Abstract
Autoimmune lymphoproliferative syndrome (ALPS) is a rare disorder of disrupted lymphocyte homeostasis. Clinical manifestations of ALPS vary but typically include autoimmune cytopenias, organomegaly, lymphadenopathy, and increased risk of malignancies. A similar spectrum of symptoms may be seen in some patients with Evans syndrome (ES), a hematologic disorder defined by autoimmune destruction of at least 2 hematologic cell types. We hypothesized that a subset of patients diagnosed with ES may have ALPS. We screened 12 children with ES by flow cytometric analysis for CD4-/CD8- (double negative) T cells (DNTs) and with the definitive test for ALPS, defective in vitro Fas-mediated apoptosis. Six of the patients had elevated DNTs, suggestive of ALPS and also had defective Fas-mediated apoptosis. The other 6 patients displayed normal T-cell apoptosis; 5 of whom had normal DNTs, and 1 had a borderline result. Thus, 7 (58%) of 12 patients with ES had elevated DNTs suggestive of ALPS, with functional confirmation in 6 of 7. This suggests that analysis of DNTs may be a sensitive first-line screening test, serving as a marker of patients who should undergo definitive testing for ALPS. Our data further suggest that a number of patients with ES may have ALPS, a novel finding with important therapeutic implications.
Introduction
Autoimmune lymphoproliferative syndrome (ALPS) is a recently described disorder of disrupted lymphocyte homeostasis. Patients with ALPS have mutations in the Fas apoptotic pathway, leading to abnormal lymphocyte survival, resulting in chronic lymphoproliferation and a breakdown in immunologic tolerance. ALPS was first characterized in 1992 in a group of patients who were found to have chronic lymphoproliferation, autoimmune manifestations, and an increased number of double-negative T cells (DNTs; cell phenotype CD4-/CD8-, CD3+, T-cell receptorαβ+ [TCRαβ+]).1 These patients shared similar clinical features with 2 mouse models of autoimmunity, lpr and gld. These mice were later found to have defective Fas-mediated apoptosis with homozygous mutations in the Fas gene and Fas ligand gene, respectively.2,3 Such patients with autoimmunity and lymphoproliferation were proposed4 and later confirmed5 to have similar genetic defects to the lpr and gld mice and were classified as having ALPS. ALPS is thought to be a rare condition, and since the original report of ALPS, only a few hundred cases have been reported.6
Apoptotic pathways are important for maintaining lymphocyte homeostasis by eliminating excess activated, antigen-driven, and autoreactive cells. Fas, a member of the tumor necrosis (TNF) receptor family, is normally highly expressed in activated B and T cells.7 Fas is activated by binding to the protein Fas ligand which is highly expressed in activated T lymphocytes. The interaction of Fas and Fas ligand leads to multiple intracellular reactions, culminating in the activation of the caspase cascade and cellular apoptosis. The Fas apoptotic pathway is crucial for the down-regulation of the immune response, and in its absence patients develop chronic lymphoid hyperplasia and autoimmunity.8 The majority of patients with ALPS have mutations in the Fas9 gene, but mutations in Fas ligand,10 caspase 8,11 and caspase 1012 have been found. No mutation is defined in up to 24% of patients.8,13,14
Clinical manifestations in patients with ALPS vary but typically include autoimmune cytopenias, lymphoproliferation with lymphadenopathy and organomegaly, and a propensity to develop secondary neoplasms. In the largest published series of patients with ALPS,15 all patients had lymphoproliferation and the majority but not all patients had autoimmune cytopenias. Risk of secondary malignancy is thought to approach 10%.15 Other infrequent manifestations include autoimmune liver and kidney disease and vasculitis.15 The current diagnostic criteria as proposed by the National Institutes of Health (NIH) ALPS group15 are listed in Table 1.
Diagnostic criteria for ALPS
Required |
| 1. Chronic nonmalignant lymphoproliferation |
| 2. Defective in vitro Fas-mediated lymphocyte apoptosis |
| 3. At least 1% TCR α/β+, CD3+, CD4-, CD8- cells (DNTs) in peripheral blood or lymphoid tissue |
| Supporting |
| 1. Autoimmune antibodies |
| 2. Mutations in Fas gene, Fas ligand gene, or caspase 8 or 10 genes |
| Adapted with permission from Bleesing et al.16 |
Required |
| 1. Chronic nonmalignant lymphoproliferation |
| 2. Defective in vitro Fas-mediated lymphocyte apoptosis |
| 3. At least 1% TCR α/β+, CD3+, CD4-, CD8- cells (DNTs) in peripheral blood or lymphoid tissue |
| Supporting |
| 1. Autoimmune antibodies |
| 2. Mutations in Fas gene, Fas ligand gene, or caspase 8 or 10 genes |
| Adapted with permission from Bleesing et al.16 |
An overlapping constellation of clinical findings can be found in patients with ALPS and patients with Evans syndrome (ES). In 1951, R.S. Evans first characterized a hematologic disorder consisting of multiple autoimmune cytopenias which was later named Evans syndrome.17 ES is defined as a disease in which patients have autoimmune destruction of at least 2 peripheral blood cell types. ES is a chronic relapsing disease associated with significant morbidity despite therapy.18 The underlying pathophysiology of ES is unknown but is thought to be secondary to generalized immune dysregulation. ES is a diagnosis of exclusion, and other confounding disorders must be ruled out before establishing the diagnosis.19 Infections, rheumatologic diseases, and malignancies can present with autoimmune cytopenias, and patients with these conditions do not, by definition, have ES.20 Patients with ES frequently have other symptoms in addition to their autoimmune manifestations, which may include lymphadenopathy, hepatomegaly, and splenomegaly. In one published series more than half of the patients with ES had evidence of lymphoid hyperactivity.21
On the basis of this clinical overlap between ALPS and ES, we hypothesized that a subset of patients diagnosed with ES may have ALPS. This is the first report examining patients with ES for ALPS.
Patients, materials, and methods
Patients
A review of clinical records identified 20 patients who were followed at the Children's Hospital of Philadelphia over the period 1999 to 2004 with a diagnosis of ES. Patients were eligible if they (1) carried the diagnosis of ES and (2) were being actively followed (defined as at least 1 visit in the past year). Patients were excluded from this study if autoimmune cytopenias were associated with systemic lupus erythematosus, malignancy, or treatment with immunosuppression following solid-organ transplantation. Similarly, patients with genetic abnormalities known to predispose to autoimmune cytopenias were excluded. Of the 20 identified patients with ES, 1 with DiGeorge syndrome, 1 with Kabuki syndrome, and 1 with Gaucher syndrome were excluded. Four patients were not available for enrollment and 1 had died. Thus, 12 patients with ES were enrolled on study. The research protocol was reviewed and approved by the institutional review board, and all enrolled patients or their parent/guardian signed the informed consent.
Flow cytometry for double-negative T cells
DNT analysis was performed at the clinical immunology laboratory at the Children's Hospital of Philadelphia (CHOP). The clinical immunology laboratory has received certification for quality assurance for performing high-complexity clinical testing under the Clinical Laboratory Improvement Amendments (CLIA 88). A normal control sample was run in tandem with each patient sample. The clinical immunology laboratory designed the testing protocol according to published techniques22 and evaluated 22 healthy adult control samples to establish normal parameters. Normal was defined as 2 standard deviations from the mean and 2.6% CD4-/CD8-, CD3+, TCRαβ+ cells was determined to be the threshold for the normal range using the gating strategy detailed in the following 2 paragraphs. In addition, the clinical laboratory periodically recalculated the mean and standard deviation based on series of tested controls. Thus far, more than 50 control samples have been tested with no change in mean or standard deviation.
Whole blood from each patient sample and normal control sample was stained in 2 tubes with monoclonal antibodies purchased from Beckman Coulter (Fullerton, CA). The first tube was an isotype control tube and contained anti-CD3–fluorescein isothiocyanate (FITC), mouse immunoglobulin G1 (IgG1)–RD1, and mouse IgG2a-PC5. The second tube contained anti-CD3–FITC, anti-CD4–RD1, anti-CD8–RD1, and anti-TCRαβ–PC5. After staining, the erythrocytes in each tube were lysed using the ImmunoPrep reagent system (Beckman Coulter) and the Coulter TQ-Prep Workstation (Beckman Coulter). The white blood cells in each tube were then analyzed on a Beckman Coulter Cytomics FC-500 flow cytometer.
A histogram of CD3-FITC versus side scatter was used to gate on the T cells (CD3+/low side scatter). The events in the T-cell gate for the isotype control tube for each patient or normal control sample were sent to a second histogram, plotting mouse IgG1-RD1 versus mouse IgG2a-PC5. Cursors were set based on the staining of the isotype control antibodies to allow for less than 2% false positives. The same cursor settings were then used for the specific antibody tube for the respective patient or normal control sample. The events in the T-cell gate for each specific antibody tube were sent to a second histogram plotting CD4-RD1 and CD8-RD1 versus TCRαβ-PC5. DNTs were the CD3+ cells that are CD4-/CD8- and TCRαβ+. The clinical immunology lab tested a normal control sample with every patient sample run. In addition, we performed DNT analysis on all of the healthy controls used in evaluating patients for defective Fas-mediated apoptosis (described in “Testing for Fas-mediated apoptosis”) to ensure no individual in our healthy control pool had elevated DNTs.
Testing for Fas-mediated apoptosis
Fas-mediated apoptosis was evaluated using published techniques.4,23,24 All patient samples were tested in tandem with a normal control sample. We used a panel of 10 different adult volunteers as healthy control subjects (12 total patients; 10 total control subjects). Each healthy control subject was used in 1 to 3 experiments. Peripheral blood mononuclear cells (PBMCs) were isolated by Ficoll gradient centrifugation and resuspended at a concentration of 106 cells/mL in RPMI 1640 with 10% fetal calf serum plus recombinant human interleukin 2 (rhIL-2; 25 U/mL; Leinco, St Louis, MO). To allow confirmatory testing, T cells were stimulated with 2 different mitogens. In one well, T cells were stimulated with phytohemagglutinin (PHA; 3 μg/mL) and restimulated with PHA (1 μg/mL) once between days 8 and 14. Apoptosis assays were performed 6 days after the second stimulation. In the second well, T cells were stimulated with phorbol 12-myristate 13-acetate (PMA; 10 ng/mL) and A23187 (Calcimycin; 500 ng/mL), and apoptosis assays were performed in 3 to 5 days. The published protocols assessing Fas-mediated apoptosis in the ALPS literature are based on PHA stimulation of T cells.4,23,24 Work has shown PMA and A23187 to be as effective in stimulating T cells for subsequently evaluating the Fas apoptotic pathway. PMA is a more potent mitogen, requiring a shorter culture period before performing assays.25 We compared the 2 mitogens in each sample. After stimulation, T cells were divided into aliquots into 24-well plates at a concentration of 50 000 cells per well. Cells were incubated with control media, anti-Fas monoclonal antibody (IgM, 10 μg/mL for PMA assay and 1 μg/mL for PHA assay; Upstate, Charlottesville, VA), steroid (either dexamethasone [10 μM; Sigma, St Louis, MO] or methylprednisolone [1 μM; Pharmacia, Piscataway, NJ]) or C2-ceramide (50 μMol; Sigma) for 24 to 48 hours. Induction of cell death was evaluated by trypan blue exclusion and verified with fluorescence activated cell sorting (FACS) analysis for Forward Scatter/Side Scatter (FSC/SSC) and 7-aminoactinomycin D (7-AAD; BD Pharmingen; San Diego, CA). Trypan blue exclusion was performed by adding 11 μL trypan blue dye (Cellgro, Herndon, VA) to 100 μL cell suspension and counting the number of viable cells using a Zeiss Axiovert 40C light microscope (Carl Zeiss, Thornwood, NY) equipped with an apochromatic 20 ×/0.30 objective lens. For FACS analysis, human lymphocytes both stimulated with mitogen and freshly obtained were used as isotype controls. Dead cells were defined as those displaying shrunken/hypergranular morphology on FSC/SSC and those that stained with 7-AAD. We used control-stimulated T cells treated with 100% ethanol to set parameters for dead cells on 7-AAD staining. To qualify as a positive test (consistent with death with stimuli) there must be at least both a 3-fold and a 40% increase in cell death by both FACS and Trypan blue.
Results
Twelve patients were enrolled in the study and were tested for ALPS. A summary of demographics and clinical findings are listed in Table 2. The majority of patients (8 of 12) had chronic disease with frequent exacerbations (> 2 times a year, requiring immunosuppressive medications). One third of the patients (4 of 12) had a history of lymphadenopathy (defined as noninfectious, nonmalignant enlargement of at least 2 nodal groups). Two of these patients had persistently enlarged lymph nodes greater than 2 cm in multiple (> 3) nodal regions. The other 2 patients had enlarged lymph nodes greater than 2 cm in multiple nodal regions; however, the enlarged nodes and the areas involved waxed and waned. One half (6 of 12) of the patients had a history of chronic organomegaly (enlarged for more than 6 months). All 4 patients with a history of lymphadenopathy also had a history of chronic organomegaly. Two patients, therefore, had isolated organomegaly. Two additional patients were noted to have splenomegaly during episodes of AIHA but did not have chronic organomegaly. One third (4 of 12) of the patients with splenomegaly had undergone splenectomy as part of the management of their cytopenias. Prior to splenectomy, all 4 of these patients had spleens which were chronically enlarged at least 3 to 4 cm. Of the other 2 patients with splenomegaly who did not undergo splenectomy, both had a persistently enlarged spleen at least 2 cm below the costal margin. Two patients had a history of intermittent hepatomegaly. All of these patients also had a history of chronic splenomegaly. One half (6 of 12) of the patients had a history of clinical chronic nonmalignant lymphoproliferation (lymphadenopathy and/or organomegaly), potentially consistent with ALPS. Twenty-five percent (3 of 12) of the patients had comorbid conditions, including atopy, nephrotic syndrome, and diabetes mellitus. No patient had a history of malignancy or life-threatening infection.
Clinical and demographic information with laboratory analysis
ID . | Age at Dx, y . | Age at enrollment, y . | AIHA . | ITP . | AIN . | Lymphadenopathy* . | Splenomegaly . | Hepatomegaly . | Therapy† . | DNT, % . | Apoptosis assay‡ . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 1 | 2 | Yes, R | Yes, C | Yes, C | No | No | No | IVIg, steroids | 7.6 | Defective |
| 2 | 11 | 18 | Yes, C | Yes, C | Yes, C | Yes | Yes | Yes | IVIg, steroids, splenectomy | 3.6 | Defective |
| 3 | 15 | 20 | Yes, C | Yes, C | Yes, C | Yes | Yes | No | IVIg, steroids, splenectomy | 5.1 | Defective |
| 5 | 13 | 13 | Yes, R | Yes, R | Yes, R | No | Yes§ | No | IVIg, steroids | 2.7 | Normal |
| 6 | 16 | 16 | No | Yes, R | Yes, R | No | No | No | None | 2.5 | Normal |
| 11 | 3 | 17 | Yes, C | Yes, C | No | No | Yes | Yes | CSA, steroids, splenectomy | 2.0 | Normal |
| 13 | 14 | 19 | Yes, C | Yes, C | Yes, C | No | No | No | IVIg, steroids, CSA | 1.1 | Normal |
| 17 | 4 | 11 | Yes, R | Yes, C | Yes, C | Yes | Yes | No | IVIg, steroids, sulfadoxine (Fansidar) | 10.5 | Defective |
| 18 | 6 | 19 | Yes, R | Yes, R | Yes, R | No | Yes§ | No | IVIg, steroids | 1.1 | Normal |
| 33 | 6 | 7 | Yes, C | Yes, C | Yes, C | Yes | Yes | No | IVIg, steroids | 8.9 | Defective |
| 38 | 10 | 14 | Yes, R | Yes, C | Yes, C | No | Yes | No | IVIg, steroids, rituximab, splenectomy | 7.7 | Defective |
| 39 | 17 | 18 | Yes, R | Yes, R | No | No | No | No | IVIg, steroids | 1.2 | Normal |
ID . | Age at Dx, y . | Age at enrollment, y . | AIHA . | ITP . | AIN . | Lymphadenopathy* . | Splenomegaly . | Hepatomegaly . | Therapy† . | DNT, % . | Apoptosis assay‡ . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 1 | 2 | Yes, R | Yes, C | Yes, C | No | No | No | IVIg, steroids | 7.6 | Defective |
| 2 | 11 | 18 | Yes, C | Yes, C | Yes, C | Yes | Yes | Yes | IVIg, steroids, splenectomy | 3.6 | Defective |
| 3 | 15 | 20 | Yes, C | Yes, C | Yes, C | Yes | Yes | No | IVIg, steroids, splenectomy | 5.1 | Defective |
| 5 | 13 | 13 | Yes, R | Yes, R | Yes, R | No | Yes§ | No | IVIg, steroids | 2.7 | Normal |
| 6 | 16 | 16 | No | Yes, R | Yes, R | No | No | No | None | 2.5 | Normal |
| 11 | 3 | 17 | Yes, C | Yes, C | No | No | Yes | Yes | CSA, steroids, splenectomy | 2.0 | Normal |
| 13 | 14 | 19 | Yes, C | Yes, C | Yes, C | No | No | No | IVIg, steroids, CSA | 1.1 | Normal |
| 17 | 4 | 11 | Yes, R | Yes, C | Yes, C | Yes | Yes | No | IVIg, steroids, sulfadoxine (Fansidar) | 10.5 | Defective |
| 18 | 6 | 19 | Yes, R | Yes, R | Yes, R | No | Yes§ | No | IVIg, steroids | 1.1 | Normal |
| 33 | 6 | 7 | Yes, C | Yes, C | Yes, C | Yes | Yes | No | IVIg, steroids | 8.9 | Defective |
| 38 | 10 | 14 | Yes, R | Yes, C | Yes, C | No | Yes | No | IVIg, steroids, rituximab, splenectomy | 7.7 | Defective |
| 39 | 17 | 18 | Yes, R | Yes, R | No | No | No | No | IVIg, steroids | 1.2 | Normal |
Chronic is defined as having an exacerbation at least 2 times a year, requiring immunosuppressive medications. Rare indicates not chronic.
Dx indicates diagnosis; AIHA, autoimmune hemolytic anemia; ITP, immune-mediated thrombocytopenia; AIN, autoimmune neutropenia; R, rare; C, chronic; IVIg, intravenous immunoglobulin; and CSA, cyclosporin A.
To qualify, patient must have lymphadenopathy not associated with infection or malignancy affecting 2 or more nodal groups
Treatment refers to any medication taken at any time since diagnosis with Evans syndrome. For specific information detailing medications taken at time of ALPS testing see “Discussion”
Defective refers to defective Fas-mediated apoptosis (consistent with diagnosis of ALPS); normal refers to appropriate Fas-mediated apoptosis
Splenomegaly present only during episodes of AIHA
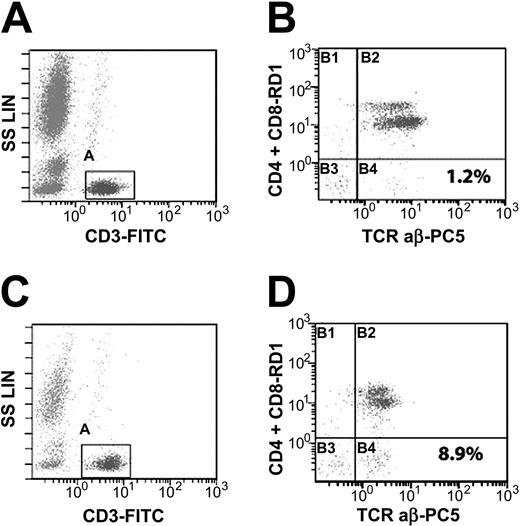
Fifty-eight percent (7 of 12) of the patients with ES had elevated DNTs. Examples of a positive and negative DNT test are depicted in Figure 1. All patients were then tested for Fas-mediated apoptosis. One half (6 of 12) of the patients had defective apoptosis consistent with the diagnosis of ALPS. An example of a test consistent with ALPS (defective Fas-mediated apoptosis) is depicted in Figure 2.
Detection of double-negative T cells and gating strategy. Peripheral blood lymphocytes were analyzed by flow cytometry for DNTs (CD4-/CD8-, CD3+, TCRαβ+). A histogram of CD3-FITC versus side scatter (A,C) was used to gate on T cells (CD3+/low SS). The events in the T-cell gate were sent to second histogram (B,D) of CD4 and CD8-RD1 versus TCRαβ-PC5. Double-negative T cells are depicted in lower right quadrant of panels B and D. Panel B represents a patient with a normal test (1.2% DNTs), and panel D represents a patient with an elevated test (8.9% DNTs). SS LIN indicates side scatter linear.
Detection of double-negative T cells and gating strategy. Peripheral blood lymphocytes were analyzed by flow cytometry for DNTs (CD4-/CD8-, CD3+, TCRαβ+). A histogram of CD3-FITC versus side scatter (A,C) was used to gate on T cells (CD3+/low SS). The events in the T-cell gate were sent to second histogram (B,D) of CD4 and CD8-RD1 versus TCRαβ-PC5. Double-negative T cells are depicted in lower right quadrant of panels B and D. Panel B represents a patient with a normal test (1.2% DNTs), and panel D represents a patient with an elevated test (8.9% DNTs). SS LIN indicates side scatter linear.
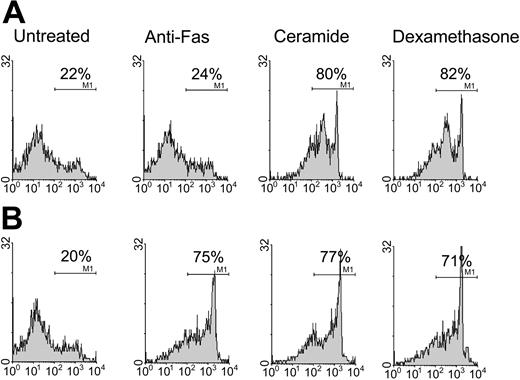
Detection of apoptosis. Fas-mediated apoptosis was analyzed by testing patient sample (A) in tandem with normal control (B). T cells were stimulated with PHA. After 14 to 21 days of culture, 106 T cells were incubated with media, anti-Fas monoclonal antibody, steroid, or C2-ceramide for 48 hours. (A) Results are an example of defective Fas-mediated apoptosis (consistent with diagnosis of ALPS) in 1 patient sample with percentages indicating proportion dead as determined by FACS analysis for 7-AAD. X-axis depicts percentage dead by 7-AAD and y-axis depicts number of cells counted. Gating strategy for dead cells is determined by comparing to control-stimulated T cells treated with ethanol and stained with 7-AAD.
Detection of apoptosis. Fas-mediated apoptosis was analyzed by testing patient sample (A) in tandem with normal control (B). T cells were stimulated with PHA. After 14 to 21 days of culture, 106 T cells were incubated with media, anti-Fas monoclonal antibody, steroid, or C2-ceramide for 48 hours. (A) Results are an example of defective Fas-mediated apoptosis (consistent with diagnosis of ALPS) in 1 patient sample with percentages indicating proportion dead as determined by FACS analysis for 7-AAD. X-axis depicts percentage dead by 7-AAD and y-axis depicts number of cells counted. Gating strategy for dead cells is determined by comparing to control-stimulated T cells treated with ethanol and stained with 7-AAD.
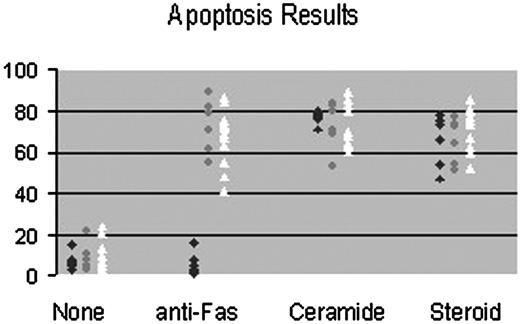
All patients described as having a test consistent with ALPS demonstrated apoptosis to C2 ceramide and to steroids but not to anti-Fas monoclonal antibody. All patients described as having a negative test demonstrated apoptosis to all 3 stimuli. The anti-Fas apoptotic response was the same in all patients and controls after both PMA and PHA T-cell stimulation (data not shown). All healthy controls demonstrated apoptosis to all 3 stimuli. Figure 3 summarizes results of apoptosis testing in patients and controls.
Summary of apoptosis results. This figure summarizes results of all patients and controls tested. Data points show percentage of death as determined by trypan blue exclusion after PHA stimulation.  represents patients with defective Fas-mediated apoptosis;, patients with normal Fas-mediated apoptosis; ▵, controls.
represents patients with defective Fas-mediated apoptosis;, patients with normal Fas-mediated apoptosis; ▵, controls.
Summary of apoptosis results. This figure summarizes results of all patients and controls tested. Data points show percentage of death as determined by trypan blue exclusion after PHA stimulation.  represents patients with defective Fas-mediated apoptosis;, patients with normal Fas-mediated apoptosis; ▵, controls.
represents patients with defective Fas-mediated apoptosis;, patients with normal Fas-mediated apoptosis; ▵, controls.
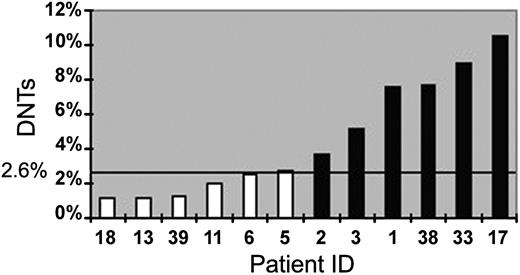
Five of 6 patients with ES with defective Fas-mediated apoptosis and elevated DNTs also had a history of lymphadenopathy or organomegaly, while 1 did not. Of the 7 patients with elevated DNTs, 6 had functional confirmation of defective Fas-mediated apoptosis. Thus, there was 1 false positive, and the specificity of the DNTs test was 85.7% in this series. Of the 6 patients with normal DNTs, none had functional confirmation of defective Fas-mediated apoptosis. Thus, there were no false negatives, and the sensitivity of the test was 100% for this series. Summary results comparing results of DNTs to apoptosis assay are depicted in Figure 4 and Table 2.
DNTs predict defective Fas-mediated apoptosis. Twelve patients with Evans syndrome were screened for ALPS with DNTs by FACS and then evaluated for ALPS by in vitro testing for defective Fas-mediated apoptosis. ▪ indicates patients with defective Fas-mediated apoptosis (consistent with ALPS); □, patients with normal apoptosis (not consistent with ALPS). The ordinate depicts percentage of DNTs and the bold line the cutoff for an elevated result (2.6%; solid horizontal line). All patients except one (number 5) with elevated DNTs had defective Fas-mediated apoptosis. All patients with normal DNTs had normal apoptosis.
DNTs predict defective Fas-mediated apoptosis. Twelve patients with Evans syndrome were screened for ALPS with DNTs by FACS and then evaluated for ALPS by in vitro testing for defective Fas-mediated apoptosis. ▪ indicates patients with defective Fas-mediated apoptosis (consistent with ALPS); □, patients with normal apoptosis (not consistent with ALPS). The ordinate depicts percentage of DNTs and the bold line the cutoff for an elevated result (2.6%; solid horizontal line). All patients except one (number 5) with elevated DNTs had defective Fas-mediated apoptosis. All patients with normal DNTs had normal apoptosis.
Pathology specimens were available for analysis on 2 patients who met diagnostic criteria for ALPS and 1 who did not. One of the biopsies from a patient with laboratory evidence of ALPS was strongly suggestive of a diagnosis of ALPS with classic features, elevated DNTs by immunohistochemical stains, paracortical expansion, and follicular hyperplasia. Another biopsy from a patient with laboratory evidence of ALPS was suggestive of ALPS but was not as conclusive. This biopsy was a poor specimen and was obtained from a normal-sized lymph node. The node showed elevated DNTs by immunohistochemical stains and follicular hyperplasia; however, because of the quality of the specimen it was also potentially consistent with a reactive node. The biopsy from the patient who did not meet laboratory diagnostic criteria for ALPS was also not consistent with ALPS, revealing a reactive lymph node with normal DNTs by immunohistochemical stains.
Discussion
We studied a group of 12 patients with Evans syndrome for the laboratory characteristics of ALPS: elevated DNTs and defective Fas-mediated apoptosis of mitogen-stimulated T cells. On the basis of this testing, we found an unexpectedly high prevalence of abnormal laboratory findings highly suggestive of ALPS among patients diagnosed with ES. Our data further suggest that DNTs may serve as a sensitive marker of patients who need further definitive testing. Identifying patients who have been diagnosed with ES who may in fact have ALPS has important prognostic and therapeutic implications. ES is poorly understood, and the exact mechanism of the disease is uncharacterized. While many case reports describe an increased risk of secondary malignancies in patients with Evans syndrome,26,27 the exact risk is not known. The mode of inheritance is unknown in Evans syndrome, and therapeutic options are limited to immunosuppressive therapy (IST). ALPS is better characterized as a result of a clear understanding of the pathophysiology. The lifetime incidence of secondary malignancy in ALPS approaches 10%,16 warranting careful observation, interval screening, and family counseling. Patients with ALPS usually inherit the disease in an autosomal dominant pattern with variable penetrance. Thus, genetic counseling plays a role for families of patients with ALPS. In addition, some evidence13 suggests an increased risk of malignancy may exist in relatives with the same Fas pathway mutation, in which the ALPS phenotype is not penetrant. Finally, while the mainstay of treatment of ALPS is similar to Evans syndrome (ie, use of IST), newer agents are becoming available which may be specific to ALPS. Pyrimethamine and sulfadoxine were shown to significantly reduce lymphoproliferation and autoimmune cytopenias in a small series of patients with ALPS.28 Currently, the National Institutes of Health (NIH) are investigating this agent in a phase 1 clinical trial. Understanding the mechanism behind ALPS has the potential to yield better-targeted therapies.
Of interest, our clinical laboratory established a different normal range for DNTs than is cited in the literature. In our laboratory, after healthy control subjects were tested, 2.6% was determined to be at the threshold for normal range, likely as a result of our highly gated analytic strategy. The normal cutoff for DNTs varies from institution to institution and different series publish different normal ranges.6,13 The current accepted definition of ALPS includes a result of more than 1% DNTs as a diagnostic criterion. Thus, it is important to know the normal values of an institutional laboratory. As is described in the literature,29 healthy adults were used to establish normal parameters for DNTs since DNTs have not been found to change with age.6
Four patients in our study were taking immunomodulating medications at the time of evaluation for DNTs. Three of these patients were taking low-dose steroids (5 mg or less) every other day. Two of these patients had elevated DNTs and defective Fas-mediated apoptosis, and 1 of these patients had normal DNTs and normal Fas-mediated apoptosis. Similar doses of steroids have not been shown to affect DNT or apoptosis analysis.30 No patient had received high-dose steroids in the 6 months prior to testing. All 3 of the patients talking low-dose steroids demonstrated apoptosis to steroids in vitro. One patient was treated with cyclosporine. This patient had normal DNTs and normal Fas-mediated apoptosis. No data exist in the literature as to the effect of cyclosporine on either test. Thus, this patient could represent a false negative. Four other patients were periodically treated with IVIg for immune cytopenias; however, no patient received IVIg within 6 weeks prior to testing for ALPS and, thus, should not have affected results.
One of the patients with defective Fas-mediated apoptosis and elevated DNTs had no history of lymphadenopathy or organomegaly. The lack of clinically identifiable lymphoproliferation in a patient with defective apoptosis is an unexpected finding, in apparent contradiction of the accepted definition of ALPS that lists lymphoproliferation as a mandatory diagnostic criterion. The identification of a patient with the triad of autoimmune cytopenias, elevated DNTs, and defective Fas-mediated apoptosis without clinical lymphoproliferation, raises the question of whether the accepted criteria for ALPS16 should be revised or whether this patient has a similar but different disease. Of note, this patient is 3 years old, and it is possible he may develop clinically identifiable lymphoproliferation with time; nevertheless, this patient would still contradict the published literature on ALPS which describes lymphoproliferation presenting prior to autoimmune cytopenias.16 Whether ALPS is a clinical diagnosis requiring lymphoproliferation or a genetic diagnosis causing defective Fas-mediated apoptosis is not yet known. Evaluating more patients with Evans syndrome for DNTs and apoptosis defects may help answer this question.
Approximately 76% of patients with ALPS have identifiable genetic mutations; however, in up to 24% of patients no mutation can be found. We did not perform genetic analysis on our patient population; however, 5 of the patients in our series have all 3 mandatory diagnostic criteria for ALPS. Thus, whether or not these patients have identifiable gene mutations would not change the results of our study.
Five patients in our series with defective Fas-mediated apoptosis and elevated DNTs had clinically identifiable lymphoproliferation consistent with the NIH criteria for diagnosis of ALPS. Of the 6 patients with Evans syndrome and normal Fas-mediated apoptosis, 5 had no history of clinically identifiable lymphoproliferation. In summary, 1 of 6 patients (16%) with defective Fas-mediated apoptosis had no lymphoproliferation and 1 of 6 patients (16%) without defective Fas-mediated apoptosis had lymphoproliferation. These findings suggest that clinically identifiable lymphoproliferation should not be used as the sole criterion for determining which patients with ES need definitive testing for ALPS.
ALPS is presumed to be a rare diagnosis with only a few hundred reported cases.6 Our finding of a high prevalence of ALPS in ES, a more common condition, argues that ALPS may be more common than previously thought. An assessment of the true prevalence of ALPS within patients diagnosed with ES awaits a larger prospective study. Because of the selection bias inherent in retrospectively assessing patients at a tertiary referral center, the prevalence of ALPS assessed by a prospective study could be lower than we have observed here.
In addition to ALPS, another disorder with autoimmunity and lymphoproliferation has been described, autoimmune lymphoproliferative disease (ALD).23 This disorder is characterized by patients with clinical lymphoproliferation similar to ALPS but with normal DNTs. These patients have defective apoptosis to both anti-Fas monoclonal antibody and ceramide, in contrast to patients with ALPS who only have defective apoptosis to anti-Fas monoclonal antibody. The patients described in our study more closely resemble ALPS than ALD since they have elevated DNTs and normal apoptosis to ceramide.
Patients with a single isolated immune cytopenia (immune thrombocytopenia purpura [ITP] or autoimmune hemolytic anemia [AIHA]) have been evaluated for elevated DNTs and/or defective Fas-mediated apoptosis.23,31 No patient in any published series with ITP or AIHA has been found to have elevated DNTs and defective Fas-mediated apoptosis (ie, the patient would not meet diagnostic criteria for ALPS). In one series, 2 patients with ITP were described with defective Fas-mediated apoptosis but had normal DNTs and no lymphoproliferation.31 Defects in Fas-mediated apoptosis have been observed in other disorders, including lupus,32 multiple sclerosis,33 and type 1 diabetes mellitus.34 No patients in our series had a comorbid disorder associated with defective Fas-mediated apoptosis except one patient who had diabetes mellitus. This patient had normal Fas-mediated apoptosis and normal DNTs.
In summary, in the group of 12 patients with ES tested in our study, 58% had elevated DNTs suggestive of ALPS, with functional confirmation in 6 of 7 tested. Our data suggest that DNTs may be a sensitive first-line screening test and may serve as a marker that identifies patients who require definitive testing. Our preliminary findings suggest a high prevalence of ALPS among patients with ES, a novel finding with important implications.
Prepublished online as Blood First Edition Paper, November 12, 2004 DOI 10.1182/blood-2004-09-3542.
Supported by the Goldman Philanthropic Partnerships and the Rockefeller Brothers Fund (D.T.T.), by a Charles E. Culpepper Biomedical Pilot Initiative grant of the Goldman Philanthropic Partnerships, and by the Sanford Chair and Weinberg Fund (S.A.G.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal