Abstract
Mantle-cell lymphoma (MCL) is characterized by poor prognosis with a median survival of only 3 to 4 years. To improve clinical outcome, the European MCL Network initiated a randomized trial comparing consolidation with myeloablative radiochemotherapy followed by autologous stem cell transplantation (ASCT) to α-interferon maintenance (IFNα) in first remission. Patients 65 years of age or younger with advanced-stage MCL were assigned to ASCT or IFNα after achievement of complete or partial remission by a cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP)–like induction therapy. According to the International Prognostic Index (IPI), 43% of patients had a low-risk, 41% a low-intermediate, 11% a high-intermediate, and 6% a high-risk profile. Sixty-two of 122 patients proceeded to ASCT and 60 received IFNα. Patients in the ASCT arm experienced a significantly longer progression-free survival (PFS) with a median of 39 months compared with 17 months for patients in the IFNα arm (P = .0108). The 3-year overall survival (OS) was 83% after ASCT versus 77% in the IFN group (P = .18). Early consolidation by myeloablative radiochemotherapy followed by ASCT is feasible and results in a significant prolongation of PFS in advanced-stage MCL. Longer follow-up is needed to determine the effect on OS.
Introduction
Advanced-stage mantle-cell lymphoma (MCL) is characterized by an aggressive clinical course and poor prognosis with a median survival of only 3 to 4 years and a low proportion of long-term survivors.1-3 Various approaches to improve this dismal outcome by conventional chemotherapy have failed. Although overall response rates of 70% to 85% and complete remission (CR) rates of 20% to 30% are often achieved, the long-term perspective has remained virtually unchanged.1,4
In an attempt to improve the outcome of MCL by new therapeutic approaches, the effect of α-interferon (IFNα) maintenance was investigated in several studies.5,6 These analyses revealed a nonsignificant tendency toward a prolonged progression-free survival (PFS). More recently, the anti-CD20 antibody rituximab was investigated as a single agent7-9 as well as in combination with chemotherapy. While the results of rituximab monotherapy revealed only a moderate activity, highly encouraging data were reported by several studies combining rituximab and chemotherapy.10-12 A recently completed prospective randomized phase 3 trial of the German Low-Grade Lymphoma Study Group (GLSG) showed a significantly better response rate after a combined immunochemotherapy regimen (rituximab and CHOP [cyclophosphamide, doxorubicin, vincristine, and prednisone]) compared with CHOP alone.11 However, the duration of remission remained rather short. More promising results have recently been achieved in various phase 2 studies implementing high-dose cytarabine. More than 80% of patients with MCL obtained a CR after a sequential CHOP-DHAP (CHOP–dexamethasone, high-dose cytarabine, and cisplatin) regimen in a study by Lefrere et al.13 Similarly, high response rates of more than 90% could be demonstrated by a dose-intensified approach of the MD Anderson Cancer Center, applying an alternating regimen of hyper-CVAD (hyper–cyclophosphamide, vincristine, adriamycin, and dexamethasone) with high-dose cytarabine and methotrexate in elderly patients not suitable for stem cell transplantation.14
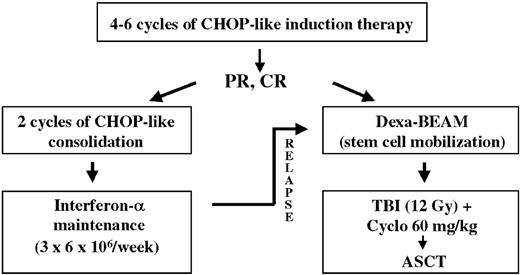
Encouraging results were also obtained by different phase 2 studies exploring the potential of consolidation by high-dose therapy followed by autologous stem cell transplantation (ASCT) to eliminate residual lymphoma cells after conventional chemotherapy.15-23 In order to define the impact of this approach more precisely, the European MCL Network embarked in 1996 on a randomized comparison of myeloablative radiochemotherapy followed by ASCT as consolidation in first remission versus IFNα maintenance in patients 65 years of age or younger after a CHOP-like induction regimen. Based on a previous analysis, which demonstrated the benefit of total body irradiation (TBI) as part of the conditioning regimen in MCL, TBI was included in the myeloablative regimen.19
Patients, materials, and methods
Inclusion criteria
Inclusion criteria comprised patients between 18 and 65 years of age with previously untreated, advanced Ann Arbor stage III and IV MCL according to the current World Health Organization (WHO) classification.24 The histologic diagnosis was confirmed by a central pathology review at one of the designated pathology reference centers (European MCL Pathology Panel). Patients with stage I or II disease as well as patients with a poor performance status (Eastern Cooperative Oncology Group [ECOG] score > 2) were not eligible. In addition, patients with seriously impaired cardiac, pulmonary, hepatic (aspartate aminotransferase/alanine aminotransferase [ASAT/ALAT] level ≥ 3 times of upper limits and/or bilirubin level ≥ 34.2 μM [2.0 mg/dL]), or renal function (creatinine level > 176.8 μM [2.0 mg/dL]) as well as pregnant or lactating women were not enrolled. Approval for our trial was obtained from the institutional review boards of the 129 participating study centers. Informed consent was provided according to the Declaration of Helsinki.
The initial diagnostic workup comprised the assessment of the extent of the disease including computed tomography (CT) scans of the neck, chest, and abdomen; abdominal ultrasound; and a bone marrow biopsy. Normal organ function was assured by the respective laboratory tests, as well as by echocardiograms and electrocardiograms.
Treatment schedule
Patients were randomized up front either to myeloablative radiochemotherapy followed by ASCT or to IFNα maintenance after completion of induction therapy (Figure 1). For initial cytoreductive therapy, different CHOP-like regimens were applied (Table 1). The vast majority of patients received CHOP (cyclophosphamide 750 mg/m2 intravenously, day 1; doxorubicin 50 mg/m2 intravenously, day 1; vincristine 1.4 mg/m2 [maximum 2 mg] intravenously, day 1; and prednisone 100 mg/m2 orally, days 1-5) or the combination of CHOP and rituximab (375 mg/m2 after prophylactic application of antipyretic and antihistamine premedication on day 0). Patients achieving a complete remission (CR) after 4 cycles of initial cytoreductive chemotherapy proceeded directly to consolidation therapy. All other patients received 6 cycles of induction therapy. Consolidation therapy was initiated only in patients who achieved a CR or a partial remission (PR) following induction therapy and if no mantle cells were detectable in the peripheral blood (based on differential blood count and morphology) and the bone marrow biopsy showed less than 20% residual lymphoma cells. Patients not achieving at least a PR after 6 cycles of induction chemotherapy were taken off study (Figure 1).
Patient characteristics
. | IFN . | ASCT . | Total . |
|---|---|---|---|
| Patients evaluable, no. | 60 | 62 | 122 |
| Median age of evaluable patients in years (range) | 55.2 (37-65) | 55.6 (35-65) | 55.6 (35-65) |
| IPI, n = 108 (%) | |||
| Low-risk | 22 (42) | 24 (44) | 46 (43) |
| Low-intermediate risk | 23 (43) | 21 (38) | 44 (41) |
| High-intermediate risk | 6 (11) | 6 (11) | 12 (11) |
| High-risk | 2 (4) | 4 (7) | 6 (6) |
| Male (%) | 42 (70) | 48 (77) | 90 (74) |
| Stage IV (%) | 47 (78) | 52 (84) | 99 (81) |
| Elevated serum LDH, n = 111 (%) | 14 (26) | 14 (25) | 28 (25) |
| ECOG score greater than 1 (%) | 3 (5) | 2 (3) | 5 (4) |
| B-symptoms (%) | 20 (33) | 25 (40) | 45 (37) |
| Induction therapy (%) | |||
| CHOP | 35 (58) | 39 (63) | 74 (61) |
| R-CHOP | 14 (23) | 18 (29) | 32 (26) |
| Other CHOP-like regimens | 11 (18) | 5 (8) | 16 (13) |
. | IFN . | ASCT . | Total . |
|---|---|---|---|
| Patients evaluable, no. | 60 | 62 | 122 |
| Median age of evaluable patients in years (range) | 55.2 (37-65) | 55.6 (35-65) | 55.6 (35-65) |
| IPI, n = 108 (%) | |||
| Low-risk | 22 (42) | 24 (44) | 46 (43) |
| Low-intermediate risk | 23 (43) | 21 (38) | 44 (41) |
| High-intermediate risk | 6 (11) | 6 (11) | 12 (11) |
| High-risk | 2 (4) | 4 (7) | 6 (6) |
| Male (%) | 42 (70) | 48 (77) | 90 (74) |
| Stage IV (%) | 47 (78) | 52 (84) | 99 (81) |
| Elevated serum LDH, n = 111 (%) | 14 (26) | 14 (25) | 28 (25) |
| ECOG score greater than 1 (%) | 3 (5) | 2 (3) | 5 (4) |
| B-symptoms (%) | 20 (33) | 25 (40) | 45 (37) |
| Induction therapy (%) | |||
| CHOP | 35 (58) | 39 (63) | 74 (61) |
| R-CHOP | 14 (23) | 18 (29) | 32 (26) |
| Other CHOP-like regimens | 11 (18) | 5 (8) | 16 (13) |
The distribution of patient characteristics is well balanced between both study arms.
IFN indicates α-interferon maintenance; ASCT, autologous stem cell transplantation; IPI, International Prognostic Index; n, number of patients; and ECOG, Eastern Cooperative Oncology Group.
Patients randomized to ASCT received intensified mobilization chemotherapy with Dexa-BEAM (dexamethasone 3 × 8 mg orally, days 1-10; BCNU [1,3-bis(2-chloroethyl)-1-nitrosourea] 60 mg/m2 intravenously, day 2; melphalan 20 mg/m2 intravenously, day 3; etoposide 75 mg/m2 intravenously, days 4-7; cytarabine 2 × 100 mg/m2 intravenously, days 4-7; granulocyte colony-stimulating factor [G-CSF] initiated on day 11). Dexa-BEAM was performed within 6 weeks after completion of induction chemotherapy. Peripheral stem cells were harvested and subsequently cryopreserved without any purging procedure. A minimum of 1.0 × 106/kg body weight (bw) CD34+ cells was required for ASCT. Myeloablative therapy was performed within 2 months of mobilization and consisted of a total body irradiation (TBI; 12 Gy; TBI was fractionated on days –6 to –4; pulmonary dosage was limited to 8 Gy) and high-dose cyclophosphamide (60 mg/kg bw intravenously, days –3 and –2) regimen. The previously harvested peripheral blood stem cells were reinfused on day 0. G-CSF was initiated on day +1.
Patients randomized to the IFNα maintenance arm received 2 additional courses of conventional chemotherapy to balance the mobilization scheme (Dexa-BEAM; Figure 1). Subsequently α-interferon was applied at a starting dose of 6 × 106 U subcutaneously 3 times weekly until progression of lymphoma. In case of occurrence of intolerable toxicity, the dosage was adapted accordingly.
Response criteria and evaluation
Response to therapy was assessed after every 2 cycles of induction therapy and prior to and after ASCT. Response evaluation included a physical examination, a complete blood count, a serum biochemistry profile, an ultrasound of the abdomen, CT scans of previously involved areas, and a bone marrow biopsy. Follow-up was performed every 3 months in both study arms, except for bone marrow biopsies and CT scans of previously involved areas which were repeated every 6 months.
Response was defined according to the International Working Group criteria,25 hence, complete remission (CR) was defined as complete absence of disease manifestations for at least 4 weeks. Partial remission (PR) was defined as at least a 50% reduction of all evaluable lymphoma manifestations, without appearance of new lesions for at least 4 weeks. Minimal response (MR) was defined as reduction of all evaluable lymphoma manifestations by less than 50%. Stable disease (SD) was defined as no reduction of evaluable lymphoma manifestations; progression (PD) was defined as increase in lymphoma-associated symptoms, the appearance of new lymphoma manifestations, or an increase in volume of lymphoma of greater than 25%. Progression-free survival (PFS) was defined for patients who achieved at least a PR after induction therapy from the end of successful induction therapy to documentation of progression or death from any cause. Overall survival (OS) was defined as the interval between the end of successful induction therapy and death from any cause. The frequency and severity of side effects was recorded according to the WHO classification.26
Randomization and statistical analysis
Randomization was carried out up front prior to induction therapy to allow smaller study centers in which the autologous stem cell transplantation could not be performed to find an appropriate transplantation center. Randomization was performed centrally, blocked, and stratified according to the number of risk factors at baseline defined by the International Prognostic Index (IPI)27 and according to the country of the participating center.
The primary study end point was the PFS after successful completion of induction therapy. This parameter was monitored continuously and analyzed by means of a sequential procedure in order to allow to stop randomization as soon as a significant difference was detected between the 2 study arms. Based on a significance level α= 0.05, the one-sided triangular test for the log-rank statistic was designed to detect a risk reduction by 65% for the PFS by ASCT with a power of 95%.
Randomized patients were evaluable for the primary analysis of the PFS if induction therapy was completed with at least a PR with no detectable mantle cells in the peripheral blood and less than 20% residual lymphoma cells in the bone marrow. In addition, either stem cell mobilization with Dexa-BEAM or consolidation therapy had to be initiated according to randomization. Patients with serious protocol violations (eg, additional therapy or ASCT with purging) were censored at the time of the protocol violation. The significance level for the main parameter was calculated with respect to the sequential design.
All randomized patients with advanced-stage MCL were included for additional intention-to-treat analyses. For the intention-to-treat analysis of PFS, time-to-treatment failure (TTF) was defined as time from randomization to failure of induction therapy (MR, SD, PD), relapse after CR or PR, and death from any cause. Patients with MR or SD after induction therapy went off study. Thus, progression was not evaluated for those patients. For the intention-to-treat analysis of OS, the survival time was calculated from date of randomization. Kaplan-Meier estimates were calculated for PFS and OS. The log-rank test was used for secondary time-to-event analyses. A multiple Cox regression analysis with backward selection applying the Wald statistic was performed for PFS. Safety data were compared with the 2-sided Fisher exact test.
The design of the triangular test, the sample size calculation, and the analysis of the main parameter were carried out using the PEST3 software (PEST Version 3; Applied Statistics Department, Reading University, Reading, Great Britain). All other statistical analyses were performed with the SAS system (SAS Version 8.02; SAS Institute, Cary, NC).
Trial conduct
The trial was carried out in accordance with the Declaration of Helsinki. All patients gave their written informed consent after having been informed about the purpose and investigational nature of the trial. Prior to initiation, the trial received approval by the responsible ethics committee.
Results
Patient characteristics
Between September 1996 and March 2004, 269 patients from 129 institutions were randomized to either ASCT or IFNα maintenance. Thirty-eight patients were subsequently excluded, as the diagnosis of MCL could not be confirmed by the central pathology review. In addition, 1 patient was excluded, as he presented with stage II disease. Of the 230 remaining cases, 52 did not achieve a remission by initial cytoreductive therapy. Furthermore, 29 are currently not evaluable for induction therapy. From the remaining 149 cases, 27 patients were not evaluable because of refusal of the assigned therapy (n = 5) or lack of documentation (n = 5); 4 patients were not suitable for the assigned therapy due to additional diseases, 5 patients had a bone marrow infiltration of greater than 20%, 1 patient had detectable mantle cells in the peripheral blood, in 1 patient no bone marrow biopsy was performed after induction therapy, 2 patients did not complete induction therapy, 1 patient was lost to follow-up, and 3 patients did not receive the assigned therapy. Hence, 122 patients are fully evaluable. Eighty-one percent of these patients were diagnosed with stage IV disease, 25% had an elevated lactate dehydrogenase (LDH) serum level, and 37% presented with B symptoms. In addition, of the 108 patients evaluable for the IPI,27 43% had a low-risk, 41% a low-intermediate, 11% a high-intermediate, and 6% a high-risk IPI. Sixty-one percent of patients received CHOP, 26% rituximab and CHOP (R-CHOP), 10% mitoxantrone-chlorambucil-prednisone (MCP), and 3% other CHOP-like chemotherapy regimens as induction therapy. The patient characteristics in the 2 study arms are comparable and are summarized in Table 1.
Response to consolidation
After initial cytoreductive therapy, 22 of 62 patients in the ASCT group had achieved a CR (35%) and 40 a PR (65%). Following consolidation therapy with myeloablative radiochemotherapy and ASCT, 44 (81%) of the 54 patients reached a CR and 9 (17%) a PR. In the IFNα group, 17 of 60 patients achieved a CR (28%) and 43 a PR (72%) after induction therapy. After 2 additional cycles of consolidating chemotherapy, 37% (n = 22) of the patients achieved a CR and 62% (n = 37) a PR.
Progression-free survival
After a median follow-up of 25 months, 27 relapses in the ASCT group and 42 in the IFN arm were observed. In addition, 3 deaths in the ASCT study arm occurred in remission: 1 patient died following Dexa-BEAM mobilization from sepsis. Similarly, 2 other patients died from infectious complications 2 months following ASCT. In the IFNα study arm, no deaths in remission could be observed.
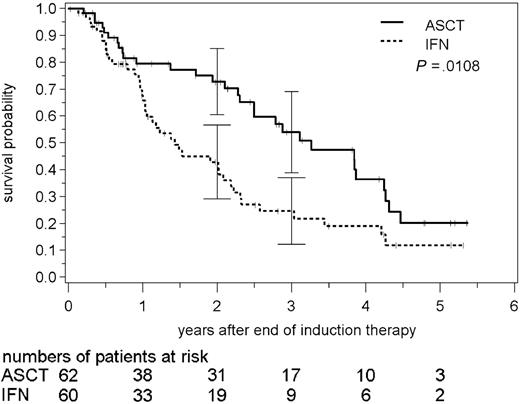
Accordingly, the PFS was significantly different in the 2 study arms. In patients receiving ASC transplants, the median PFS was 39 months and the 3-year PFS rate was 54% (95% confidence interval between 39% to 69%). In contrast, in the IFNα study arm, the median PFS was only 17 months and the PFS rate was 25% (12% to 37%) after 3 years (P = .0108; Figure 2). In the intention-to-treat analysis of all randomized patients, median TTF was 29 months in the ASCT arm (n = 114) compared with 15 months in the IFNα arm (n = 116; P = .0023).
Progression-free survival after high-dose radiochemotherapy followed by autologous stem cell transplantation (ASCT) and interferon-α (IFN) maintenance in MCL. Patients assigned to stem cell transplantation experience significantly longer progression-free survival (log-rank test). Solid line indicates ASCT; broken line, IFN. Vertical bars indicate 95% confidence intervals for progression-free survival.
Progression-free survival after high-dose radiochemotherapy followed by autologous stem cell transplantation (ASCT) and interferon-α (IFN) maintenance in MCL. Patients assigned to stem cell transplantation experience significantly longer progression-free survival (log-rank test). Solid line indicates ASCT; broken line, IFN. Vertical bars indicate 95% confidence intervals for progression-free survival.
Additionally, the PFS was analyzed according to induction therapy. Patients who received CHOP (n = 74) and subsequently underwent ASCT (n = 39) had a median PFS of 46 months and a 3-year PFS of 62% (44% to 79%) compared with a median PFS of only 23 months and a 3-year PFS of 27% (11% to 43%) in patients receiving IFNα (n = 35; P = .019). In patients who were treated initially by the combination of CHOP and rituximab (n = 32), the detected differences between ASCT (2-year PFS 51%; 21% to 82%; median not yet reached) and IFNα (2-year PFS 44%; 8% to 79%; median PFS 17 months) were much smaller (P = .73; median follow-up time only 12 months).
We also investigated PFS according to the response to initial induction chemotherapy. Patients who received transplants in CR (n = 22) had a median PFS of 46 months and a 3-year PFS of 71% (48% to 93%) in comparison to only 24 months and 19% (0% to 38%) in the IFN study group (n = 17), respectively (P = .0019; Figure 3A). In patients who received ASC transplants in PR (n = 40), the median PFS was 33 months and the 3-year PFS was 45% (26% to 64%) in comparison to 15 months and 29% (13% to 45%), respectively, in patients with IFNα maintenance (n = 43; P = .122; Figure 3B).
PFS according to the response to initial induction chemotherapy in patients who received transplants in CR and in PR. (A) Progression-free survival after high-dose radiochemotherapy followed by autologous stem cell transplantation (solid line) and interferon-α maintenance (broken line) in the subgroup of patients with CR following induction therapy. Patients assigned to stem cell transplantation experience significantly longer progression-free survival (log-rank test). (B) Progression-free survival after high-dose radiochemotherapy followed by autologous stem cell transplantation and interferon-α maintenance in the subgroup of patients with PR after induction therapy. Bars indicate 95% confidence interval.
PFS according to the response to initial induction chemotherapy in patients who received transplants in CR and in PR. (A) Progression-free survival after high-dose radiochemotherapy followed by autologous stem cell transplantation (solid line) and interferon-α maintenance (broken line) in the subgroup of patients with CR following induction therapy. Patients assigned to stem cell transplantation experience significantly longer progression-free survival (log-rank test). (B) Progression-free survival after high-dose radiochemotherapy followed by autologous stem cell transplantation and interferon-α maintenance in the subgroup of patients with PR after induction therapy. Bars indicate 95% confidence interval.
The analysis of PFS according to the IPI27 demonstrated an advantage of ASCT especially in the intermediate-risk subgroup. In patients receiving ASC transplants (n = 27), the median PFS was 46 months and the 3-year PFS was 62% (39% to 86%) compared with 15 months and 13% (0% to 26%) in the IFNα arm (n = 29; P = .0069). In the low-risk IPI subgroup (n = 46), the median PFS was 51 months after ASCT (n = 24) and 36 months after IFNα maintenance (n = 22; P = .38).
A Cox regression analysis was performed to independently evaluate the effect of the parameters included in the IPI (elevated serum LDH level, extranodal involvement > 1 site, ECOG performance status score ≥ 2), randomization to induction therapy (CHOP vs R-CHOP vs CHOP-like induction regimen), response to induction therapy (CR vs PR), sex, B symptoms, and the choice of consolidation therapy (ASCT vs IFNα maintenance), on the PFS. This analysis identified only ASCT (hazard ratio of 0.42; P = .0015) and a low IPI (hazard ratio of 0.54; P < .0001) to be independently associated with an improved PFS.
Overall survival
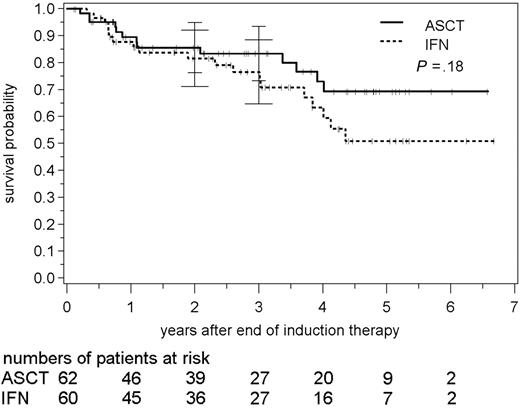
After a median follow-up of 34 months, 32 patients have died (26%; 13 patients in the ASCT study group and 19 in the IFN arm). The survival probability following consolidation with myeloablative radiochemotherapy and ASCT was 86% (76% to 95%) after 2 years compared with 82% (71% to 92%) in the IFN group (Figure 4). The estimated 3-year survival was 83% (73% to 93%) following ASCT and 77% (65% to 88%) in the IFN group, respectively (P = .18; medians not yet reached).
Overall survival following autologous stem cell transplantation and interferon-α maintenance, respectively. Bars indicate 95% confidence intervals.
Overall survival following autologous stem cell transplantation and interferon-α maintenance, respectively. Bars indicate 95% confidence intervals.
Twelve patients in the IFN arm received ASC transplants as salvage therapy in first relapse. Eight of these patients were alive at the date of this analysis, whereas 4 patients died 49, 177, 210, and 952 days after secondary ASCT. Censoring IFN patients at the date of secondary ASCT did not alter the result of the overall survival (3-year survival 83% vs 79%; P = .27).
The intention-to-treat analysis of all randomized patients resulted in a 3-year OS with randomization of 76% (66% to 85%; median not yet reached) in the ASCT arm (n = 114) compared with 68% (58% to 78%; median 56 months) in the IFN arm (n = 116; P = .16).
So far, no significant differences of the OS were observed between patients who received transplants in CR and PR. The 3-year OS for patients who received ASC transplants in CR was 94% (84% to 100%) compared with 77% (63% to 91%) for patients who received transplants in PR (P = .52).
Toxicity
As expected, acute toxicity was significantly higher in the ASCT study arm. Anemia (91%; 45% grades 3 and 4), leukocytopenia (97%; 95% grades 3 and 4), granulocytopenia (85%; 84% grades 3 and 4), and thrombocytopenia (94%; 91% grades 3 and 4) according to the WHO classification26 occurred in the vast majority of cases (Table 2). Infections due to cytopenia occurred in 85% of cases (23% grades 3 and 4) in the ASCT group compared with only 22% (2% grades 3 and 4) in the IFNα study arm. Accordingly, mortality due to infectious complications was 5% in the ASCT study arm, whereas no patient died from infections during IFNα maintenance. Other nonhematologic toxicity also occurred more frequently in patients receiving stem cell transplantation. Mucositis in 74%, gastrointestinal side effects in 66%, pulmonary toxicity in 17%, renal toxicity in 17%, and liver toxicity in 48% of cases were more frequent in ASCT patients. In contrast, muscle and bone pain as well as depression occurred more often during IFNα maintenance (Table 2).
Acute hematologic and nonhematologic toxicity according to the WHO classification26following ASCT and IFN, respectively
WHO toxicity . | IFN, % . | ASCT, % . |
|---|---|---|
| Acute hematologic toxicity | ||
| Anemia | ||
| Grades I/II | 21 | 45 |
| Grades III/IV | 0 | 45 |
| Leukocytopenia | ||
| Grades I/II | 43 | 2 |
| Grades III/IV | 42 | 95 |
| Granulocytopenia | ||
| Grades I/II | 30 | 2 |
| Grades III/IV | 36 | 84 |
| Thrombocytopenia | ||
| Grades I/II | 8 | 3 |
| Grades III/IV | 2 | 91 |
| Acute nonhematologic toxicity | ||
| Mucositis | ||
| Grades I/II | 9 | 41 |
| Grades III/IV | 0 | 33 |
| Infections | ||
| Grades I/II | 20 | 62 |
| Grades III/IV | 2 | 23 |
| Nausea | ||
| Grades I/II | 15 | 55 |
| Grades III/IV | 0 | 11 |
| Diarrhea | ||
| Grades I/II | 7 | 31 |
| Grades III/IV | 4 | 16 |
| Alopecia | ||
| Grades I/II | 22 | 2 |
| Grades III/IV | 54 | 81 |
| Liver | ||
| Grades I/II | 14 | 40 |
| Grades III/IV | 0 | 8 |
| Renal | ||
| Grades I/II | 0 | 15 |
| Grades III/IV | 0 | 2 |
| Pulmonary | ||
| Grades I/II | 0 | 11 |
| Grades III/IV | 0 | 6 |
| Muscle/bone pain | ||
| Grades I/II | 20 | 19 |
| Grades III/IV | 6 | 0 |
| Depression | ||
| Grades I/II | 9 | 7 |
| Grades III/IV | 6 | 2 |
WHO toxicity . | IFN, % . | ASCT, % . |
|---|---|---|
| Acute hematologic toxicity | ||
| Anemia | ||
| Grades I/II | 21 | 45 |
| Grades III/IV | 0 | 45 |
| Leukocytopenia | ||
| Grades I/II | 43 | 2 |
| Grades III/IV | 42 | 95 |
| Granulocytopenia | ||
| Grades I/II | 30 | 2 |
| Grades III/IV | 36 | 84 |
| Thrombocytopenia | ||
| Grades I/II | 8 | 3 |
| Grades III/IV | 2 | 91 |
| Acute nonhematologic toxicity | ||
| Mucositis | ||
| Grades I/II | 9 | 41 |
| Grades III/IV | 0 | 33 |
| Infections | ||
| Grades I/II | 20 | 62 |
| Grades III/IV | 2 | 23 |
| Nausea | ||
| Grades I/II | 15 | 55 |
| Grades III/IV | 0 | 11 |
| Diarrhea | ||
| Grades I/II | 7 | 31 |
| Grades III/IV | 4 | 16 |
| Alopecia | ||
| Grades I/II | 22 | 2 |
| Grades III/IV | 54 | 81 |
| Liver | ||
| Grades I/II | 14 | 40 |
| Grades III/IV | 0 | 8 |
| Renal | ||
| Grades I/II | 0 | 15 |
| Grades III/IV | 0 | 2 |
| Pulmonary | ||
| Grades I/II | 0 | 11 |
| Grades III/IV | 0 | 6 |
| Muscle/bone pain | ||
| Grades I/II | 20 | 19 |
| Grades III/IV | 6 | 0 |
| Depression | ||
| Grades I/II | 9 | 7 |
| Grades III/IV | 6 | 2 |
IFN indicates α-interferon maintenance; and ASCT, autologous stem cell transplantation.
Discussion
Similar to follicular lymphoma, conventional chemotherapy is a noncurative approach in advanced-stage III or IV MCL.1,3,4 In addition, MCL is characterized by a rapidly progressive course and poor clinical outcome. Thus, new therapeutic strategies are urgently warranted to further improve the prognosis of MCL. One of the recently established approaches in the therapy of lymphoid malignancies is intensive consolidation with high-dose therapy followed by ASCT. Due to its potential curative impact, stem cell transplantation has been accepted as standard therapy in relapsed aggressive non-Hodgkin lymphoma.28 In indolent lymphomas, encouraging results were also reported, although a curative potential has not been demonstrated so far.29-31 In MCL, several phase 2 studies investigated the efficacy of this approach; however, the published results differ substantially.15-23 Hence, Freedman et al20 reported that 68% of patients relapsed after a median time of 21 months. Similarly, Ketterer et al16 could also not show a benefit of high-dose therapy. In contrast, Dreger et al32 observed a 2-year event-free survival and OS of 77% and 100%, respectively, in previously untreated patients. These promising results were confirmed in 2 recent analyses.21,22 The different results in these studies are most probably due to different time points of transplantation (first remission vs second or subsequent remission) and other patient selection criteria. So far, no randomized trial investigated the impact of stem cell transplantation on the long-term outcome of patients with MCL. Thus, the European MCL Network embarked on a randomized trial comparing myeloablative radiochemotherapy followed by ASCT to an IFNα maintenance following initial CHOP-like cytoreductive chemotherapy. Based on a previously published analysis, which demonstrated the benefit of total body irradiation (TBI) as part of the conditioning regimen in MCL, TBI was included in the myeloablative regimen.19 The primary study end point was the PFS after achievement of a PR or CR after initial chemotherapy.
After CHOP-like induction therapy, 74% of patients with MCL achieved either a PR or a CR. Thus, our results are in line with previous studies that reported overall response rates of 65% to 75%.33,34 Dexa-BEAM mobilization chemotherapy as well as consolidation radiochemotherapy followed by ASCT was feasible and well tolerated in the vast majority of patients. However, as expected, toxicity was significantly higher in the ASCT study arm in comparison to IFNα. Hematologic toxicity with anemia, thrombocytopenia, and granulocytopenia was the main adverse event following ASCT. In contrast, depression and muscle and bone pain were more frequently observed in patients receiving IFNα. However, toxicity was acceptable in both study arms. Three patients (5%) in the ASCT arm died of infectious complications. Thus, our data are in line with previous studies, which reported the feasibility of myeloablative therapy in patients with MCL.21,35,36 In a recent study of Gianni et al,35 only one toxic death occurred following a rituximab-containing high-dose therapy. Similarly, in the study of Andersen et al,21 only one patient died from infectious complications.
The current multicenter trial of the European MCL Network demonstrates a significant improvement of PFS in advance-stage MCL by myeloablative radiochemotherapy followed by ASCT versus IFNα maintenance. Currently, 30 events were observed after ASCT compared with 42 events in the IFNα group. Accordingly, the 3-year PFS following ASCT was 54% and only 25% in the interferon arm (P = .0108). At this time, this advantage did not result in a significant difference in OS with a 3-years OS of 83% versus 77%. However, due to the low number of events so far, a longer follow-up is needed to evaluate the definite impact of ASCT on the OS.
We cannot exactly determine the contribution of the Dexa-BEAM regimen that was applied as mobilization scheme prior to ASCT on the improvement of PFS. Thus, to balance both study arms, patients in the IFNα maintenance arm received 2 additional courses of induction therapy.
Our trial also suggests that the impact of ASCT could depend on the remission status prior to transplantation. In our trial, the median PFS was 46 months in CR patients compared with 33 months in patients who received ASC transplants in PR.
The best conditioning regimen for ASCT is currently unknown. One major obstacle may be the risk of secondary hematologic neoplasias, especially after TBI. However, in a prospective evaluation of the incidence of therapy-related myelodysplastic syndrome/therapy-related acute myeloid leukemia (t-MDS/t-AML) in indolent lymphoma following an identical consolidating regimen, only 3.8% of patients developed a t-MDS after 5 years.37 Thus, we believe that the benefit of a significantly improved PFS will not be diminished by a slight increase of secondary hematologic neoplasias.
Even after such a dose-intensified consolidation, the vast majority of patients with MCL will eventually relapse. One major obstacle of stem cell transplantation is the contamination of the harvested stem cells with circulating lymphoma cells. Thus, in vitro as well as in vivo purging procedures have been introduced to eliminate residual lymphoma cells.35,38 Especially antibody-based in vivo purging seems to be a very efficient approach. In previously untreated patients with MCL, Gianni et al35 reported an OS of 89% at 54 months following an in vivo purging with rituximab and subsequent high-dose consolidation. However, such an intensified approach is only feasible in one half of patients with MCL, as the median age is 65 years. Another innovative approach is the application of radioactively labeled (131iodine or 90yttrium) anti-CD20 antibodies. Different studies achieved remarkably high and long-lasting remissions in relapsed or refractory patients with MCL.39,40 Gopal et al39 investigated the efficacy of the 131iodine-labeled anti-CD20 antibody tositumomab in 16 pretreated patients with MCL in combination with high-dose chemotherapy followed by ASCT. High overall response rates of 100% with 91% CR as well as a remarkable estimated 3-year OS of 93% was reported. Thus, it is tempting to speculate that a combined radioimmunochemotherapy followed by ASCT might further improve the long-term outcome of MCL. This concept is currently being investigated in various phase 2 studies.
In summary, myeloablative radiochemotherapy is a feasible and effective treatment option in the therapy of MCL when applied in first remission. Although no differences in the OS are currently detectable, ASCT significantly improved the PFS in comparison to IFNα. Thus, myeloablative radiochemotherapy may be recommended to patients 65 years of age or younger in first remission after an induction therapy containing rituximab, as recent data suggest significantly improved remission rates compared with chemotherapy alone.11 However, since the majority of patients will still relapse after ASCT, additional measures such as in vivo purging with rituximab or antibody maintenance are required to further improve the prognosis of patients with MCL.
Appendix
The following persons and institutions participated in this study: D. Bron, Institut Jules Bordet, Bruxelles, Belgium; A. Bosly, Université Catholique de Louvain, Ivoir, Belgium; P. Vandenberg, G. Verhoef, University Hospital Gasthuisberg, Leuven, Belgium; A. MacWhannell, New Cross Hospital, Wolverhampton, England; B. Corront, Centre Hospitalier d'Annecy, Annecy, France; J. F. Dor, Centre Hospitalier Antibes Juan-les-Pins, Antibes, France; G. Lepeu, Hôpital Henri Duffaut, Avignon, France; P. Agapé, Centre Hospitalier Docteur Duchenne, Boulogne sur Mer, France; O. Reman, Centre Hospitalier Universitaire Clemenceau, Caen, France; M. Blanc, Centre Hospitalier de Chambéry, Chambéry, France; F. Kohser, Centre Hospitalier Louis Pasteur, Colmar, France; G. Tertian, Hôpital de Bicêtre, Le Kremlin-Bicêtre, France; C. Kulekci, Hôpital Marc Jacquet, Melun, France; C. Platini, Groupement des Hôpitaux de Thionville, Metz, France; G. Marit, Hôpital Haut-Lévêque, Pessac, France; Y. Kerneis, F. Morva, F. Rousseau, Centre Hospitalier René Dubos, Pontoise, France; M. R. Boisseau, Hôpital Purpan, Toulouse, France; G. Unverferth, W. Langer, F. Püschel, Kreiskrankenhaus Aurich, Aurich, Germany; D. Hennesser, Vinzenz-Pallotti Hospital, Bergisch Gladbach, Germany; E. Thiel, T. Fietz, Universitätsklinikum Benjamin Franklin, Berlin, Germany; K. P. Hellriegel, H. H. Fülle, R. Simon, Krankenhaus Moabit, Berlin, Germany; K. Possinger, O. Sezer, Universitätsklinik Charité/Campus Mitte, Berlin, Germany; W.-D. Ludwig, H. Harder, Robert Rössle Klinik, Berlin, Germany; J. Potenberg, E. Aulbert, Evangelisches Waldkrankenhaus Spandau, Berlin, Germany; H. Vetter, S. Fronhoffs, Medizinische Poliklinik der Universität, Bonn, Germany; B. Wörmann, G. Jordan, A. Pies, Städtisches Klinkum Braunschweig, Braunschweig, Germany; F. Fiedler, A. Hänel, Klinikum Chemnitz Krankenhaus Küchwald, Chemnitz, Germany; M. Grundeis, Schwerpunktpraxis Onkologie, Hämatologie, Chemnitz, Germany; M. Lößner, Carl-Thiem-Klinikum, Cottbus, Germany; R. Haas, Universitätsklinik Düsseldorf, Düsseldorf, Germany; M. Gramatzki, Klinikum der Universität, Erlangen, Germany; R. Fuchs, S. Wehle-Ilka, J. Wiegand, St-Antonius-Hospital, Eschweiler, Germany; U. Dührsen, H. Nückel, Medizinische Klinik und Poliklinik, Essen, Germany; J. G. Saal, D. Hartwigsen, U. Strack, St-Franziskus-Hospital, Flensburg, Germany; T. Reiber, D. Semsek, Praxis für Innere Medizin, Freiburg, Germany; R. Mertelsmann, J. Finke, Medizinische Universitätsklinik, Freiburg, Germany; W. Faßbinder, H. G. Höffkes, Klinikum Fulda Medizinische Klinik III, Fulda, Germany; G. C. Schliesser, Hämatologische Praxis, Giessen, Germany; B. Glaß, Universitätsklinikum Göttingen, Göttingen, Germany; H. Eimermacher, Katholisches Krankenhaus, Hagen, Germany; S. Kraus, I. Hausbrandt, St Salvator Krankenhaus, Halberstadt, Germany; H. J. Schmoll, H. H. Wolf, Klinikum der Universität Halle, Halle, Germany; H. J. Hurtz, R. Rohrberg, R. Behrends, Schwerpunktpraxis, Halle, Germany; N. Schmitz, P. Dreger, Allgemeines Krankenhaus St Georg, Hamburg, Germany; S. Hegewisch-Becker, Universitätsklinik Eppendorf, Hamburg, Germany; H. Schmidt, Kreiskrankenhaus Hameln, Hameln, Germany; H. A. Dürk, B. Schmid, S. Weibrecht, St-Marien-Hospital, Hamm, Germany; A. Ganser, D. Peest, Medizinische Hochschule Hannover, Hannover, Germany; F. Henne, Internist, Hechingen, Germany; H. Dietzfelbinger, Privatklinik Dr R. Schindlbeck, Herrsching, Germany; U. Basler, B. Sievers, Städtisches Krankenhaus Hildesheim, Hildesheim, Germany; J. Th. Fischer, S. Wilhelm, R. Ehrhardt, Städtisches Klinikum Karlsruhe, Karlsruhe, Germany; J. Mezger, G. Göckel, St-Vincentius-Krankenhäuser, Karlsruhe, Germany; F. A. Mosthaf, K. Zutavern-Bechtold, M. Procaccianti, Gemeinschaftspraxis Hämatologie/Internistische Onkologie, Karlsruhe, Germany; S. Siehl, U. Söling, Gemeinschaftspraxis Hämatologie/Internistische Onkologie, Kassel, Germany; M. Kneba, Universitätsklinikum Kiel, Kiel, Germany; Th. Eisenhauer, H. Nolte, Städtisches Klinikum Kemperhof, Koblenz, Germany; M. Hallek, V. Diehl, A. Engert, P. Staib, I Medizinische Universitätsklinik, Köln, Germany; M. Planker, M. Busch, M. Hipp, Städtische Krankenanstalten, Krefeld, Germany; A. Schwarzer, Gemeinschaftspraxis für Hämatologie/Onkologie, Leipzig, Germany; S. Fetscher, Städtisches Krankenhaus Süd, Lübeck, Germany; M. Uppenkamp, M. Hoffmann, Klinikum der Stadt, Ludwigshafen, Germany; M. Wiermann, Universitätsklinikum Magdeburg, Magdeburg, Germany; C. Huber, T. Fischer, G. Heß, Universitätsklinikum Mainz, Mainz, Germany; R. Hehlmann, E. Lengfelder, C. Kuhn, III Medizinische Klinik Mannheim, Mannheim, Germany; A. Pfeiffer, M. Mennicke, Klinikum Memmingen, Memmingen, Germany; H. Bodenstein, H.-H. Wöltjen, Klinikum II, Minden, Germany; M. Becker, C. Kreisel-Büstgens, Gemeinschaftspraxis Hämatologie/Internistische Onkologie, Minden, Germany; R. Götz, P. Jehner, Krankenhaus Bethanien, Moers, Germany; H. E. Reis, D. Kohl, D. Berkovic, Kliniken Maria Hilf, Mönchengladbach, Germany; C. Lunscken, Hämatologische Praxis, Mülheim an der Ruhr, Germany; R. Forstpointner, Klinikum Grosshadern der Universität, München, Germany; R. Hartenstein, N. Brack, Städtisches Krankenhaus München-Harlaching, München, Germany; D. Schlöndorff, J. Walther, U. Seybold, Klinikum Innenstadt, München, Germany; W. Abenhardt, F. J. Tigges, D. Bosse, Onkologische Praxis im Elisenhof, München, Germany; W. E. Berdel, Universitätsklinikum Münster, Münster, Germany; H. Rühle, N. Grobe, F. Jungmichel, Klinikum Neubrandenburg, Neubrandenburg, Germany; P. Ehscheidt, St-Elisabeth-Krankenhaus, Neuwied, Germany; H. Wandt, J. Wortmann, Klinikum Nord, Nürnberg, Germany; H.-J. Illiger, Klinikum Oldenburg, Oldenburg, Germany; L. Theilmann, B. Sandritter, Städtisches Klinikum Pforzheim, Pforzheim, Germany; P. Weber, S. Perino, Medizinische Klinik des Siloah Krankenhauses, Pforzheim, Germany; R. Pasold, F. Rothmann, A. Haas, Ernst-Von-Bergmann-Klinik, Potsdam, Germany; G. Kautzsch, A. Rupprecht, Sankt-Josefs-Krankenhaus Potsdam, Potsdam, Germany; R. Andreesen, S. Krause, S. Mayer, Universitätsklinik Regensburg, Regensburg, Germany; E.-D. Kreuser, Krankenhaus Barmherzige Brüder, Regensburg, Germany; M. Freund, Universitätsklinik Rostock, Rostock, Germany; M. Baldus, Internistische Schwerpunktpraxis, Rüsselsheim, Germany; H. G. Mergenthaler, J. Schleicher, Katharinenhospital, Stuttgart, Germany; E. Heidemann, J. Kaesberger, Diakonissenkrankenhaus, Stuttgart, Germany; H. Fiechtner, Praxis für Hämatologie/Onkologie, Stuttgart, Germany; H. G. Biedermann, W. Larisch, Kreiskrankenhaus Traunstein, Traunstein, Germany; C. B. Kölbel, K. J. Weber, H. Kirchen, Krankenhaus der Barmherzigen Brüder, Trier, Germany; L. Labedzki, H. J. Bias, Kreiskrankenhaus Waldbröl, Waldbröl, Germany; S. Vedder, J. Rövekamp, St-Christophorus-Krankenhaus Werne, Werne, Germany; M. Sandmann, G. Becker, Kliniken St Antonius, Wuppertal, Germany; K. Wilms, H. Rückle-Lanz, M. Wilhelm, Medizinische Poliklinik der Universität, Würzburg, Germany; G. Schott, Heinrich-Braun-Krankenhaus Zwickau, Zwickau, Germany; M. Federico, Policlinical Centre, Modena, Italy; F. Nobile, Azienda Ospedaliera, Reggio-Calabria, Italy; M. Martelli, Universita degli Studi di Roma, Roma, Italy; P. Milone, S. Giovanni Battista di Torino, Torino, Italy; H. L. Haak, Leyenburg Hospital, Den Haag, The Netherlands; K. J. Heering, Groene Hart Ziekenhuis, Gouda, The Netherlands; M. Fickers, Atrium Medisch Centrum, Heerlen, The Netherlands; and R. Willemze, Leiden University Medical Center, Leiden, The Netherlands.
Prepublished online as Blood First Edition Paper, December 9, 2004; DOI 10.1182/blood-2004-10-3883.
Supported in part by a grant of the Deutsche Krebshilfe (project no. 70-2208-Hi 2), the European Community (no. LSHC-CT-2004-503351), and the Bundesministerium für Bildung und Forschung Kompetenznetz Maligne Lymphome (no. 01 GI 9994).
A complete list of the members of the German European MCL Network appears in the “Appendix.”
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We would like to thank the European MCL Pathology Panel (Coordinator Reza Parwaresch) for the pathologic review.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal