Abstract
Hereditary hemochromatosis (HH) is an autosomal recessive disease that leads to parenchymal iron accumulation. The most common form of HH is caused by a single amino acid substitution in the HH protein, HFE, but the mechanism by which HFE regulates iron homeostasis is not known. In the absence of transferrin (Tf), HFE interacts with transferrin receptor 1 (TfR1) and the 2 proteins co-internalize, and in vitro studies have shown that HFE and Tf compete for TfR1 binding. Using a cell line lacking endogenous transferrin receptors (TRVb cells) transfected with different forms of HFE and TfR1, we demonstrate that even at low concentrations Tf competes effectively with HFE for binding to TfR1 on living cells. Transfection of TRVb cells or the derivative line TRVb1 (which stably expresses human TfR1) with HFE resulted in lower ferritin levels and decreased Fe2+ uptake. These data indicate that HFE can regulate intracellular iron storage independently of its interaction with TfR1. Earlier studies found that in HeLa cells, HFE expression lowers Tf-mediated iron uptake; here we show that HFE lowers non–Tf-bound iron in TRVb cells and add to a growing body of evidence that HFE may play different roles in different cell types.
Introduction
Hereditary hemochromatosis (HH) is a common autosomal recessive disease of iron metabolism characterized by gradual accumulation of excess iron in organs such as the liver, heart, pancreas, and thyroid, resulting in symptoms, including hepatic cirrhosis and hepatocellular carcinoma, cardiomyopathy and arrhythmias, diabetes, and hypogonadotropic hypogonadism.1,2 The most common form of HH is caused by a single base pair mutation in the HH gene that results in the substitution of tyrosine for cysteine at amino acid position 260 (C260Y; The numbering system for both HFE and low-density lipoprotein receptor [LDLR] is based on the mature protein and does not include the signal sequence) in the mature protein, HFE.3 HFE is an atypical major histocompatibility complex (MHC) class 1–related protein. The C260Y mutation disrupts a disulfide bond in its α3 domain, which abrogates its association with the β2-microglobulin (β2M) light chain and subsequent trafficking to the cell surface.4 Although the importance of functional HFE-β2M heterodimers in the maintenance of iron homeostasis has been further documented in studies using HFE knock-out mice,5,6 the mechanism by which HFE regulates iron metabolism in cells is still unknown.
In vitro and in vivo studies show that HFE associates with transferrin receptor 1 (TfR1) at neutral pH7-10 and that the binding sites for HFE and Tf overlap in TfR.11-13 In vitro experiments demonstrate that HFE competes with Tf for binding to TfR1 at concentrations of diferric Tf lower than 100 nM.11 These experiments explain the early observation that HFE appears to lower the binding affinity of TfR to diferric Tf.7,14 Indeed, in HeLa, HEK293, and H1299 cells, expression of exogenous HFE results in the lowering of intracellular iron levels. However, HeLa and H1299 cells expressing HFE have about 30% lower rates of Tf-mediated iron uptake than parental controls, even in the presence of sufficiently high concentrations of diferric Tf that HFE does not affect the uptake of Tf.15,16 In addition, the W81A HFE mutant regulates Tf-mediated iron uptake to the same extent as wild-type HFE in HeLa cells,17 despite showing a 5000-fold lower affinity for TfR1 binding.18 These studies show that the effect of HFE on iron homeostasis does not depend solely on the interaction with TfR1 and is more complex than simple competition between HFE and Tf for binding to TfR1.
In this study we took advantage of a Chinese hamster ovary cell line, TRVb cells, which lacks endogenous transferrin receptors.19 These cells were stably transfected with different combinations of human HFE, β2M, and TfR cDNAs, and flow cytometry was used to study the competition between Tf and HFE for binding to TfR1 in intact cells. Our results show that this competition occurs even at concentrations of Tf well below those found in the blood. We went on to examine the role of HFE in iron homeostasis in cells lacking TfR1 and found that HFE can lower intracellular iron levels independently of TfR1.
Materials and methods
Plasmids and subcloning
The pCB6 plasmids encoding HFE-GFP-LDLR and HFE-GFP were gifts from Dr Pamela Bjorkman (California Institute of Technology, Pasadena). The HFE constructs were subcloned via Asp718/HindIII digestion into the pCDNA3.1+ hygro plasmid (Invitrogen, BV Carlsbad, CA). The HFE-GFP-LDLR construct contains the entire coding sequence of HFE, a Leu/Gln linker region, enhanced green fluorescent protein (EGFP), and the cytoplasmic domain of the LDLR (Lys 790-Ala 839).
Cell culture
TRVb cells (which express no endogenous TfR1), TRVb1 cells (TRVb transfected with human TfR1),19 and TRVb32-8 cells (TRVb transfected with a mutated TfR1 lacking amino acids 3-59 of the cytoplasmic domain)20 were gifts from Dr Timothy McGraw (Cornell Medical College, Ithaca, NY). The TRVb1/HFE/β2M and TRVb1/HFE-GFP/β2M cell lines were generated by cotransfection of TRVb1 cells with pCDNA 3.1 hygro+ HFE and pBA encoding β2M (gift from Dr John Feder, Bristol-Meyers Squibb, Princeton, NJ), or with pCDNA3.1 hygro+ HFE-GFP and pBA β2M. The TRVb32-8/HFE-LDLR-GFP/β2M cell line was generated by cotransfection of TRVb32-8 cells with pCDNA3.1 hygro+ HFE-LDLR-GFP and pBA β2M. TRVb/HFE/β2M cells were generated by cotransfection of TRVb cells with pCDNA3.1 hygro+ HFE and pBA β2M. Cells were selected with hygromycin (300 μg/mL) and subcloned once. TRVb cells were maintained in F12 medium (Sigma, St Louis, MO) supplemented with 10% fetal bovine serum (FBS; Hyclone, Logan, UT), 2 mg/mL glucose, and 10 μM ferric Fe+3 nitrilotriacetate (Fe-NTA). TRVb1 cells and TRVb/HFE/β2M were maintained in the same growth medium with 400 μg/mL G418 (Geneticin; Calbiochem, San Diego, CA) and 300 μg/mL hygromycin (Sigma), respectively. TRVb32-8/HFE-LDLR-GFP/β2M, TRVb1/HFE-GFP/β2M, and TRVb1/HFE/β2M cell lines were maintained in growth medium with both G418 and hygromycin.
For Tf or Fe-NTA treatments, cells were incubated with 1 to 3 mg/mL (12.5-37.5 μM) human diferric Tf (Intergen, Chicago, IL) or 150 μM Fe-NTA for 24 hours to load cells to induce similar levels of ferritin, then analyzed as described in the following analysis. Fe-NTA was always freshly prepared at a ratio of 1:40 as described previously.17 At this ratio of Fe to NTA the iron remains in solution longer. Treatment of cells over this time period did not alter their growth or viability (data not shown). The concentrations given in the text refer to the concentration of Fe in the solution. Tf saturation was confirmed by measuring the solution absorbance at 465 and 280 nm (100% saturation OD465/OD280 = 0.045).
Immunodetection
Subconfluent cells grown in 35-mm dishes were washed twice with phosphate-buffered saline (PBS; pH 7.4) and solubilized with lysis buffer (20 mM HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid) pH 7.4, 1 mM sodium citrate, 0.5% (vol/vol) NP-40, 10 mM sodium pyrophosphate, 50 mM β-glycerophosphate, 50 mM sodium fluoride, 5 mM EDTA (ethylenediaminetetraacetic acid), and 1 mM sodium orthovanadate plus 2 mM benzamidine, 40 μg/mL leupeptin, 40 μg/mL soybean trypsin inhibitor, 0.2 mM phenylmethylsulfonyl fluoride (PMSF), and 4 μg/mL pepstatin on ice. Lysates were centrifuged at 13 000g in a microcentrifuge for 5 minutes at 4°C to remove nuclei and assayed for total protein by bicinchoninic acid protein assay (Pierce, Rockford, IL). Lysates (normalized to total protein) were denatured by incubation with an equal volume of 2 × Laemmli buffer (125 mM Tris (tris(hydroxymethyl)aminomethane)–HCl [pH 6.8], 4% sodium dodecyl sulfate [SDS], 20% glycerol, and 10% 2-mercaptoethanol21 for 5 minutes at 94°C prior to electrophoresis on 12% polyacrylamide gels under reducing conditions. The proteins were transferred to nitrocellulose, checked for protein loading by PonceauS staining, and blocked with 5% nonfat milk in 0.1 M Tris-HCl, 0.15 M NaCl, pH 7.4, 0.05% Tween-20 buffer. Immunoblot analysis was performed using sheep anti-TfR1 serum (1:5000 dilution),22 mouse monoclonal anti–β-actin (1:2000; Chemicon, Temecula, CA), rabbit anti-HFE serum (1:1000 dilution; Dr Pamela Bjorkman, Caltech), sheep anti–human ferritin (1:100 dilution; The Binding Site Ltd, Birmingham, United Kingdom), 1:10 000 no. 137 rabbit anti-HFE serum (Dr John Feder, Bristow Meyers-Squibb, Princeton, NJ), and mouse anti-β2M (1:10 000 dilution; Immunotech, Cedex, France) followed by the appropriate secondary antibody conjugated to horseradish peroxidase and chemiluminescence (SuperSignal; Pierce, Rockford, IL).
Soluble HFE treatment
TRVb and TRVb1 cells were treated with 0.5 μM soluble HFE (sHFE; a kind gift from Dr Pamela Bjorkman, Caltech) overnight as previously described23 with or without the addition of 150 μM Fe-NTA. The effects of sHFE on iron homeostasis in these cells were evaluated by immunodetection of ferritin.
Flow cytometry analysis
Cells were washed twice with PBS (pH, 7.4) and released from the dish with dissociation buffer (Sigma). Cells (1 × 106/mL) were incubated with Ham F12 medium containing 10% FBS with or without 1 μg/mL to 10 mg/mL (12.5 nM-125 μM) diferric Tf for 30 minutes at 37°C in 5% CO2. After treatment, cells were centrifuged for 5 minutes at 1000g at 4°C, washed once with Hanks buffer supplemented with 3% FBS and 10 mM HEPES, and then incubated with monoclonal anti-TfR1 (1:200 dilution) or rabbit anti-HFE serum (1:200 dilution; Dr Pamela Bjorkman) for 30 minutes at 4°C to bind cell-surface TfR1 or HFE. After washing, the cells were incubated with phycoerythrin-conjugated anti–mouse immunoglobulin G (IgG; 1:200 dilution; Caltag, Burlingame, CA) or anti–rabbit (1:200 dilution) antibodies for 30 minutes at 4°C. Following a final wash with supplemented Hanks buffer, flow cytometric analysis was performed in a Becton Dickinson FACSCalibur flow cytometer. The median fluorescence of the IgG1 control at the same concentration as the monoclonal anti-TfR1 was less than one tenth of the anti-TfR1 signal. The median fluorescence of rabbit preimmune serum at the same dilution as the anti-HFE serum was subtracted from the HFE-positive signal.
Electrophoretic mobility shift assay
Iron-responsive element (IRE) RNA binding activity was quantitated as described previously.24,25 Cells were pretreated with 1 mg/mL (∼ 12.5 μM) diferric Tf, 150 μM Fe-NTA, or 100 μM desferal overnight. RNA binding assays were performed at 4°C with cell lysate (10 μg total protein) in 25 μL incubated with 25 μL binding buffer (5% glycerol, 1 mM magnesium acetate, 20 mM HEPES pH 7.6, 20 μg/mL nuclease-free bovine serum albumin) containing 50 000 cpm of 32P-labeled RNA with or without 2.4% 2-mercaptoethanol (2ME). After 30 minutes, 0.45 mg/mL heparin was added, and the mixture was incubated on ice for another 10 minutes. The complex was resolved on 7% polyacrylamide (60:1 acrylamide/bis) gels, and bands were detected and quantitated on a PhosphorImager (Amersham, Piscataway, NJ) using IP Lab Gel 1.5 software.
55Fe+2 uptake assay
Reduction of 55Fe+3 to 55Fe+2 was performed according to an established method26 with some modification. Briefly, 55FeCl3 (Perkin Elmer Life Sciences, Boston, MA) was incubated with ascorbate at a ratio of 1:100 for about 20 minutes to allow complete reduction of 55Fe+3 to 55Fe+2. Incubation buffer (25 mM Tris, 140 mM NaCl, 5.4 mM KCl, 5 mM glucose, 25 mM 2-N-morpholino ethane sulfonic acid, 1.8 mM CaCl2, pH 5.5) was then made up to a final concentration of 10 μM 55Fe using the freshly prepared 55Fe+2 and immediately added to subconfluent cells in 35-mm dishes. The cells were incubated at 37°C in a 5% CO2 atmosphere, and 55Fe+2 uptake was measured at 0, 15, 30, and 60 minutes. Cells were washed 4 times with 2 mL ice-cold PBS plus 5 mM EDTA (Sigma) to remove surface-bound iron. Cellular 55Fe was measured after solubilization of cells in 0.1 N NaOH. The specific 55Fe+2 uptake by cells was determined by subtracting the nonspecific uptake in the corresponding samples incubated at 4°C. Having established that the uptake was linear for at least an hour, only the time point at 30 minutes was used for comparison between cell lines. Each point represents the average of 3 dishes.
To verify the stability of Fe+2, we used both ferrozine and 1,10-phenanthroline, both of which form specific complexes with Fe+2, to demonstrate by spectrophotometry that Fe+2 remained in its reduced and soluble form for at least 2 hours under the conditions of our experiments (data not shown). In stably transfected HeLa cells overexpressing divalent metal transporter-1 (DMT1), DMT1-mediated Fe+2 uptake was maximal at pH 5.5 and at 10 μM Fe+2 (data not shown). The rate of Fe+2 uptake was about 100-fold higher in DMT1-transfected HeLa cells than in untransfected parent cells and was not significantly altered by the presence of 40-fold excess NTA or 100-fold excess citrate (with respect to Fe+2 concentration) (data not shown). These results indicate that 55Fe+2 uptake measured by the methods described is ferrous iron specific and does not result from uptake of low molecular weight 55Fe+3 complexes, as has been reported in reticulocytes.27
Results
Tf competes with HFE for binding to the TfR
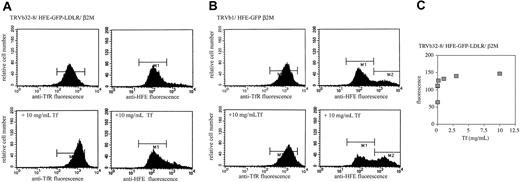
TRVb32-8 cells express a truncated form of human TfR1 lacking its internalization motif as well as most of the cytoplasmic domain.20 The truncated TfR1 does not concentrate in clathrincoated pits and internalizes 5- to 10-fold less efficiently than wild-type TfR1.20,28-30 TRVb32-8 cells were transfected with β2M and an HFE C-terminal chimera containing GFP followed by the cytoplasmic domain of the low-density lipoprotein receptor (LDLR), which contains a Tyr-based internalization signal. HFE has no obvious internalization sequence on its cytoplasmic domain and presumably internalizes via its association with TfR1.7 Thus, in the TRVb32-8/HFE-GFP-LDLR/β2M cell line, endocytosis of the truncated TfR1 is driven by its interaction with HFE-GFP-LDLR. In at least 2 cell lines approximately 25% of the TfR1 pool is present on the cell surface and 75% in internal compartments.22,29 Thus, if Tf effectively competes with HFE for binding to the truncated TfR1, TfR1 would be expected to redistribute to the cell surface upon incubation of TRVb32-8/HFE-GFP-LDLR/β2M cells with diferric Tf. The population of cells expressing HFE (GFP-positive cells) was analyzed for cell surface TfR1 by labeling cells at 4°C with a monoclonal antibody to TfR1. In agreement with our hypothesis, preincubating these cells with Tf caused an increase in TfR1 on the cell surface (Figure 1A). Tf did not interfere with the binding of the monoclonal antibody to TfR1 by either flow cytometry or immunoprecipitation experiments (results not shown). Cell surface TfR1 was 2.7-fold higher following incubation with Tf compared with incubation in the absence of Tf (Table 1). Since the HFE chimera carries the internalization signal, Tf would not be expected to affect the distribution of HFE; as predicted, no redistribution of HFE was observed (Figure 1A; Table 1). The redistribution of TfR1 to the cell surface depended on the concentration of Tf, and more than one half of the maximal effect was detected at concentrations of Tf from 1 to 10 μg/mL (Figure 1C). Conversely, when TfR1 has an intact internalization signal and HFE does not, then incubation of cells with Tf would be expected to displace HFE from TfR1 and lead to the accumulation of HFE on the cell surface without affecting the distribution of TfR1. To test this case, TRVb1 cells expressing wild-type human TfR1 were transfected with human β2M, and an HFE chimera containing GFP on its cytoplasmic domain and GFP-positive cells were analyzed for cell surface HFE by incubating the cells at 4°C with an antibody to HFE. Incubation of cells with Tf at 37°C prior to flow cytometry analysis resulted in an increase in the level of HFE at the cell surface but had no effect on the amount of TfR1 at the cell surface (Figure 1B; Table 1). The increase in fluorescence at the cell surface still showed a competition between Tf and HFE for binding but was not as pronounced as in the TRVb32-8/HFE-GFP-LDLR/β2M cells. This observation is most likely explained by both cell lines expressing more HFE than TfR1, so that when HFE relies on TfR1 for internalization some of the excess HFE will remain on the cell surface, causing a lower relative increase in fluorescence than when HFE carries the internalization signal and there is no excess of TfR1.
Tf competes with HFE for binding to TfR1. Cells were incubated in the presence or absence of 10 mg/mL (∼ 125 μM) Tf and subjected to flow cytometry analysis as described in “Materials and methods,” using antibodies to detect TfR1 and HFE in separate experiments. (A) Increase in cell-surface TfR1 but not HFE in TRVb32-8/HFE-GFP-LDLR/β2M cells incubated with Tf. TRVb32-8 cells express a form of TfR1 lacking the endocytic signal, so internalization of this mutant TfR1 is dependent on interaction with HFE-GFP-LDLR, in which the cytoplasmic domain of LDLR mediates constitutive endocytosis. (B) Increase in cell-surface HFE but not TfR1 in TRVb1/HFE-GFP/β2M cells incubated with Tf. (C) Concentration dependence of the change in cell surface TfR1 in TRVb32-8/HFE-GFP-LDLR/β2M cells incubated with Tf. Cells were incubated in complete medium containing 0, 0.001, 0.01, 0.1, 1, 3, or 10 mg/mL diferric Tf, and cell surface TfR1 was measured by flow cytometry (the values for 0.001 and 0.01 were nearly identical and appear as a single point in this particular experiment). These experiments were repeated 3 times using 3 different clones for each cell line with similar results.
Tf competes with HFE for binding to TfR1. Cells were incubated in the presence or absence of 10 mg/mL (∼ 125 μM) Tf and subjected to flow cytometry analysis as described in “Materials and methods,” using antibodies to detect TfR1 and HFE in separate experiments. (A) Increase in cell-surface TfR1 but not HFE in TRVb32-8/HFE-GFP-LDLR/β2M cells incubated with Tf. TRVb32-8 cells express a form of TfR1 lacking the endocytic signal, so internalization of this mutant TfR1 is dependent on interaction with HFE-GFP-LDLR, in which the cytoplasmic domain of LDLR mediates constitutive endocytosis. (B) Increase in cell-surface HFE but not TfR1 in TRVb1/HFE-GFP/β2M cells incubated with Tf. (C) Concentration dependence of the change in cell surface TfR1 in TRVb32-8/HFE-GFP-LDLR/β2M cells incubated with Tf. Cells were incubated in complete medium containing 0, 0.001, 0.01, 0.1, 1, 3, or 10 mg/mL diferric Tf, and cell surface TfR1 was measured by flow cytometry (the values for 0.001 and 0.01 were nearly identical and appear as a single point in this particular experiment). These experiments were repeated 3 times using 3 different clones for each cell line with similar results.
Summary of flow cytometry data
. | TfR1 . | TfR1 + 10 mg/mL Tf . | HFE . | HFE + 10 mg/mL Tf . | Fold increase, TfR1 . | Fold increase, HFE . |
|---|---|---|---|---|---|---|
| TRVb 32-8/HFE-GFP-LDLR/β2M | 376 ± 18 | 1028 ± 143 | 162 ± 16 | 222 ± 35 | 2.7 | 1.3 |
| TRVb1/HFE-GFP/β2M | 1014 ± 84 | 1250 ± 73 | 208 ± 13 | 376 ± 25 | 1.2 | 1.8 |
. | TfR1 . | TfR1 + 10 mg/mL Tf . | HFE . | HFE + 10 mg/mL Tf . | Fold increase, TfR1 . | Fold increase, HFE . |
|---|---|---|---|---|---|---|
| TRVb 32-8/HFE-GFP-LDLR/β2M | 376 ± 18 | 1028 ± 143 | 162 ± 16 | 222 ± 35 | 2.7 | 1.3 |
| TRVb1/HFE-GFP/β2M | 1014 ± 84 | 1250 ± 73 | 208 ± 13 | 376 ± 25 | 1.2 | 1.8 |
Data are changes in cell surface TfR and HFE (mean fluorescence units). These results were determined from 3 independent experiments for each cell line.
HFE expression in TRVb1 cells decreases intracellular ferritin levels
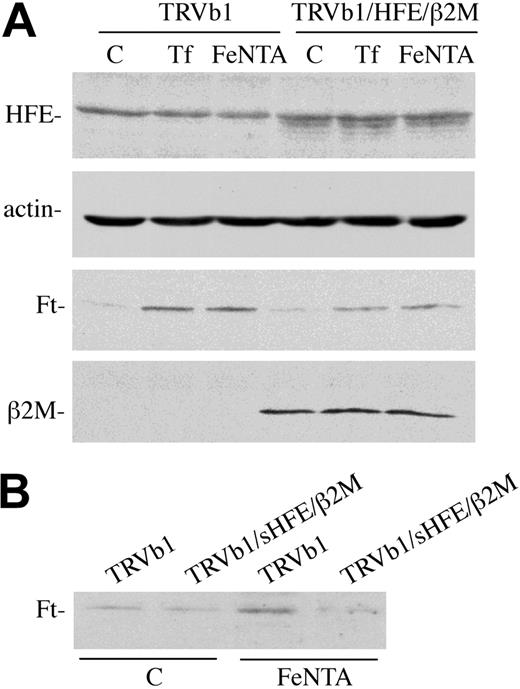
Expression of exogenous HFE lowers intracellular iron stores and decreases ferritin levels in HeLa and HEK293 cells,15,23 whereas in some monocytic and a colonic cell line, HT29, it increases ferritin levels.31,32 We, therefore, used 3 methods to examine the effect of HFE expression on the iron status of TRVb1 cells. In the first method, qualitative observations were made on the effect of HFE/β2M on the ferritin levels of the cell lines examined. Ferritin levels in TRVb1 and TRVb1/HFE/β2M cells are very low, but when similar ferritin levels were achieved by the addition of either 3 mg/mL diferric Tf or 150 μM Fe-NTA to the medium, HFE expression significantly decreased ferritin levels relative to untransfected controls. Ferritin levels were not only lower in diferric Tf-treated TRVb1/HFE/β2M cells compared with TRVb1 cells but also in Fe-NTA–treated cells (Figure 2A). Ferritin levels increased in TRVb1 cells with both Tf and Fe-NTA treatment. To differentiate between the possibilities that the lower ferritin levels seen were due to clonal variation and that HFE/β2M expression was responsible for the lower ferritin levels detected in the TRVb1 HFE/β2M cells, TRVb1 cells were incubated with soluble HFE (sHFE) overnight. Treatment of cells with sHFE decreased ferritin levels in TRVb1 cells (Figure 2B), ruling out the chance that the variation in ferritin levels between clonal cell lines was the cause in the differences in ferritin levels. These results indicate that HFE/β2M expression can regulate the accumulation of iron derived from both Tf-mediated and non–Tf-mediated uptake mechanisms.
HFE decreases intracellular ferritin levels in TRVb1 cells. (A) HFE expression. TRVb1 or TRVb1/HFE/β2M cells were left untreated or incubated with 3 mg/mL diferric Tf or 150 μM Fe-NTA for 24 hours prior to harvesting. Cell lysates (45 μg total protein) were subjected to SDS–polyacrylamide gel electrophoresis (PAGE), transferred to nitrocellulose, and probed with antibodies to TfR1, actin, HFE (no. 137), ferritin (Ft), or β2M. The no. 137 rabbit anti-HFE serum gives a nonspecific band just above the HFE doublet. Actin was used as a loading control. (B) sHFE treatment. TRVb1 cells were treated with 0.5 μM sHFE overnight in the presence (Fe-NTA) or absence (C) of 150 μM Fe-NTA. Cell lysates were then prepared and used for immunodetection of ferritin levels as described for panel A. These experiments were repeated 3 times using 3 different clones for each cell line with similar results.
HFE decreases intracellular ferritin levels in TRVb1 cells. (A) HFE expression. TRVb1 or TRVb1/HFE/β2M cells were left untreated or incubated with 3 mg/mL diferric Tf or 150 μM Fe-NTA for 24 hours prior to harvesting. Cell lysates (45 μg total protein) were subjected to SDS–polyacrylamide gel electrophoresis (PAGE), transferred to nitrocellulose, and probed with antibodies to TfR1, actin, HFE (no. 137), ferritin (Ft), or β2M. The no. 137 rabbit anti-HFE serum gives a nonspecific band just above the HFE doublet. Actin was used as a loading control. (B) sHFE treatment. TRVb1 cells were treated with 0.5 μM sHFE overnight in the presence (Fe-NTA) or absence (C) of 150 μM Fe-NTA. Cell lysates were then prepared and used for immunodetection of ferritin levels as described for panel A. These experiments were repeated 3 times using 3 different clones for each cell line with similar results.
HFE expression in TRVb1 cells increases the binding of IRP to IRE
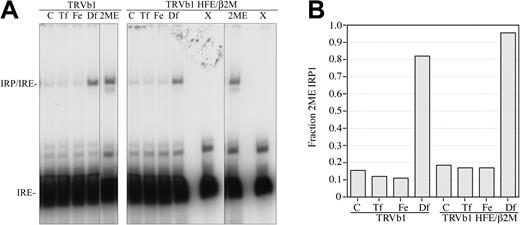
The lower ferritin levels in TRVb1 cells expressing HFE/β2M suggested that the cells had diminished intracellular iron. Ferritin synthesis is regulated by iron regulatory proteins (IRPs; reviewed by Hentze and Kuhn33 ). Electrophoretic mobility shift assays were used as a second method as an indicator of intracellular iron status. The amount of IRPs binding to the IREs of ferritin mRNA reflects intracellular iron status. We, therefore, evaluated the effect of HFE/β2M expression on IRP binding to IREs in TRVb1 cells. Cell extracts normalized to total protein were mixed with a 32P-labeled IRE probe containing the 5′ untranslated region of L-ferritin. The expression of HFE/β2M in TRVb1 cells increased the fraction of IRPs bound to IREs compared with TRVb1 cells, indicating a lower intracellular iron pool, relative to controls (Figure 3). Treatment of both TRVb1 and TRVb1/HFE/β2M cells with 1 mg/mL diferric Tf or 150 μM Fe-NTA resulted in decreased IRE/IRP binding relative to total IRPs detected when extracts were treated with 2-mercaptoethanol, but, even in the presence of high concentrations of Tf or Fe-NTA, IRE/IRP fraction was slightly higher in TRVb1/HFE/β2M than in TRVb1 cells. These results indicate that HFE can play a role in decreasing the iron pool in the presence of concentrations of Tf that would be expected to diminish the TfR1-HFE interaction and are consistent with our flow cytometry analysis showing competition between Tf and HFE over a wide range of Tf concentrations.
HFE expression in TRVb1 cells increases binding of IRP to IRE. Cells were left untreated (C) or incubated with 1 mg/mL diferric Tf (Tf), 150 μM Fe-NTA (Fe), or 100 mM desferal (Df) for 16 to 24 hours prior to harvesting. (A) Gel shift assay. Cell lysates (10 μg total protein) were incubated with approximately 50 000 cpm of 32P-labeled RNA probe, and the reaction mixtures were resolved on 7% polyacrylamide (60:1 acrylamide/bis) gels. The lysates of cells treated with desferal were also mixed with incubation buffer containing 2-mercaptoethanol to measure maximal IRP binding in each cell line (2ME). A separate lane was loaded with probe alone as a control (X). Samples containing 2ME were run at the same time on a separate gel. Gels were exposed to x-ray film for the same period of time. (B) Quantitation of the bands in panel A. The radioactivity in each band was detected and quantitated on a PhosphorImager (Amersham, Piscataway, NJ) using IP Lab Gel 1.5 software. The amount of radioactivity in each band was normalized to the total IRP binding (2ME-treated extracts of Df-treated cells) in each cell line. This experiment was repeated once for each cell line with a similar result.
HFE expression in TRVb1 cells increases binding of IRP to IRE. Cells were left untreated (C) or incubated with 1 mg/mL diferric Tf (Tf), 150 μM Fe-NTA (Fe), or 100 mM desferal (Df) for 16 to 24 hours prior to harvesting. (A) Gel shift assay. Cell lysates (10 μg total protein) were incubated with approximately 50 000 cpm of 32P-labeled RNA probe, and the reaction mixtures were resolved on 7% polyacrylamide (60:1 acrylamide/bis) gels. The lysates of cells treated with desferal were also mixed with incubation buffer containing 2-mercaptoethanol to measure maximal IRP binding in each cell line (2ME). A separate lane was loaded with probe alone as a control (X). Samples containing 2ME were run at the same time on a separate gel. Gels were exposed to x-ray film for the same period of time. (B) Quantitation of the bands in panel A. The radioactivity in each band was detected and quantitated on a PhosphorImager (Amersham, Piscataway, NJ) using IP Lab Gel 1.5 software. The amount of radioactivity in each band was normalized to the total IRP binding (2ME-treated extracts of Df-treated cells) in each cell line. This experiment was repeated once for each cell line with a similar result.
HFE acts independently of TfR1 to lower ferritin levels in TRVb cells
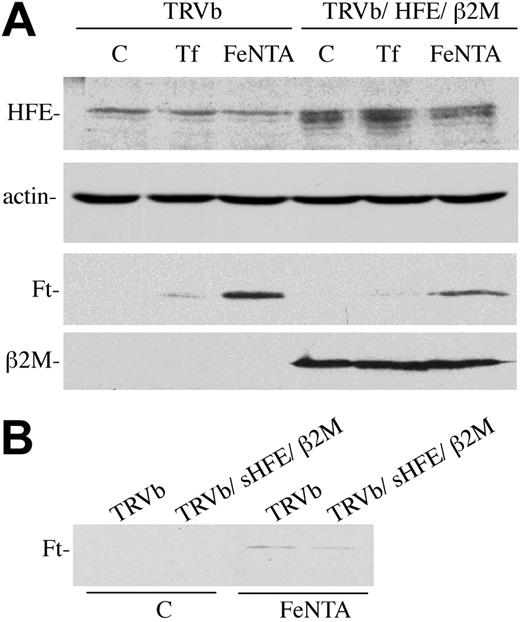
The lack of endogenous transferrin receptors in TRVb cells allowed us to determine whether HFE/β2M acted through association with TfR1 or independently of TfR1 in decreasing intracellular iron levels. Cells were transfected with HFE and β2M and treated with 3 mg/mL diferric Tf or 150 μM Fe-NTA for 24 hours (Figure 4A), and ferritin levels were used as a qualitative marker of intracellular iron levels. In the absence of TfR1, cells expressing HFE and β2M had lower ferritin levels than the parental TRVb cells. The effect was most pronounced in the Fe-NTA–treated cells. Since TRVb cells do not express endogenous TfR1, they are not expected to respond to Tf at all; the slight effect that was observed is probably due to nonspecific pinocytosis of Tf and release of iron at such a high concentration of Tf. Incubation of TRVb cells overnight with soluble HFE also resulted in lowered ferritin levels (Figure 4B), indicating that the lower ferritin levels were not a result of clonal variation of ferritin within cells.
HFE lowers ferritin levels in TRVb cells. (A) HFE expression. TRVb or TRVb/HFE/β2M cells were left untreated (C) or incubated with 3 mg/mL diferric Tf or 150 mM Fe-NTA for 24 hours prior to harvesting. Cell lysates (30 μg total protein) were subjected to SDS-PAGE, transferred to nitrocellulose, and probed with antibodies to HFE (no. 137), actin, Ft, or β2M. The no. 137 rabbit anti-HFE serum gives a nonspecific band just above the HFE doublet. Actin was used as a loading control. (B) sHFE treatment. TRVb cells were treated with 0.5 μM sHFE overnight in presence (Fe-NTA) or absence (C) of 150 μM Fe-NTA. Cell lysates were then prepared and used for immunodetection of ferritin levels as described in panel A. This experiment was repeated 3 times using 3 different clones for each cell line with similar results.
HFE lowers ferritin levels in TRVb cells. (A) HFE expression. TRVb or TRVb/HFE/β2M cells were left untreated (C) or incubated with 3 mg/mL diferric Tf or 150 mM Fe-NTA for 24 hours prior to harvesting. Cell lysates (30 μg total protein) were subjected to SDS-PAGE, transferred to nitrocellulose, and probed with antibodies to HFE (no. 137), actin, Ft, or β2M. The no. 137 rabbit anti-HFE serum gives a nonspecific band just above the HFE doublet. Actin was used as a loading control. (B) sHFE treatment. TRVb cells were treated with 0.5 μM sHFE overnight in presence (Fe-NTA) or absence (C) of 150 μM Fe-NTA. Cell lysates were then prepared and used for immunodetection of ferritin levels as described in panel A. This experiment was repeated 3 times using 3 different clones for each cell line with similar results.
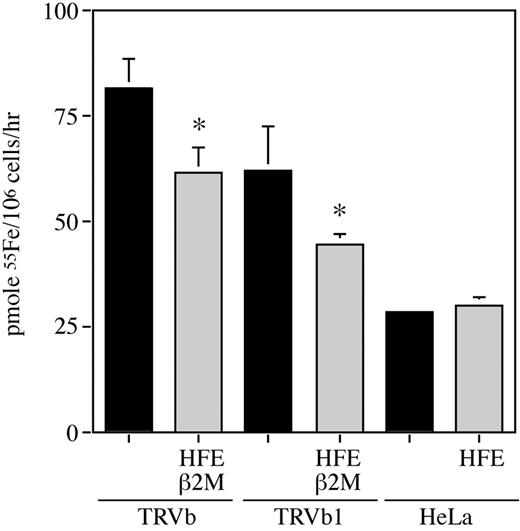
Expression of HFE in TRVb cells decreases ferrous iron accumulation in cells
To determine the mechanism by which HFE/β2M mediates lower intracellular iron levels in cells, iron uptake studies were performed. Cells were incubated in buffer containing 10 μM 55Fe and 1 mM ascorbic acid (at pH 5.5, which is the optimal pH for DMT1-mediated iron uptake) for 30 minutes before being washed and lysed; intracellular 55Fe was measured by liquid scintillation counting of the lysates. Expression of HFE significantly decreased ferrous iron accumulation in both TRVb and TRVb1 cells but not in HeLa cells (Figure 5). These results indicate that HFE/β2M regulates iron accumulation in TRVb cells differently than in HeLa cells even though both cell lines have lower intracellular iron levels in response to HFE expression. Quantitative polymerase chain reaction (PCR) analysis did not reveal any difference in DMT1 levels in response to HFE expression in TRVb or HeLa cells (data not shown), which implies that HFE might regulate iron uptake via a post-translational mechanism or act on an as yet unidentified protein involved in iron uptake. Because we measured iron uptake for 30 minutes, HFE could be increasing iron efflux from cells. No detectable iron efflux could be measured from these cells (results not shown).
Expression of HFE in TRVb cells decreases Fe+2 uptake. Cells (∼ 1 × 106 in 35-mm dishes) were incubated with 1 mL incubation buffer (25 mM Tris, 140 mM NaCl, 5.4 mM KCl, 5 mM glucose, 25 mM 2-N-morpholino ethane sulfonic acid, 1.8 mM CaCl2, pH 5.5) containing 1 mM ascorbic acid and 10 μM 55Fe+2 for 30 minutes at 37°C, washed with ice-cold PBS plus 5 mM EDTA, and lysed in 0.1 M NaOH. 55Fe uptake was determined by liquid scintillation counting of the lysates. Student t test demonstrated that ferrous iron uptake in the TRVb cells was significantly higher than in cells expressing TRVb/HFE/β2M (*P < .01). The same finding was true in TRVb1 cells versus TRVb1/HFE/β2M. This experiment was repeated 4 times using 3 different clones for each cell line with similar results. Error bars indicate standard deviation (SD).
Expression of HFE in TRVb cells decreases Fe+2 uptake. Cells (∼ 1 × 106 in 35-mm dishes) were incubated with 1 mL incubation buffer (25 mM Tris, 140 mM NaCl, 5.4 mM KCl, 5 mM glucose, 25 mM 2-N-morpholino ethane sulfonic acid, 1.8 mM CaCl2, pH 5.5) containing 1 mM ascorbic acid and 10 μM 55Fe+2 for 30 minutes at 37°C, washed with ice-cold PBS plus 5 mM EDTA, and lysed in 0.1 M NaOH. 55Fe uptake was determined by liquid scintillation counting of the lysates. Student t test demonstrated that ferrous iron uptake in the TRVb cells was significantly higher than in cells expressing TRVb/HFE/β2M (*P < .01). The same finding was true in TRVb1 cells versus TRVb1/HFE/β2M. This experiment was repeated 4 times using 3 different clones for each cell line with similar results. Error bars indicate standard deviation (SD).
Discussion
In vitro experiments using truncated forms of TfR and HFE11,12 or solubilized full-length HFE and TfR17 have demonstrated that Tf and HFE compete with each other for binding to TfR1. These studies also indicated that at concentrations of diferric Tf in the blood (∼ 1-4 mg/mL; ∼ 12.5-60 μM) no HFE would be expected to bind TfR1. Since HFE and TfR1 are integral membrane proteins, the possibility exists that the effective concentration of HFE in the vicinity of TfR1 is greater than that of soluble proteins such as Tf. We tested this possibility in living cells using CHO-derived cell lines expressing either wild-type or truncated TfR1 together with either wild-type HFE or a chimeric HFE fused to the LDLR cytoplasmic domain. In these experiments, Tf did not affect the cell-surface levels of whichever protein carried the internalization signal (wild-type TfR1 or HFE-LDLR), but caused a redistribution to the surface of its binding partner (TfR1-32/8 or wild-type HFE), demonstrating direct competition between soluble Tf and cell-surface HFE for binding to cell-surface TfR1. These observations are in agreement with previous in vitro data7,12,18 and imply that Tf can compete with HFE for binding to TfR1 even in tissues in which Tf levels could be orders of magnitude lower than the levels in serum. Both Kupffer cells and hepatocytes are exposed to serum concentrations of Tf. Such concentrations of Tf would be expected to reduce the association between HFE and TfR1, but as Figure 1C shows, it does not completely abrogate it: approximately 16% of the total change in fluorescence occurs between 0.1 and 3 mg/mL diferric Tf. Thus, even in the liver, competition between HFE and Tf for TfR1 binding may be relevant to iron homeostasis.
The results from the inhibition of ferrous iron uptake in cells expressing HFE/β2M but not TfR1 strongly indicate that HFE regulates ferritin and intracellular iron levels by a mechanism independent of its interaction with TfR1. They are in agreement with studies showing that the W81A HFE mutant (which has a greatly reduced affinity for TfR1) has the same effect as wild-type HFE on the iron phenotype of HeLa cells17 and with evidence for a TfR1-independent effect on macrophage iron phenotype.31 These data lead us to speculate that the target(s) of HFE action might be involved in iron import. We, therefore, examined whether HFE affects Fe2+ uptake, which is mediated by iron transporters such as DMT1. In the TRVb cells HFE/β2M expression inhibits ferrous iron accumulation over 30 minutes. These results differ from the results that we and other investigators observed in HeLa cells in which HFE inhibits Tf-mediated iron uptake,15,24,34,35 HEK293,36 and H129916 cells. The degree to which HFE expression inhibits Fe+2 accumulation is, however, comparable to the decrease in Tf-mediated iron uptake in HeLa cells.
Our results differ from those of Waheed et al,37 who found that, while expression of wild-type HFE lowered intracellular iron levels in TRVb1 cells, simultaneous overexpression of β2M resulted in a higher iron phenotype. This led the investigators to conclude that the lower iron phenotype somehow resulted from a lack of β2M expression. More recent results, however, are consistent with our findings: cotransfection with β2M did not alter the effect of HFE on iron phenotype in H1299 cells.16 Further, our earlier studies show that in HeLa cells, which show a lower iron phenotype when overexpressing exogenous HFE, β2M levels increase as HFE expression increases.17
A growing body of evidence indicates that the effect of HFE expression in a variety of cell lines and in different tissues is cell-type dependent. Expression of wild-type HFE lowers intracellular iron levels in TRVb, HEK293, HeLa, and H1299 cells.16 It does so in TRVb cells by lowering Fe+2 accumulation, whereas in HeLa cells it lowers Tf-mediated iron uptake.15,35 In contrast, in macrophages,38 in the macrophage-derived THP1 cell line31 and in a colonic carcinoma cell line, HT29,32 HFE expression results in the accumulation of iron resulting in higher ferritin levels within the cell. The accumulation of iron is the consequence of an inhibition of iron efflux from these cell types. Differences in response to HFE have been recorded even between cells of similar lineage, whereas macrophage-like THP-1 cells show increased ferritin and decreased TfR1 levels, monocyte-like U937 cells show the opposite response when treated with soluble HFE or infected with recombinant vaccinia expressing HFE.31 Cell-type specificity in vivo has been observed as well. In the early stages of HH due to the C260Y HFE mutation, hepatocytes accumulate excess iron, whereas Kupffer cells are iron-deficient (reviewed by Chorney et al39 ). Both cell types normally express HFE.40,41
Since HFE can result in either the accumulation or depletion of intracellular iron stores, we speculate that it can interact with either the proteins involved in the import or export of iron and that the differences seen between cell lines and in tissues depends on the ratios of exporters and importers and TfR1. HFE appears to cause accumulation of intracellular iron in cell lines that exhibit active iron efflux.31,38 In the absence of evidence for iron efflux in ovary-derived cells our present results showing that expression of HFE/β2M lowers intracellular iron stores in TRVb cells are consistent with this speculation. In this context it is important to note that TRVb cells were selected for their ability to survive without transferrin receptors, so that alternative mechanisms of iron acquisition may be up-regulated for cell survival. Such mechanisms may be relatively unimportant in HeLa cells, which take up about 5 times as much iron from Tf as from Fe-NTA,35 but they could be important in hepatocytes that have robust non-Tf mediated iron uptake. Both the relative contributions of the 2 pathways to iron acquisition and the potential for ferrous iron uptake to be up-regulated if Tf-mediated uptake drops may serve to mask any effect of HFE on non–Tf-mediated iron uptake in HeLa cells. In TRVb cells, the absence of Tf receptors may reveal HFE effects that cannot be observed in HeLa cells. This idea is consistent with the growing body of evidence indicating that HFE activity is cell-type specific and may depend on the relative levels and/or activities of other molecules involved in iron homeostasis. The challenge now becomes to identify the proteins responsible for the cell-type dependence of HFE effects on iron homeostasis.
Prepublished online as Blood First Edition Paper, November 4, 2004; DOI 10.1182/blood-2004-03-1204.
Supported by grants from the National Institutes of Health (R01NIDDK 54488 [C.A.E.] and HL 069133 [W.H.F.]) and by a grant from the National Heart, Lung, and Blood Institute (NHLBI) Training Program in Molecular Hematology (T32-HL00781) (H.C.).
H.C. and A.-S.Z. both contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Pamela Bjorkman for the HFE constructs and C. William Hooker for careful reading of the manuscript and helpful discussions. We also thank Dr Anthony Bakke for training on the Becton Dickinson GACScan flow cytometer, Emily L. Anderson for excellent technical assistance, Dr Timothy McGraw for the TRVb cell lines, and Dr John Feder for the HFE and β2M plasmid and antibody no. 137 to HFE.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal