Abstract
Transfusion-related acute lung injury (TRALI) is a life-threatening adverse effect of transfusion that is occurring at increasing incidence in the United States and that, in the past 2 reporting years, has been the leading cause of transfusion-related death. TRALI and acute lung injury (ALI) share a common clinical definition except that TRALI is temporally and mechanistically related to the transfusion of blood/blood components. In prospective studies, 2 patient groups, 1 requiring cardiac surgery and 1 with hematologic malignancies and undergoing induction chemotherapy, were predisposed. Two different etiologies have been proposed. The first is a single antibody-mediated event involving the transfusion of anti-HLA class I and class II or antigranulocyte antibodies into patients whose leukocytes express the cognate antigens. The second is a 2-event model: the first event is the clinical condition of the patient resulting in pulmonary endothelial activation and neutrophil sequestration, and the second event is the transfusion of a biologic response modifier (including lipids or antibodies) that activates these adherent polymorphonuclear leukocytes (PMNs), resulting in endothelial damage, capillary leak, and TRALI. These hypotheses are discussed, as are the animal models and human studies that provide the experimental and clinical relevance. Prevention, treatment, and a proposed definition of TRALI, especially in the context of ALI, are also examined.
Historical background
Transfusion-related acute lung injury (TRALI) was first reported in 19511 and 1957,2 and findings from the initial case series were published in 1966.3 In 19704 and 1971,5 it was postulated that leukoagglutinins to HLA and non-HLA antigens were etiologic in TRALI reactions; however, it was not until 1985, with the report of a series of 36 patients,6 that TRALI was recognized as a distinct clinical entity. With more aggressive transfusion support and increased recognition of this syndrome, TRALI has become a common clinical complication of transfusion. In the past 2 reporting years, it has been named the leading cause of transfusion-related death in the United States.7
Clinical presentation and treatment
Although TRALI develops within 6 hours of transfusion,6,8-10 most occurrences take place during transfusion or within the first 1 or 2 hours after it.8,9,11-14 Clinical findings of TRALI consist of the rapid onset of tachypnea, cyanosis, dyspnea, and fever (1°C or higher.).6,9 Although hypotension was reported in most patients in the early clinical studies of TRALI, it is not a consistent finding.6,11,12 Auscultation of the lungs reveals diffuse crackles and decreased breath sounds, especially in dependent areas.6,9 Physiologic findings include acute hypoxemia, with PaO2/FiO2 less than 300 mm Hg, and decreased pulmonary compliance despite normal cardiac function.6,9,12,15 Radiographic examination reveals diffuse, fluffy infiltrates consistent with pulmonary edema (Figure 1).6,9 In short, the clinical diagnosis of TRALI is identical to that of acute lung injury (ALI), as described by Bernard et al16 (Table 1). Treatment consists of aggressive respiratory support, including supplemental oxygen and mechanical ventilation.6,9,12 Milder forms of TRALI have been described that require prompt delivery of supplemental oxygen alone.11,12,17,18 As with ALI and the acute respiratory distress syndrome (ARDS), there is no role for treatment with corticosteroids or diuretics.9,12,18-20 One infant was successfully treated with extracorporeal membrane oxygenation (ECMO) for a particularly severe clinical presentation.21
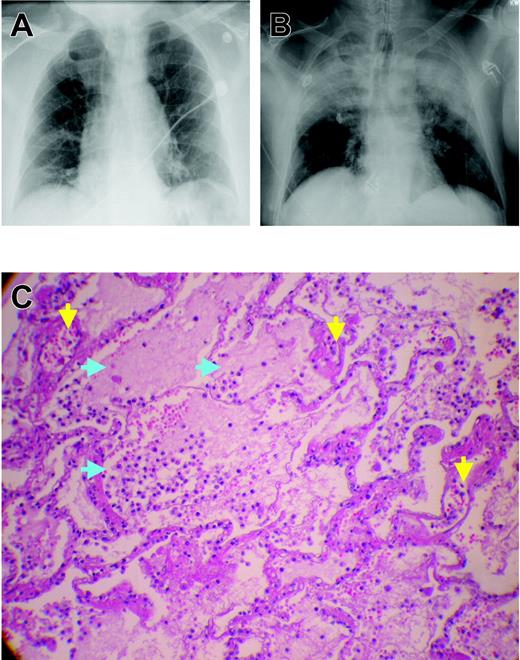
Chest x-rays and microscopic pathology examination of a patient with fatal TRALI. (A) Chest x-ray of the patient before surgery demonstrating low lung volumes with subsegmental bibasilar atelectasis, no evidence of pleural effusion, focal consolidation, or pneumothorax, and normal cardiomediastinal silhouette. (B) Chest radiograph at the time TRALI was recognized, which demonstrates extensive bilateral areas of consolidation in the mid and upper lobes of the lung consistent with aspiration or edema with a normal cardiac silhouette, new since the previous examination earlier on the same day. The endotracheal tube tip is 6 cm above the carina, the nasogastric tube is coiled within the hypopharynx before extending to the proximal trachea, and the right internal jugular introducer sheath tip overlies the proximal superior vena cava. These extensive areas of consolidation in mid and upper lobes are most concerning for noncardiogenic pulmonary edema. (C) Plastic-embedded histologic sections of the lungs at autopsy were stained with hematoxylin and eosin, toluidine blue, or Jones stains, and were examined by light microscopy under an OptiPhot-2 microscope equipped with a 20 ×/0.4 objective lens (Nikon, Melville, NY). Images were photographed with a Nikon CoolPix 4500 camera and acquired with Apple Mac OS × 10.3.5 (Apple, Cupertino, CA) running Portfolio 7 software (Extensis, Portland, OR). There is significant extravasation of PMNs into the alveoli and air spaces with interstitial and intra-alveolar edema (blue arrows). Toluidine blue and Jones stains demonstrate dilated capillaries and a prominence of inflammatory neutrophils in the capillaries and air spaces (yellow arrows). Histologic findings are consistent with early acute respiratory distress syndrome.
Chest x-rays and microscopic pathology examination of a patient with fatal TRALI. (A) Chest x-ray of the patient before surgery demonstrating low lung volumes with subsegmental bibasilar atelectasis, no evidence of pleural effusion, focal consolidation, or pneumothorax, and normal cardiomediastinal silhouette. (B) Chest radiograph at the time TRALI was recognized, which demonstrates extensive bilateral areas of consolidation in the mid and upper lobes of the lung consistent with aspiration or edema with a normal cardiac silhouette, new since the previous examination earlier on the same day. The endotracheal tube tip is 6 cm above the carina, the nasogastric tube is coiled within the hypopharynx before extending to the proximal trachea, and the right internal jugular introducer sheath tip overlies the proximal superior vena cava. These extensive areas of consolidation in mid and upper lobes are most concerning for noncardiogenic pulmonary edema. (C) Plastic-embedded histologic sections of the lungs at autopsy were stained with hematoxylin and eosin, toluidine blue, or Jones stains, and were examined by light microscopy under an OptiPhot-2 microscope equipped with a 20 ×/0.4 objective lens (Nikon, Melville, NY). Images were photographed with a Nikon CoolPix 4500 camera and acquired with Apple Mac OS × 10.3.5 (Apple, Cupertino, CA) running Portfolio 7 software (Extensis, Portland, OR). There is significant extravasation of PMNs into the alveoli and air spaces with interstitial and intra-alveolar edema (blue arrows). Toluidine blue and Jones stains demonstrate dilated capillaries and a prominence of inflammatory neutrophils in the capillaries and air spaces (yellow arrows). Histologic findings are consistent with early acute respiratory distress syndrome.
Clinical criteria for the diagnosis of ALI and TRALI16
Insidious, acute onset of pulmonary insufficiency |
| Profound hypoxemia, PaO2/FiO2 less than 300 mm Hg* |
| Bilateral fluffy infiltrates consistent with pulmonary edema |
| Pulmonary artery wedge pressure less than or equal to 18 mm Hg† |
| No clinical evidence of left atrial hypertension |
Insidious, acute onset of pulmonary insufficiency |
| Profound hypoxemia, PaO2/FiO2 less than 300 mm Hg* |
| Bilateral fluffy infiltrates consistent with pulmonary edema |
| Pulmonary artery wedge pressure less than or equal to 18 mm Hg† |
| No clinical evidence of left atrial hypertension |
If arterial blood gas values are unavailable, pulse oximetry less than 90% meets the criterion for hypoxemia
Irrespective of the pulmonary end-expiratory pressure (PEEP)
The mortality rate from TRALI ranges from 5% to 25%; rates in the lower end of the range are more common.9,11,14,20 Most patients recover within 72 hours; however, the data regarding TRALI are limited, and the attendant morbidity and mortality may be underappreciated because of lack of recognition and underreporting.9,11,14,20 Autopsy specimens have demonstrated pulmonary findings consistent with ARDS, including widespread leukocyte infiltration with interstitial and intra-alveolar pulmonary edema, hyaline membrane formation, and destruction of the normal lung parenchyma (Figure 1).11,22-26 In addition, in epidemiologic studies of ARDS, blood transfusion was implicated as the most common risk factor for the genesis of ARDS, and a number of these patients may represent severe cases of TRALI.22
Differential diagnosis
The differential diagnosis of patients who have pulmonary insufficiency after transfusion must include circulatory overload, anaphylactic transfusion reactions, and transfusion of blood products contaminated with bacteria.9,14,20 Transfusion-associated circulatory overload (TACO) develops within minutes to hours of transfusion as respiratory distress with tachypnea, tachycardia, hypertension, and cyanosis.27,28 All blood components have been implicated in TACO, and it rapidly responds to aggressive diuresis and ventilatory support.28 Anaphylactic transfusion reactions involve respiratory distress related to bronchospasm manifested by tachypnea, wheezing, cyanosis, and severe hypotension.29 Facial and truncal erythema and edema are common with urticaria, characteristically involving the head, neck, and trunk.29 The respiratory distress from anaphylactic transfusion reactions is related to laryngeal and bronchial edema rather than to pulmonary edema, as in TRALI.29 These reactions occur rapidly during the transfusion of any type of protein-containing blood component and may occur after the transfusion of small volumes of blood.29 Transfusion-related bacterial sepsis after transfusion of contaminated peripheral red blood cells (PRBCs) or platelet concentrates manifests as fever, hypotension, and vascular collapse, which may include respiratory distress, and must be considered in patients with pulmonary insufficiency who have undergone transfusion.30 Last, although some symptoms and signs of immediate hemolytic transfusion reaction may overlap with TRALI, they are easily distinguished by the presence of hemolysis.9,13
Incidence and patient predisposition
In North America, the reported incidence of TRALI is 1 in 5000 to 1 in 1323 transfusions,6,11,12 with newer data from Quebec reporting an incidence of 1 in 100 000 to 1 in 10 000 (depending on the transfused product).31 In Europe, TRALI is rarer, with reported incidences of 1.3 in 1 000 000 to 1 in 7900.32-35 Although the true incidence of TRALI remains unknown, it is unlikely to be calculated until a consensus definition can be reached.
Although no specific patient groups are predisposed to TRALI, Van Buren et al36 first postulated that the clinical status of the patient played a significant role in the pathogenesis. In a retrospective series of 10 TRALI patients compared with 10 patients with uncomplicated febrile or urticarial transfusion reactions, investigators hypothesized that TRALI was the result of 2 independent insults, similar to animal models of ALI.11,37,38 All patients in the TRALI group (10 of 10) had an antecedent “first event” including recent (within 72 hours) major surgery, active infection (bacterial or viral), massive transfusion (replacement of total blood volume daily for 4 days), or cytokine administration (granulocyte–colony-stimulating factor [G-CSF] or granulocyte-monocyte stimulating factor) compared with the control group, in which only 2 of 10 patients had a possible predisposing condition (recent surgery and G-CSF administration) (P < .05).11 The authors postulated that these first events might have predisposed patients to TRALI through activation of the pulmonary endothelium, resulting in polymorphonuclear leukocyte (PMN) sequestration in the lungs.11 Transfusion was the second event that activated these sequestered PMNs, which were adherent to the pulmonary vasculature, resulting in endothelial damage, capillary leak, and TRALI.11 Three of the 4 proposed predisposing conditions appeared to have clinical relevance with respect to TRALI because other groups have postulated that these same clinical conditions may increase patient susceptibility to TRALI. They are (1) massive transfusion has been implicated as a risk factor for TRALI in patients receiving solid organ transplants39 ; (2) follow-up information from the Mayo Clinic, in its series of 36 TRALI patients, revealed that all these patients had undergone recent (within 48 hours) surgery20 ; and (3) cytokine administration was proposed as the predisposing event in a report of a patient with antibody-negative TRALI.40 In addition, active infection is the most common predisposing clinical condition for the development of ALI and may also be associated with TRALI.16
A nested case-control study compared 46 consecutive patients with TRALI to 225 hospital patients who received transfusions during the same time interval and did not experience any adverse effects.12 Compared with controls who underwent transfusion, 2 patient groups were at particular risk for TRALI: those in the induction phase of treatment for hematologic malignancies (P < .0004) and those with cardiovascular disease who required bypass surgery (P < .0006).12 In addition, other patient groups, including those receiving fresh frozen plasma (FFP) for coumadin reversal and those with thrombotic thrombocytopenic purpura and widespread endothelial activation, appear to be at risk for TRALI.6,17,20,39,41,42 TRALI has also been reported in patients requiring transplantation of bone marrow and solid organ; ALI in these patients should be considered an untoward reaction related to the graft or to supportive care.43-50 Further prospective analyses of patients who have undergone transfusion are required to properly identify patients who are at risk for TRALI.
Implicated blood components
In published series of TRALI, plasma-containing blood components are most commonly implicated with whole blood–derived platelet concentrates (WB-PLTs),20 which have caused the largest number of these reactions, followed by FFP,6,9,20 PRBCs,6,9,20 whole blood,6,9,20 apheresis platelet concentrates (A-PLTs),9,40,47,51 granulocytes,52-54 cryoprecipitate,9,43 and intravenous immunoglobulin (IVIG).55 IVIG has the capacity to cause TRALI if it contains a significant amount of antileukocyte antibodies directed against antigens on host leukocytes, but given that there is only a single case report of IVIG-related TRALI, this may be a rare event.55 Although the plasma fraction of blood or blood components rather than the cellular constituents appears to be etiologic in TRALI, two of the most frequently implicated products (PRBCs and WB-PLTs) do not contain large amounts of plasma.13 However, although WB-PLT pools may contain significant amounts of plasma, the relative amount of plasma from each donor, the source of the antileukocyte antibody or biologic response modifier that may cause TRALI, remains small.13
Pathogenesis
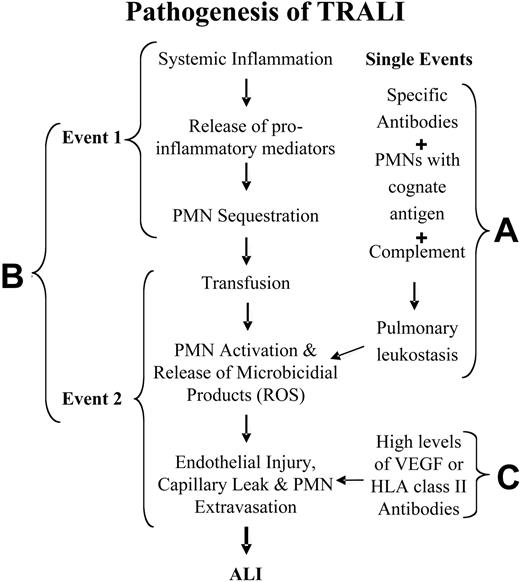
Two basic mechanisms have been proposed for the pathogenesis of TRALI for immunocompetent hosts, and another mechanism has been hypothesized for neutropenic patients.6,11,17,51 For patients with normal absolute neutrophil counts, adherence and activation of neutrophils leads to endothelial damage, capillary leak, and ALI (Figure 2A-B). The first hypothesis is that TRALI is secondary to a single antibody-mediated event (Figure 2A). In this model, TRALI is caused by the passive infusion of donor antibodies directed against recipient antigens on the surfaces of their leukocytes or the infusion of donor leukocytes into a recipient with antibodies directed against these donor leukocytes.6,9,17 The second hypothesis postulates that TRALI is caused by at least 2 independent events (Figure 2B).11,12,20,26 The first event relates to the underlying clinical condition of the patient such that the patient has pulmonary endothelial activation resulting in pulmonary sequestration of neutrophils.11,12,20,26 Transfusion is the second event; it involves the infusion of specific antibodies directed against adherent PMNs in the lung or against other biologic response modifiers, including lipophilic compounds, that cause activation of the microbicidal arsenal of these primed, adherent PMNs, resulting in endothelial damage, capillary leak, and TRALI.11,12,20,26 In addition, albeit rarely, TRALI has been reported in neutropenic patients.51 In these patients it is thought that TRALI is caused by the infusion of vascular endothelial growth factor (VEGF), an effective permeability factor,56-59 or by the infusion of antibodies against HLA class II antigens that reside on pulmonary vascular endothelium and cause endothelial shape change and fenestration (Figure 2C).60-62 Each of these mechanisms in neutropenic patients might elicit mild pulmonary leak.
Pathogenesis of TRALI. (A) TRALI may be precipitated by a single clinical event, the infusion of donor antibodies directed against host leukocytes, which causes complement activation, pulmonary leukostasis, PMN activation, endothelial damage, capillary leak, and ALI. (B) TRALI may be the result of 2 clinical events: the first consists of the clinical condition of the host, which causes pulmonary endothelial activation and adherence of PMNs, resulting in pulmonary sequestration. The second event, consisting of the transfusion of biologic response modifiers (biologically active lipids, antibodies directed against specific HLA class I or II or granulocyte antigens) in the blood component then activates these adherent PMNs and precipitates TRALI in predisposed patients. ROS indicates reactive oxygen species. (C) In neutropenic patients, TRALI may be precipitated by agents that directly cause endothelial fenestration, including high levels of VEGF or high levels of HLA class II antibodies directed against antigens on the pulmonary endothelium.
Pathogenesis of TRALI. (A) TRALI may be precipitated by a single clinical event, the infusion of donor antibodies directed against host leukocytes, which causes complement activation, pulmonary leukostasis, PMN activation, endothelial damage, capillary leak, and ALI. (B) TRALI may be the result of 2 clinical events: the first consists of the clinical condition of the host, which causes pulmonary endothelial activation and adherence of PMNs, resulting in pulmonary sequestration. The second event, consisting of the transfusion of biologic response modifiers (biologically active lipids, antibodies directed against specific HLA class I or II or granulocyte antigens) in the blood component then activates these adherent PMNs and precipitates TRALI in predisposed patients. ROS indicates reactive oxygen species. (C) In neutropenic patients, TRALI may be precipitated by agents that directly cause endothelial fenestration, including high levels of VEGF or high levels of HLA class II antibodies directed against antigens on the pulmonary endothelium.
Antibody-mediated TRALI caused by HLA class I and antigranulocyte antibodies
In 1985, Popovsky and Moore6 proposed the infusion of donor antibodies to explain TRALI. This study documented donor antibodies to granulocytes in 89% of these cases and antibodies to HLA antigens in 72% of cases examined.6 Most of the granulocyte antibodies did not exhibit specificity, but 59% of the HLA class I antibodies did.6 These findings have been confirmed by a number of other groups, and approximately 50% of donor antileukocyte antibodies display specific reactivity to recipient antigens.9,36,63,64 This pathogenesis has also been postulated for the transfusion of antigranulocyte antibodies.9,36,63,64 This infusion of leukoagglutinins is thought to cause complement activation, resulting in PMN influx into the lung, followed by activation of these PMNs and release of cytotoxic agents, in turn resulting in endothelial damage, capillary leak, and pulmonary damage.6,9,64 Thus, these antibodies are likely to be cytotoxic antibodies to confer such biologic activity when recognition of a leukocyte antigen occurs.6,12,27,65-67 In addition, TRALI can be caused by the binding of recipient antibodies to discrete antigens on transfused donor granulocytes; however, the number of viable PMNs is an issue, and such a mechanism represents only 10% of TRALI cases.9,68 This mechanistic sequence has particular relevance to patients receiving granulocyte transfusions and in whom alloantibodies, episodes of pulmonary leukostasis, and ALI develop.68
One particularly good example of antibody-mediated TRALI was reported by Dykes et al45 in a woman who underwent lung transplantation. After the transfusion of 2 U PRBCs, she became dyspneic and had marked hypoxia, and chest x-ray revealed a unilateral “white-out” of the transplanted lung. Antibodies to HLA-B44 were present in the donor of the second PRBC unit, and the antigen was expressed on the transplanted lung but not on the uninvolved lung. The patient did recover after intensive support, and this case provides an elegant example of antibody-mediated TRALI.45
Animal models of antibody-mediated TRALI. The relevance of these observations was reported in an ex vivo rabbit model of TRALI that demonstrated ALI characterized by severe pulmonary edema resulted from the infusion of a mixture of human PMNs {HNA-3a (5b) positive}, human HNA-3a antibodies, and rabbit plasma as a complement source.67 In this ex vivo model pulmonary edema occurred 3 to 6 hours after the infusion of the admixture; however, if any of the 3 components was deleted (HNA-3a antibodies, HNA-3a-positive PMNs, or complement), lung pulmonary edema would not occur.67 Furthermore, if immunoglobulins with indeterminate antigen specificity were infused with complement and human PMNs, ALI was not observed.67 Recently, Bux et al69 have updated this model and have demonstrated that using antigranulocyte antibodies and PMNs that have the cognate antigens may cause pulmonary edema without the addition of a complement source.
Modeling constraints. Although antibodies to HLA class I or granulocyte antigens explain many TRALI cases, a number of problems with this antibody-mediated mechanism remain. In the original description, only 59% of the immunoglobulins identified demonstrated antigen specificity, and in published series of TRALI, only approximately 50% of the implicated antibodies demonstrated specificity for recipient antigens.6,9 Because such nonspecific antibodies did not cause TRALI in the ex vivo animal model, the significance of these immunoglobulins, especially in the context of TRALI, is undefined.67 The precise mechanism for antibody-mediated TRALI is unknown6,9 ; moreover, in a number of cases of TRALI, an antibody is not present either in the donor or in the recipient,7,9-12,40,65 and recently a case of autologous TRALI has been reported.15 Even when antibodies are transfused into patients with the cognate antigens on the surfaces of their leukocytes, most patients do not acquire TRALI.18,36,70 Last, the ex vivo animal model of TRALI by Seeger et al67 introduced PMNs into the lung perfusate. This model used sterile plastic tubing, which is known to effectively prime PMNs in clinical situations including cardiac bypass and dialysis.71-73 Thus, if one considers the effects of the tubing on the circulating PMNs, this may be seen as a 2-event model: the first is the “inflammatory effect” of the tubing on the HNA-3a+ PMNs, and the second is the introduction of the HNA-3a antibodies with rabbit plasma, a complement source.67,71-73
TRALI secondary to the infusion of class II HLA antibodies
Kopko et al17 have postulated that TRALI is caused by the infusion of HLA class II antibodies with specificity for class II antigens in the recipient, and these findings have been confirmed by other groups.74,75 Furthermore, they demonstrated in vitro that HLA class II antibodies implicated in TRALI could activate circulating monocytes that expressed these antigens, causing synthesis of significant amounts of TNF-α, IL-1β, and tissue factor over a 4-hour time period compared with monocytes incubated with control sera.76 These cytokines have the capacity to activate PMNs, leading to endothelial damage capillary leak and TRALI.10,76 In addition, because HLA class II antigens may be expressed on endothelial cells (ECs), especially after inflammatory stimuli,61,62 these investigators questioned whether the infusion of HLA class II antibodies into a recipient with cognate antigen expression on the pulmonary endothelium may manifest TRALI because of endothelial activation, changes in cellular shape, fenestration, and capillary leak.76 Such a hypothesis is attractive, especially in the neutropenic patient who develops TRALI, but further work is required to test this hypothesis.
Modeling constraints. The infusion of HLA class II antibodies into patients who express the cognate antigens represents an attractive model for TRALI but raises a number of questions. First, though the synthesis of cytokines by circulating monocytes has the potential to cause TRALI, there is a significant time delay (4 hours) for the production of these inflammatory mediators; moreover, in these studies, the cytokines were intracellular and were not released extracellularly.76 Second, this model has relevance only if the infused antibody specifically recognizes a recipient antigen.76 Moreover, one interesting phenomenon is that cytokine-activated PMNs express HLA class II antigens, whereas resting PMNs express these antigens at very low levels or not at all.77-80 Therefore, if PMNs become primed and adherent, do they then express HLA class II antigens that may be recognized by HLA class II antibodies infused with the transfusion, and, if so, would not ligation of these antigens on primed, adherent PMNs then cause activation of the microbicidal arsenal, endothelial damage, and TRALI?81,82 Such a mechanism is plausible but would require 2 events: the first would cause adherence of PMNs to the pulmonary microvasculature, and the second would be the passive infusion of specific HLA class II antibodies directed against the class II antigens on the cell surfaces of the primed, sequestered PMNs.10,81,83 Despite the plausibility of this mechanism, one must remember that in vitro cytokine exposure of 72 hours is required for surface expression of HLA class II antigens on PMNs77-81 and that there may be significant differences between the effects of cytokines on leukocytes in vitro and in vivo.84 Moreover, investigation of a case of HLA class II antibody–mediated TRALI did not demonstrate the appearance of such HLA class II antigens on the surfaces of the patient's intravascular leukocytes.23 Further work is required to elucidate the mechanism in HLA class II antibody–mediated TRALI.
In addition, in the past few years the technology for leukocyte antibody detection has demonstrably changed and has increased the power and the specificity of the assay, especially with the use of flow cytometry. This improved technology has led to more precise definition of the antigen/antibody pairs that have resulted in TRALI. If one examines these implicated conjugates of the past 3 years, relatively few antibodies appear to be etiologic in TRALI (Patricia Kopko, presented at the Consensus Conference on TRALI, Toronto, Canada, April 2004). These antibody/antigen pairs include anti–HNA-3a and HNA-3a, granulocyte antigen, anti–HLA-A2 Creg and HLA-A2 Creg HLA, anti–HLA-B12 and HLA-B12, multiple HLA class II antibody/antigen pairs, and a mixture of HLA class I and class II antibody/antigen pairs. These results are different from the data presented by Popovsky and Moore, and other investigators, that demonstrate 83% concordance of HLA class I and granulocyte antibodies in patients with TRALI.6 These findings suggest that only specific antigens may be etiologic in TRALI and that such antigens may be responsible for signal transduction in leukocytes that leads to the synthesis and release of cytokines, EC damage, and TRALI.
Two-event model of TRALI. All proposed models of TRALI in immunocompetent patients implicate the PMN as the effector cell.6,11,12,17,20,26 Thus, it is important to understand PMN physiology, especially the interaction of PMNs with pulmonary vascular endothelium (Figure 3A)85-87 and PMN-mediated cell damage leading to ALI (Figure 3B). Moreover, the underlying clinical condition of the patient is important, as demonstrated in 3 “look-back” studies, including those of Van Buren et al36 with a donor with HNA-2b antibodies, Kopko et al18 with a donor with HNA-3a antibodies, and Nicolle et al70 with 2 donors with multiple HLA class II antibodies, which demonstrated that most transfused patients did not develop TRALI even though their leukocytes contained the cognate antigens.
Normal and abnormal neutrophil physiology. (A) Normal PMN emigration from the vasculature to the site of infection or inflammation in the tissues. In response to an infection (grouped ovals) in the tissues, inflammatory signals (stage 1, arrows) diffuse through the tissues to the vasculature and activate the vascular endothelium, causing release of chemokines (stage 2, 4-pointed stars), which attract PMNs to the endothelial surface (stages 3-4).20,102-104 Attraction is followed by selectin-mediated PMN rolling (stage 3) and β2-integrin/ICAM-1–mediated firm adhesion of PMNs to ECs (stage 4).20,102-104 These PMNs, which have undergone a change from a nonadhesive to an adhesive phenotype, are now primed (stage 4).20 Priming of PMNs enhances the microbicidal function of PMNs to a subsequent stimulus and changes the activity of PMNs such that stimuli that normally do not cause the activation of quiescent neutrophils are able to activate primed PMNs.20,88 It is important to note that priming is part of the orderly process of PMN transmigration to the tissues. Although there are benefits to enhanced PMN function, including efficient destruction of pathogens, it is clear priming may be detrimental to the host and may lead to PMN-mediated organ injury, especially ARDS.20 The PMNs then pass by diapedesis through the endothelial layer (stage 5), orient by chemotaxis to the site of infection (stage 5), and phagocytize (stage 6) and destroy the bacterial invaders (stage 6).20,102-104 (B) PMN-mediated tissue injury. If the orderly process of PMN transmigration is altered by a stimulus coming from the intravascular space (4-pointed stars, crosses, triangles) rather than the tissues, these intravascular stimuli activate vascular ECs (arrows) and cause attraction (stage 2), selectin-mediated rolling (stage 3), firm adhesion through the ICAM-1/β2-integrin interaction (stage 4), and priming of PMNs (stage 4).20 However, because there are no signals to cause diapedesis and PMN chemotaxis into the tissues, the PMNs become sequestered in the microvasculature.20 These primed, hyperreactive leukocytes may be activated by stimuli (stage 5, large triangle with diamonds) that normally have no effect, including antibodies directed against specific leukocyte antigens or the lipids that accumulate during routine storage of cellular blood components.20 Activation of these adherent PMNs causes endothelial damage (stage 5, ECs with lines) with lines, capillary leak (large arrow, stage 6), and organ injury.20
Normal and abnormal neutrophil physiology. (A) Normal PMN emigration from the vasculature to the site of infection or inflammation in the tissues. In response to an infection (grouped ovals) in the tissues, inflammatory signals (stage 1, arrows) diffuse through the tissues to the vasculature and activate the vascular endothelium, causing release of chemokines (stage 2, 4-pointed stars), which attract PMNs to the endothelial surface (stages 3-4).20,102-104 Attraction is followed by selectin-mediated PMN rolling (stage 3) and β2-integrin/ICAM-1–mediated firm adhesion of PMNs to ECs (stage 4).20,102-104 These PMNs, which have undergone a change from a nonadhesive to an adhesive phenotype, are now primed (stage 4).20 Priming of PMNs enhances the microbicidal function of PMNs to a subsequent stimulus and changes the activity of PMNs such that stimuli that normally do not cause the activation of quiescent neutrophils are able to activate primed PMNs.20,88 It is important to note that priming is part of the orderly process of PMN transmigration to the tissues. Although there are benefits to enhanced PMN function, including efficient destruction of pathogens, it is clear priming may be detrimental to the host and may lead to PMN-mediated organ injury, especially ARDS.20 The PMNs then pass by diapedesis through the endothelial layer (stage 5), orient by chemotaxis to the site of infection (stage 5), and phagocytize (stage 6) and destroy the bacterial invaders (stage 6).20,102-104 (B) PMN-mediated tissue injury. If the orderly process of PMN transmigration is altered by a stimulus coming from the intravascular space (4-pointed stars, crosses, triangles) rather than the tissues, these intravascular stimuli activate vascular ECs (arrows) and cause attraction (stage 2), selectin-mediated rolling (stage 3), firm adhesion through the ICAM-1/β2-integrin interaction (stage 4), and priming of PMNs (stage 4).20 However, because there are no signals to cause diapedesis and PMN chemotaxis into the tissues, the PMNs become sequestered in the microvasculature.20 These primed, hyperreactive leukocytes may be activated by stimuli (stage 5, large triangle with diamonds) that normally have no effect, including antibodies directed against specific leukocyte antigens or the lipids that accumulate during routine storage of cellular blood components.20 Activation of these adherent PMNs causes endothelial damage (stage 5, ECs with lines) with lines, capillary leak (large arrow, stage 6), and organ injury.20
Accumulation of PMN priming activity in stored blood
During routine storage of cellular components, an effective PMN priming activity accumulates that is lipophilic, as determined by its solubility in chloroform.88,89 Separation and characterization of this activity in whole blood (WB), PRBCs, and platelet concentrates demonstrated that this activity consisted of a mixture of lysophosphatidylcholines (lyso-PCs).88,89 These compounds effectively prime the PMN oxidative burst and can activate primed, adherent PMNs in vitro.88-91 In addition, an in vitro model of TRALI that used human pulmonary microvascular endothelial cells (HMVECs) as targets demonstrated that 2 events were required for PMN cytotoxicity.91 The first was HMVEC activation, which caused significant PMN adherence to the HMVEC surface that required chemokines for PMN attraction and firm adherence through the PMN β2-intergrins and the ICAM-1 on HMVECs.88 This PMN adherence mimics pulmonary sequestration of PMNs.91 The introduction of lyso-PCs from stored blood, the second event, activated these adherent PMNs, causing HMVEC death; however, if the PMNs were not adherent to the HMVECs, even with the addition of lyso-PCs, no PMN cytotoxicity occurred.91 In addition, PMN cytotoxicity in this model could be abrogated by inhibitors of the respiratory burst.91 These studies provide in vitro evidence that TRALI may be the result of 2 independent events and that endothelial activation resulting in firm adhesion of PMNs to endothelium is required.91
Two-event animal model of TRALI. The 2-event model of TRALI has been verified in an animal model.26,92 In this model, rats were treated with endotoxin (lipopolysaccharide [LPS]) for 2 hours to approximate active infection, one of the predisposing clinical conditions associated with TRALI.26,92 LPS activates the pulmonary vascular endothelium, which results in pulmonary sequestration of PMNs, confirmed by the pulmonary histology.26,92 The lungs were then isolated and perfused with buffer controls or 5% plasma from day 0 or day 42 PRBCs or day 0 or day 5 plasma from both WB-PLTs and A-PLTs.26,92 Vehicle-pretreated animals did not evidence acute lung injury with any of the perfusates.26,92 However, lungs from LPS-pretreated animals perfused with 5% plasma from day 42 PRBC units or day 5 WB-PLT or A-PLTs, but not plasma from identical PRBCs, WB-PLTs, or A-PLTs from day 0 or buffer controls, caused acute lung injury, as documented by pulmonary edema, lung histology demonstrating PMN-mediated ALI, and increases in leukotriene concentrations.26,92 In addition, both the lipid fraction and purified lipids from stored, but not fresh, PRBCs, WB-PLTs, and A-PLTs were etiologic in producing TRALI.26,92 Thus, the plasma and the lipids from stored blood products caused TRALI in this model.26,92
Modeling constraints. The 2-event model requires PMNs and requires that these PMNs be sequestered in the pulmonary vasculature. Therefore, apparently healthy patients who experience TRALI seem to be obviated from this pathogenesis. However, patients who require transfusion are not healthy by definition. Moreover, a study of the PMNs from 5 “healthy” donors, by history, demonstrated that their PMNs were grossly primed, as determined by the appearance and activity of these PMNs. In fact, all these donors acquired infections, either viral or bacterial, over the next 24 hours.90,93 Thus, it may be difficult to determine whether patients who undergo transfusion are indeed healthy.
Relevance of the 2-event model to clinical TRALI
A retrospective clinical study of TRALI patients demonstrated effective PMN priming activity in the patients' plasma at the time TRALI was recognized that had not been in the patient's pretransfusion typing serum; it was postulated that the clinical condition of the patient was important as a first event.11 Furthermore, no PMN priming activity was present in the plasma of patients with febrile or urticarial reactions, the PMN priming activity was lipid, and 2 of 3 predisposing conditions—massive transfusion and recent surgery, present in the TRALI patients but not in the controls—have since been implicated by other groups as predisposing conditions.11,20,39
In a prospective analysis of TRALI, the role of cytotoxic HLA class I and class II and antigranulocyte antibodies were examined.12 Of the donors tested, only 1 of 28 exhibited an antibody with specificity (HLA-A26) similar to that of positive controls.12 The implicated blood products demonstrated significant plasma PMN priming activity compared with similar products from the same facility and identical storage time that did not cause transfusion reactions.12 There was PMN priming activity in all TRALI patients at the time of recognition that consisted of neutral lipids and lyso-PCs.12 In addition, the roles of IL-6 and IL-8 were examined; each increased during storage, but only IL-6 was significantly increased in the TRALI patients compared with the pretransfusion sample.12 Thus, in this series, TRALI was caused by 2 events. The first was the clinical condition of the patient, and the second was the infusion of bioactive lipids in the stored blood component.12
Merging of the mechanisms of TRALI in immunocompetent patients
Recent preliminary data have provided evidence that antigranulocyte antibodies, namely antibodies directed against HNA-3a, were able to rapidly prime the fMLP-activated respiratory burst, analogous to lipids that accumulate during routine blood storage.94 In addition, these antibodies did not prime the PMNs through non-specific activation of Fc receptors because pretreatment of HNA-3a+ PMNs with F(ab')2 fragments directed against CD16, CD32, and CD64 had little effect on the priming activity of the HNA-3a antibodies.94 Moreover, antibodies to the HNA-3a locus did not prime HNA-3a- PMNs.94 Therefore, antibodies may have similar effects on recipient PMNs because other biologic response modifiers, including lipophilic compounds, which have the capacity to directly prime PMNs in vitro,87 cause PMN cytotoxicity in vitro91 and elicit PMN-mediated ALI in a 2-event animal model of TRALI.26,92 Further work is required to corroborate these findings and to determine whether these antibodies may directly cause PMN cytotoxicity in vitro.
Prevention
Decreasing blood usage will likely diminish TRALI because a number of untoward clinical outcomes are related to transfusion and must be considered to maximize patient outcome, especially for ill patients who may require transfusions.95 Moreover, applying consistent transfusion guidelines may decrease unnecessary transfusions and the morbidity associated with such needless patient exposures to blood products.95-98 In addition, many investigators, transfusion medicine professionals, and the American Association of Blood Banks advocate temporary disqualification of donors implicated in TRALI reactions until leukocyte antibody testing can be completed.9,13,76,82 If these donors have antibodies to high-frequency leukocyte antigens, such as HNA-3a, HLA-A2, and HLA-B12, they should be disqualified from plasma or platelet donation; otherwise, if these findings are negative, they should be returned to the donor pool.9,13,76,82 Although these precautions seem reasonable, there are a number of questions with this approach: What is the expense of such testing? Who will pay? Should all donors in implicated pools of WB-PLTs be temporarily disqualified? These and other questions must be answered through the use of multi-institutional, prospective studies.
To make the blood supply safer, the United Kingdom has disqualified all multiparous females from plasma donation because of the possibility that plasma from females may be the major factor in TRALI. Although some data have implicated plasma from females as less safe than plasma from males,99 recent data have demonstrated that plasma from females is not disproportionately implicated in common transfusion reactions.100 Much of the data do not support the disqualification of multiparous female donors at this time,12,100,101 and such disqualification may be disastrous to blood centers, in which these persons make up 20% to 30% of the donor pool.12,101
For scheduled major surgical procedures requiring transfusions, washing of cellular components removes antibodies, lipids, and other biologic response modifiers (BRMs) from the plasma fraction.13,102 Fresher products may be used to obviate the effects of lipids in patients at high-risk who are not neutropenic. Using PRBCs for less than 14 days85 and platelet concentrates for less than 2 days86 may avert many of the effects of these compounds, which accumulate during storage because there is not a significant accumulation of PMN priming activity during shorter storage times for PRBCs and platelet concentrates, respectively.85,86 In addition, a strategy to decrease the amount of plasma in blood components has demonstrated a decrease in TRALI; however, the study interval in at least one of these series is only 15 months.103
Definition
Because of the increase in TRALI cases in the United States, the National Heart Lung and Blood Institute of the National Institutes of Health convened a working group in May 2003 to arrive at a consensus definition. Furthermore, TRALI was the subject of a 2-day Canadian Consensus Conference sponsored by Canadian Blood Services and Héma-Quebec,8 and a number of European groups, including the British and the Dutch, are developing a consensus definition of this life-threatening adverse event. TRALI is acute lung injury temporally related to transfusion. It is not physiologically different from other forms of ALI or ARDS, and it is important to note that autopsy findings from fatal TRALI cases demonstrate that TRALI is indeed PMN mediated and that autopsy findings are similar to those of patients with ALI/ARDS. Thus, the diagnosis of TRALI should be considered in all cases of respiratory distress with significant hypoxemia (PaO2/FiO2 less than 300 mm Hg) temporally related to transfusion and should satisfy the criteria for the diagnosis of ALI (Table 1). Therefore, TRALI may or may not occur with the presence of risk factors for ALI, as long as the observed respiratory insufficiency is temporally related to the transfusion (Table 2). Risk factors for ALI should not exclude TRALI, but transfusion must be the inciting event. Moreover, worsening pulmonary function after transfusion in a patient with compromised respiratory status should also be considered to indicate TRALI. Patients who undergo transfusion are not healthy, and it is sometimes difficult to discern when a patient is in the early stage of an acute illness.93
Definition of TRALI
TRALI without clinical risk factors for ALI |
| New ALI temporally related to transfusion* |
| Worsening of preexisting pulmonary insufficiency temporally related to transfusion |
| TRALI with clinical risk factors for ALI |
| New ALI temporally related to transfusion* |
| New ALI thought to be mechanistically related to transfusion† |
| Worsening of preexisting pulmonary insufficiency temporally related to transfusion |
| Pulmonary insufficiency satisfies the criteria for the diagnosis of ALI.16 |
TRALI without clinical risk factors for ALI |
| New ALI temporally related to transfusion* |
| Worsening of preexisting pulmonary insufficiency temporally related to transfusion |
| TRALI with clinical risk factors for ALI |
| New ALI temporally related to transfusion* |
| New ALI thought to be mechanistically related to transfusion† |
| Worsening of preexisting pulmonary insufficiency temporally related to transfusion |
| Pulmonary insufficiency satisfies the criteria for the diagnosis of ALI.16 |
ALI occurred during transfusion or within 6 hours of the completion of transfusion
Recognition by the clinical care team that ALI is most likely caused by transfusion
Conclusions
TRALI may be caused by the passive infusion of antibodies directed against host leukocytes, of biologic response modifiers into a susceptible host, or of permeability factors.6,12,17,51 The single-event antibody-mediated pathophysiology of TRALI appears reasonable4-6,17,36,44,64 but does not explain why TRALI may occur without the infusion of specific antibodies directed against host leukocytes, especially autologous TRALI,15 and why TRALI does not occur even when there is an antibody infused into patients with the cognate antigen.18,36,70 Such data may lead one to consider that TRALI may be a multifactorial syndrome brought on by at least 2 distinct clinical events. The first is activation of the pulmonary endothelium resulting in PMN sequestration,10,12,20,83 and the second is activation of these primed adherent PMNs resulting in endothelial damage, capillary leak, and ALI.10,12,36,83,104 This 2-event model comprises antibody-mediated and nonantibody-mediated TRALI. Preliminary data indicate that antibody-mediated and 2-event models of TRALI may cause ALI by the capacity to prime host PMNs.94 Further work is required to define the effects of this life-threatening syndrome, to identify patients at particular risk, and to arrive at a consensus definition so that clinicians and nursing staff may properly diagnose TRALI.
Prepublished online as Blood First Edition Paper, November 30, 2004; DOI 10.1182/blood-2004-07-2929.
Supported in part by the Bonfils Blood Center, the National Heart Lung and Blood Institute (grant HL59355), the National Institutes of Health, and the Departments of Pediatrics and Surgery, University of Colorado School of Medicine, Denver.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal