show 2 red abl signals and 2 green bcr signals. Leukemic cells show a single red and green signal representing normal abl and bcr genes and the yellow signal representing fusion of abl and bcr genes (original magnification × 1000). The magnifications/numerical apertures of the objective lenses used were as follows: for panels A-F, 40×/0.65; for panels G-H, 100×/1.25.
Morphologic analysis of murine BM and spleen engrafted with CML cells. (A) Histologic section of tibia of NOD/SCID mouse irradiated but not infused with human cells at day 62 (original magnification × 400). (B) Histologic section of tibia of mouse with human cells detectable at day 62. Hypercellular marrow with proliferation of megakaryocytes is shown (original magnification × 400). (C) Histologic section of spleen from control mouse showing monomorphic lymphoid population (original magnification × 400). (D) Histologic section of spleen of mouse with human cells detectable at day 62. Infiltration with megakaryocytes is shown (original magnification, ×400). (E) Cytospin preparation of bone marrow from control mouse (original magnification × 400). (F) Cytospin preparation of bone marrow of mouse with human cells detected at day 62 showing prominent myeloid cells (original magnification × 400). (G) Cells detected by FISH with dual probes for bcr and abl showing no signal in cells from the bone marrow of control mouse (original magnification × 1000). (H) Differential engraftment of normal and leukemic CML cells in NOD/SCID mice detected by dual probes for bcr and abl genes. Normal cells show 2 red abl signals and 2 green bcr signals. Leukemic cells show a single red and green signal representing normal abl and bcr genes and the yellow signal representing fusion of abl and bcr genes (original magnification × 1000). The magnifications/numerical apertures of the objective lenses used were as follows: for panels A-F, 40×/0.65; for panels G-H, 100×/1.25.
Characterization of leukemic engraftment
Patient . | Engrafted CD33+ cells in BM, % . | BCR/ABL+ cells by FISH, %* . | Human CD45- VWF+ ECs . | BCR/ABL+ ECs . |
|---|---|---|---|---|
| 3 | 25 | 71 | +† | + |
| 5 | 53 | 85 | + | + |
| 7 | 79 | 94 | + | + |
| 7 | 59 | 79 | + | + |
| 13 | 53 | 77 | + | -‡ |
| 13 | 61 | 82 | + | + |
Patient . | Engrafted CD33+ cells in BM, % . | BCR/ABL+ cells by FISH, %* . | Human CD45- VWF+ ECs . | BCR/ABL+ ECs . |
|---|---|---|---|---|
| 3 | 25 | 71 | +† | + |
| 5 | 53 | 85 | + | + |
| 7 | 79 | 94 | + | + |
| 7 | 59 | 79 | + | + |
| 13 | 53 | 77 | + | -‡ |
| 13 | 61 | 82 | + | + |
ECs indicates endothelial cells.
Percentage of human cells that are BCR/ABL+.
Human cells in vascular wall that are CD45- VWF+ or BCR/ABL+.
Human CD45- VWF+ cells in vascular wall that are BCR/ABL-.
Flk1+CD31–CD34– cells from patients with CML give rise to endothelial cells in vivo
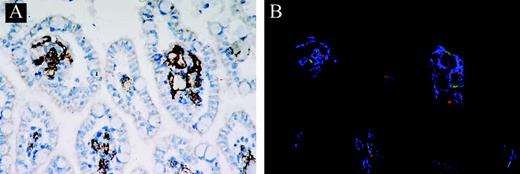
Lovesick and colleagues' (Paris et al21 ) studies provided evidence that extensive microvascular injury was the critical lesion in the gastrointestinal tract syndrome after whole body radiation. To determine whether expanded clonal cells from patients with CML' BM could contribute to neoangiogenesis in injured gastrointestinal tract of the mice killed 2 months after cell transplantation, triple-color immunofluorescence was used to discriminate between endothelial and hematopoietic cells in the same tissue sections. As expected, in the majority (5/6) of the mice in 6 mice with an engraftment level of 25% to 79% of CD33+ human cells, engraftment of hematopoietic cells was accompanied by engraftment of cells expressing endothelial cell markers, as detected by staining with antibodies to human VWF and CD45 (Figure 5; Table 3), whereas in control mice that did not receive transplants, we could not detect donor-derived endothelial cells in small intestinal sections (data not shown). Most importantly, the BCR/ABL fusion gene could be detected in these donor-derived endothelial cells that incorporated into foci of neoangiogenesis (Table 3).
Immunofluorescence staining of human Flk1+CD31–CD34––derived endothelial cells. (A) Deparaffinized section of small intestine was stained with antimouse VWF antibody (original magnification × 200). (B) Small intestine was stained with antimouse VWF antibody, antihuman VWF antibody, or antihuman CD45 antibody. Confocal microscopic images of antimouse VWF (blue), antihuman VWF (green), and antihuman CD45 (red) are shown (original magnification × 200). A 20×/0.40 objective lens was used.
Immunofluorescence staining of human Flk1+CD31–CD34––derived endothelial cells. (A) Deparaffinized section of small intestine was stained with antimouse VWF antibody (original magnification × 200). (B) Small intestine was stained with antimouse VWF antibody, antihuman VWF antibody, or antihuman CD45 antibody. Confocal microscopic images of antimouse VWF (blue), antihuman VWF (green), and antihuman CD45 (red) are shown (original magnification × 200). A 20×/0.40 objective lens was used.
Secondary transplants
One of the major features of stem cells is the ability to self-renew.22-24 This can be assayed by secondary transplants.24 We therefore tested the ability of human CML cells in primary NOD/SCID mice to reinitiate the disease in secondary recipients. BM cells (6 × 107), recovered from 3 primary recipients that had been confirmed to have CML-like disease 2 months after transplantation, were infused into secondary irradiated NOD/SCID recipients (2 × 107 cells per mouse). After 12 weeks, secondary recipients became ill and cachectic due to high levels of CML cells in the BM and spleen (Table 4), and donor-derived endothelial cells incorporating into foci of neoangiogenesis were also detected in the gastrointestinal tract (Table 4). Furthermore, BM cells recovered from 3 secondary recipients still gave rise to colonies in semisolid media, indicating that there were primitive human stem cells in SCID mice that received primary and secondary transplants (data not shown).
Engraftment levels in NOD/SCID mice receiving secondary transplantation
. | . | Phenotype of engrafted cells . | . | . | . | . | . | . | . | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | . | BM* . | . | Spleen* . | . | . | . | . | . | |||
| Patient . | Animal . | CD34 . | CD33 . | CD33 . | CD3 . | CD45- VWF+ cells . | . | BCR/ABL+ cells FISH . | . | |||
| 5 | 2 | 2.5 | 55 | ND | 49 | +† | + | + | + | |||
| 7 | 3 | 4.9 | 71 | 0 | 53 | + | + | + | + | |||
| 13 | 5 | 1.6 | 59 | ND | 57 | + | + | + | + | |||
| SR-1 | 1.8 | 47 | 2 | 47 | + | + | + | + | ||||
| SR-2 | 3.1 | 81 | 3 | 51 | + | + | + | + | ||||
| SR-3 | 2.4 | 62 | 2 | 62 | + | + | + | + | ||||
. | . | Phenotype of engrafted cells . | . | . | . | . | . | . | . | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | . | BM* . | . | Spleen* . | . | . | . | . | . | |||
| Patient . | Animal . | CD34 . | CD33 . | CD33 . | CD3 . | CD45- VWF+ cells . | . | BCR/ABL+ cells FISH . | . | |||
| 5 | 2 | 2.5 | 55 | ND | 49 | +† | + | + | + | |||
| 7 | 3 | 4.9 | 71 | 0 | 53 | + | + | + | + | |||
| 13 | 5 | 1.6 | 59 | ND | 57 | + | + | + | + | |||
| SR-1 | 1.8 | 47 | 2 | 47 | + | + | + | + | ||||
| SR-2 | 3.1 | 81 | 3 | 51 | + | + | + | + | ||||
| SR-3 | 2.4 | 62 | 2 | 62 | + | + | + | + | ||||
For SR-1, SR-2, and SR-3, bone marrow cells from animals 2, 3, and 5 were collected and transplanted into secondary recipients (2 × 107 cells per mouse).
Results are percentage of CD45+ cells expressing the denoted antigen.
Human cells that are CD45- VWF+ or BCR/ABL+.
Discussion
CML is a clonal hematopoietic stem cell disorder characterized by the t(9;22) chromosome translocation and resultant production of the constitutively activated BCR/ABL tyrosine kinase. Characterized clinically by marked myeloid proliferation, it invariably terminates in an acute leukemia. Interferon-α, STI571 (a BCR/ABL tyrosine kinase inhibitor), and stem cell transplantations are the standard therapeutic options. But interferon-α is only effective in some patients and improves survival to some degree,25-27 and chemotherapeutic intervention does not result in prolonged overall survival.28,29 Blast crisis CML is even more chemotherapy insensitive.30-33 The reason is possibly due to some unknown biology of the CML stem/progenitor cells. In general, cure can only be achieved after allogeneic BM transplantation, but age and donor availability restrict this therapy to approximately 25% of patients.25
It has been proposed that CML may be initiated by transforming events that take place in hematopoietic stem cells.34-36 Based on this concept, we developed a primary human model for CML by the transduction of b3a2 BCR/ABL cDNA in normal CD34+ hematopoietic progenitor cells. The transduction of CD34+ hematopoietic progenitor cells with the b3a2-BCR/ABL cDNA recreates most, if not all, phenotypic abnormalities seen in primary CML CD34+ cells.37 On the other hand, CML may also arise from more committed progenitors caused by mutations and/or selective expression of genes that enhance their otherwise limited self-renewal capabilities.38 More recently, detection of the BCR/ABL gene in endothelial cells in patients with CML suggests that BCR/ABL mutation may take place in the putative hemangioblast cells, a very primitive cell population with both hematopoietic and endothelial differentiation potential.9 If this is proven to be true, it may provide new insights into the pathogenesis of CML and have significant implication for the development of new therapies. Identification of such a cell at the single-cell level is necessary to prove this hypothesis. Moreover, CML stem/progenitor cell identification and purification will provide powerful diagnostic and prognostic tools in the clinic.
Although it was anticipated that the hemangioblast would be present only during the earliest stages of vascular development in the yolk sac, accumulating evidence now indicates that hematopoietic cells with hemangioblast activity persist even in adulthood.10,39-41 In our previous study, we isolated Flk1+ cells from fetal bone marrow and showed that they could give rise to erythrocyte and endothelial cells at the single-cell level. Flk1, also known as vascular endothelial growth factor receptor 2 (kinase domain receptor [KDR]), is closely related to the ontogenic development of hematopoietic and endothelial lineages. Knockout of the Flk1 gene causes a combined defect of hematopoiesis and endothelial cell growth,42,43 possibly mediated by a defect of the embryonic hemangioblast Flk1+ precursor of both lineages. Consistent with this hypothesis, Flk1+ cells isolated from differentiating embryonic stem cells and the aorta-gonad-mesonephros region generate mixed endothelial-hematopoietic colonies in unicellular culture.44,45
In this study, we have successfully established a model of CML in NOD/SCID mice with lower cell doses than has been reported for the SCID and NOD/SCID mouse model of CML.16,46 In our study, infusion of 1 × 105 cells resulted in successful engraftment in 86% of mice 2 months after transfusion. Of these, 62% of the mice had greater than 10% human cells in their BM. The ability to detect engraftment of leukemic cells is critical in an animal model in evaluating cell selection or purging techniques as therapeutic modalities for CML. Just as Hughes and coworkers (Lewis et al16 ) did, we also used FISH to detect BCR/ABL+ cells in engrafted mice as a measure to show leukemic engraftment. This allows direct visualization and enumeration of cells and avoids the reliance on proliferating cells necessary for cytogenetic analysis. FISH analysis of the CML cells (Flk1+CD31–CD34– clonal cells) from 4 patients before infusion showed they were BCR/ABL+. We analyzed 6 specimens by FISH for the BCR/ABL gene and found that a mean level of 81% of cells were human leukemic cells. This wide range in the level of leukemic engraftment may reflect preferential engraftment of leukemic stem cells, numbers of which are different in different individuals. Besides, morphologic analysis of murine BM, spleen, and peripheral blood engrafted with CML patient–derived Flk1+CD31–CD34– clonal cells showed similar characteristics to those in patients with CML. More importantly, the clonal Flk1+CD31–CD34– cells had extensive self-renewal potential and retained leukemia stem cell properties as shown by their ability to recreate the CML-like disease in secondary SCID recipients. In addition, the Flk1+CD31–CD34– cells could also incorporate into foci of vascular endothelium and contribute to neoangiogenesis. Considering the in vitro results that showed that single Flk1+CD31–CD34– could generate blood cells and endothelial cells, we conclude that CML stem cells can be traced at least to hemangioblasts, earlier than the pluripotent HSCs suggested by Lewis et al.16
The most important implication of this study is that the identification of CML-initiating cells or CML stem cells beyond HSCs will provide new insights into the molecular mechanisms underlying CML and lead to new targets for more efficient drug design and chemotherapy. In addition, purified true leukemia stem cells will also provide a target for immune-based therapies and biologic response modifiers. We are currently establishing complementary DNA libraries from these cells to characterize gene expression profiles in CML hemangioblasts and compare them with those from normal stem cells and more committed CML-CFUs. The recent development of lentiviral vectors for rapid and efficient transduction of highly purified populations of cells, including the nonproliferating HSCs, will allow efficient testing of candidate genes obtained from the microarray and investigations of their roles in leukemogenesis.
Prepublished online as Blood First Edition Paper, December 9, 2004; DOI 10.1182/blood-2004-07-2514.
Supported by grants from the “863 Projects” of Ministry of Science and Technology of PR China (no. 2002AA205061); China Medical Board of New York, Stem Cell Biology, Engineering (Grant no. 01-748); National Natural Science Foundation of China (no. 30070284); National Key Project for Basic Research of China (no. 001CB5099); and Beijing Ministry of Science and Technology (no. 2002-489). R.C.H.Z. is a Cheung Kong Scholar in PR China.
B.F. and C.Z. contributed equally to this study.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We would like to thank the staff at the State Key Lab of Sino-American Collaborative Laboratory, Experimental Haematology, Institute of Haematology and Blood Diseases Hospital and the Center of Excellence in Tissue Engineering. The expert technical assistance of Shaoguang Yang, Huixiang Li, and Changlu Ma is highly appreciated.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal