Abstract
RhD is a major blood group and the most important target antigen in hemolytic disease of the newborn (HDN). The aims of this study were to establish a humanized mouse model of responses to the RhD protein and to test whether these could be prevented by the induction of immune tolerance. HLA-DR15 is a major restricting element for human T-helper (Th) cells specific for RhD protein, and expression of this HLA-DR transgene was found to confer on mice the ability to respond to immunization with purified RhD protein. Synthetic peptides containing dominant Th cell epitopes, previously identified from studies of human alloimmunized donors, were administered to the nasal mucosa of transgenic mice before immunization with purified RhD protein. Treatment with each of the 4 dominant peptides, RhD52-66, RhD97-111, RhD117-131, and RhD177-191, inhibited T-cell priming and prevented antibody responses to the RhD protein. The ability to induce such active tolerance offers the prospect of peptide immunotherapy as a replacement for passive immune globulin in the prophylaxis of HDN.
Introduction
The RhD antigen is a highly immunogenic and clinically important human blood group. Approximately 10% of pregnancies are at risk for hemolytic disease of the newborn (HDN) caused by RhD incompatibility,1 and current prophylaxis is dependent on passive RhD immune globulin. Fuller understanding of the immune response to RhD will enable the design of alternative strategies to prevent HDN.
Most immunoglobulin G (IgG) antibody responses are dependent on T-cell help, and the production of antibodies specific for red blood cells (RBCs),2 including anti-D,3 is no exception. T-helper (Th) cells recognize short antigen-derived peptides displayed by specialized antigen-presenting cells (APCs), and it is now clear that the context in which such recognition takes place determines whether a specific immune response is activated or tolerized.4,5 Methods to manipulate Th recognition of the RhD protein to favor tolerance are of particular interest because this would permit antigen-specific intervention to prevent HDN, without the risks associated with the use of blood products. Mucosal administration of helper epitopes is an approach that has been used to induce systemic tolerance to antigens of pathogenic relevance in models of transplantation,6 autoimmunity,4,5 and asthma.7 In many cases, the induction of such tolerance has been attributed to active mechanisms of immune regulation, particularly the stimulation of regulatory T cells.6,8,9
Dominant epitopes from the RhD protein that induce the proliferation of Th cells from alloimmunized donors in vitro have previously been mapped by us.3 In particular, peptides RhD52-66, RhD97-111, RhD117-131, and RhD177-191 were each able to stimulate Th cells in vitro from more than 50% of alloimmunized donors, with responses to at least 1 sequence in every donor. The question arises as to whether administering these peptides by a tolerogenic route could prevent responses to the entire RhD protein in vivo. To address this possibility, we first developed a humanized model of responsiveness to the RhD protein. The HLA-DRB1*1501 allele is significantly overrepresented (47.6%; χ2 = 4.269; P = .039) in RhD-negative donors who have produced anti-D antibodies in response to exposure to RhD-positive RBCs,10 and HLA-DR is the major restricting locus for Th cells specific for RhD protein epitopes.3 Mice transgenic for HLA-DR15 were selected for this study, predicting that the RhD protein would generate a specific immune response in this strain. Each of the dominant peptides was then tested for the ability to induce tolerance to the RhD protein by administration to the nasal mucosa.
Study design
Generation of transgenic mice
All animal experiments were carried out under a Project License granted by the United Kingdom Home Office and were approved by the University of Aberdeen Local Ethical Committee. The generation of HLA-DR15 transgenic mice is described in full detail elsewhere.11 Briefly, fertilized oocytes from (C57/BL6 × CBA) F2 matings were microinjected with construct containing linearized HLA-DRA1*0101 cosmid along with linearized clone 11, a DRB1*1501 cosmid cloned from a SuperCOS library made from the DR15, DR4 cell line ROF-NL and characterized for the presence of DR15 coding sequence, along with extensive 3′ and 5′ flanking sequence. Positive founder number 24 was mated for 6 generations with C57/BL6 Ab0 mice to yield mice on a C57/BL6 background and mouse class II knockout. Polymerase chain reaction (PCR) was used to confirm the presence of the HLA-DR15 transgenes and the absence of the wild-type genes.
Expression of the human DR15 major histocompatibility class 2 molecule on APCs was confirmed in experimental mice by flow cytometry. A vacutainer containing sodium heparin (Becton Dickinson, Oxford, United Kingdom) was filled with 5 mL Hanks balanced salt solution (HBSS) without sodium or magnesium (Invitrogen, Paisley, United Kingdom). Approximately 25 μL blood was taken by tail bleed into 500 μL heparinized HBSS, and peripheral blood mononuclear cells (PBMCs) were isolated by density centrifugation over Ficoll-Hypaque (Nycomed, Oxford, United Kingdom). Cells were washed in HBSS and then incubated for 15 minutes with rat anti–mouse CD16/32 (Becton Dickinson) to block murine Fc receptors. They were then incubated for another 30 minutes with 20 μL mouse anti–human HLA-DR conjugated to FITC (Becton Dickinson) and 20 μL rat anti–mouse I-A/I-E (Becton Dickinson). The expression of human and mouse major histocompatibility complex (MHC) class II molecules was analyzed with a FACSVantage flow cytometer (Becton Dickinson).
Antigens
Peptides were synthesized (Department of Biochemistry, University of Bristol, United Kingdom), corresponding to the sequence of the 30-kDa Rh protein associated with expression of the D blood group antigen, as previously published.12 RhD protein was purified from R2R2 RBCs by immunoprecipitation, as previously published.13
Administration of antigens
Mice transgenic for HLA-DR15 and at least 12 weeks of age were used. To immunize, 100 μL of 2 μg/mL purified RhD protein was injected subcutaneously into the base of the tail, followed 2 weeks later by an intraperitoneal booster with 100 μL of 2 μg/mL purified RhD protein. For the induction of tolerance, 50 μL of 2 μg/mL previously identified7 dominant RhD peptides—p6 (RhD52-66), p13 (RhD97-111), p17 (RhD117-131), or p28 (RhD177-191)—was administered to the nasal mucosa of HLA-DR15 transgenic mice 2 weeks before immunization.
Measurement of anti–human red blood cell antibody
Mice were tail-bled at 2-week intervals and by cardiac puncture at the end of the experimental schedule. Sera were complement inactivated and stored frozen for up to 6 months before testing. To detect the production of mouse anti–human RBC-specific antibody, 2 methods were used: a gel column indirect antiglobulin test (IAT; Diamed AG, Cressier, Switzerland) and a highly sensitive, quantitative indirect enzyme-linked antiglobulin test14-16 (IELAT).
Gel column indirect antiglobulin test
RhD-positive RBCs (R2R2 phenotype) and RhD-negative RBCs (rr phenotype) were used to screen the candidate sera. All reagents were allowed to equilibrate to room temperature before 10 μL serum was added to 20 μLof each of the 2% RBC suspensions. Cells were incubated for 15 minutes at 37°C before 20 μL of 1 μg/mL anti–mouse IgG (γ-chain specific (Sigma, Dorset, United Kingdom) was added to each of the serum-treated RBC suspensions. The cells were incubated for another 30 minutes at 37°C before each test sample was layered into individual wells on a neutral gel card (Diamed) and was centrifuged at 910 rpm for 10 minutes. Results were scored from 0 to 5 by a blinded observer.
Indirect enzyme-linked antiglobulin test
IELAT was based on a modification of our published method.16,17 Round-bottomed microtiter plates (Nunc, Roskilde, Denmark) were blocked in phosphate-buffered saline (PBS), pH 7.4, containing 0.2% bovine serum albumin (BSA), before 25 μL of 2% vol/vol washed human R2R2- or rr-typed RBCs was added. Test sera were diluted 1:5 in PBS and were incubated at 25 μL per well with triplicate RBC samples for 1 hour at 37°C and were washed 3 times in PBS–BSA before fixing for 30 minutes in 0.15% glutaraldehyde (Sigma) to prevent lysis of the cells in the alkaline conditions required later in the test. The fixed RBCs were transferred to fresh, preblocked, 96-well plates and were washed before incubation with 50 μL per well of 1 μg/mL goat anti–mouse IgG γ-chain–specific antibody (Sigma) for 1 hour at 37°C. After washing, the plates were incubated with 50 μL per well 1 μg/mL rabbit anti–goat IgG alkaline phosphatase (Sigma) for 1 hour at 37°C and washed, and 100 μL phosphatase substrate solution was then incubated in each well for 1 hour at 37°C. After pelleting of the RBCs by centrifugation, 50 μL each supernatant was transferred to the wells of fresh, flat-bottomed microtiter plates (Nunc), and absorbance was measured at 405 nm, with 492 nm as a reference, using a multiscan plate reader (Labsystems, Basingstoke, United Kingdom). The results, from triplicate serum samples, are expressed as the difference in the mean absorbance (ΔOD) between samples taken from RhD-immunized and PBS-immunized negative control mice. PBS-immunized mice were studied in parallel to provide baseline negative values because such groups control for any changes in the environment of the mice, or increasing age, during the experiments.
T-cell proliferation assays
Spleens were taken from immunized transgenic mice 2 weeks after boosting with purified RhD protein, and single-cell suspensions were generated by homogenization. T-cell proliferative responses were measured as previously described.12 The cells were incubated at a concentration of 1.25 × 106 splenocytes/mL in the alpha modification of Eagle medium (Invitrogen), supplemented with 0.5% complement-inactivated murine serum. Peak proliferation was estimated from the incorporation of 3H-thymidine in triplicate microtiter wells 5 days after stimulation with antigen. Results are presented as mean counts per minute (cpm) ± SD of the triplicate sample or as a stimulation index (SI) expressing the ratio of mean cpm in stimulated versus unstimulated control cultures. An SI greater than 3 is interpreted as representing a significant positive response.18
Results and discussion
Responses of HLA-DR15 transgenic mice to purified RhD protein
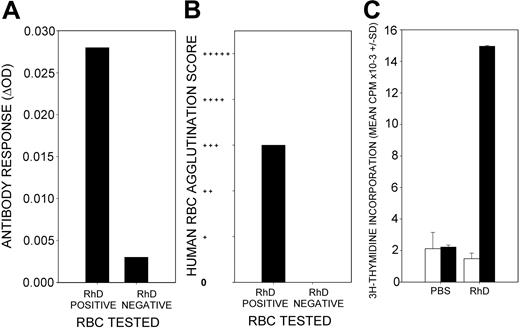
To test the tolerogenic potential of the 4 dominant RhD peptides, it was necessary to develop an animal model of immune responses to the RhD protein that did not induce specific antibody in wild-type rodents. Because the HLA-DRB1*1501 allele is significantly overrepresented in RhD-negative donors who produced anti-D antibodies in response to exposure to RhD-positive RBCs,10 we predicted that the RhD protein would generate a specific immune response in mice transgenic for HLA-DR15. This expectation was borne out by the results because 2 immunizations of the DR15 transgenic mice with purified RhD protein, 2 weeks apart, generated IgG antibody responses against human RBCs. All 12 mice tested positive by the IELAT, and 10 of 12 also tested positive by agglutination. A representative example is shown in Figure 1. Importantly, most of the antibody was specific for the RhD protein, as evidenced by much stronger reactivity with RhD-positive than the RhD-negative RBCs (Figure 1A-B). In addition, reactive sera that had been adsorbed with RhD-negative RBCs retained reactivity to RhD-positive RBCs (results not shown). The IAT and IELAT are complementary tests that use different secondary antibodies to detect RBC-bound IgG; therefore, their scores do not necessarily correspond.14,19 Priming of Th cells was demonstrated by the ability of splenocytes from the immunized mice to proliferate in vitro in response to stimulation with purified RhD protein (Figure 1C). It should be noted that the response did not require the addition of any adjuvant.
Responsiveness of HLA-DR15 transgenic mice to immunization with purified RhD protein. A representative example is shown (n = 12). RhD-immunized HLA-DR15 transgenic mice produce serum antibodies that bind to human RBCs, as detected by IELAT (A) and agglutination (B). Most such antibodies bind to RhD-positive, but not to -negative, RBCs. Splenocytes isolated from RhD-immunized mice proliferate in vitro in response to stimulation with the RhD protein (▪) compared with unstimulated control cultures (□) (C). There is no proliferative response to RhD protein by splenocytes isolated from PBS-immunized control mice. Error bars represent SD of triplicates.
Responsiveness of HLA-DR15 transgenic mice to immunization with purified RhD protein. A representative example is shown (n = 12). RhD-immunized HLA-DR15 transgenic mice produce serum antibodies that bind to human RBCs, as detected by IELAT (A) and agglutination (B). Most such antibodies bind to RhD-positive, but not to -negative, RBCs. Splenocytes isolated from RhD-immunized mice proliferate in vitro in response to stimulation with the RhD protein (▪) compared with unstimulated control cultures (□) (C). There is no proliferative response to RhD protein by splenocytes isolated from PBS-immunized control mice. Error bars represent SD of triplicates.
Neither antibodies reactive with the RhD protein nor splenocyte proliferative responses to this antigen were detected in negative control transgenic mice immunized with saline. Furthermore, wild-type mice of the same strain, but not expressing the DR15 transgene, failed to produce antibody after RhD immunization (Figure 2A-B), and their splenocytes proliferated weakly in response to stimulation with RhD protein in vitro compared with those of DR15-positive mice (Figure 2C).
Responsiveness of wild-type mice to immunization with purified RhD protein. A representative example is shown (n = 4). In contrast to the DR15 transgenic mice, wild-type mice (WT) of the same background strain immunized with RhD protein do not produce anti-RhD antibodies detectable by IELAT (A) or IAT (B). Splenocytes isolated from RhD-immunized wild-type control mice proliferate weakly in vitro in response to stimulation with the RhD protein (□) compared with DR15 transgenic mice (▪) (C).
Responsiveness of wild-type mice to immunization with purified RhD protein. A representative example is shown (n = 4). In contrast to the DR15 transgenic mice, wild-type mice (WT) of the same background strain immunized with RhD protein do not produce anti-RhD antibodies detectable by IELAT (A) or IAT (B). Splenocytes isolated from RhD-immunized wild-type control mice proliferate weakly in vitro in response to stimulation with the RhD protein (□) compared with DR15 transgenic mice (▪) (C).
Prevention of antibody responses to the RhD protein by nasal administration of dominant RhD peptides
The tolerogenic route tested here was intranasal administration because this offers convenient delivery to a mucosal site and, compared with oral dosing, avoids degradation in the digestive tract. A single dose of each candidate RhD peptide was administered to the nasal mucosa of DR15 transgenic mice 2 weeks before the first RhD immunization.
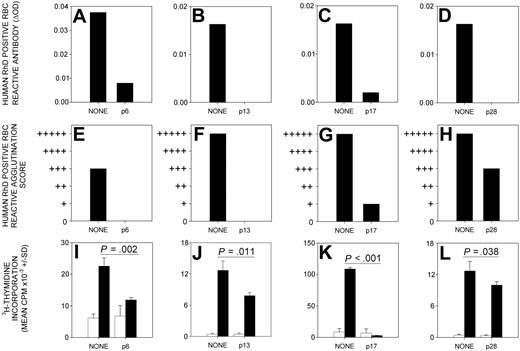
The production of anti-RhD protein antibodies by DR15 transgenic mice immunized with RhD protein was abrogated by the previous nasal administration of each of the 4 candidate tolerogenic peptides—p6 (RhD52-66), p13 (RhD97-111), p17 (RhD117-131), and p28 (RhD177-191) (Figure 3A-H). This ability to induce tolerance was specific to the dominant RhD peptides because antibody responses were unaffected by inhalation of a control RhD peptide (p50, RhD302-316) that does not induce proliferative responses by alloreactive human Th cells (results not shown).
Administration of dominant RhD peptides through the nasal mucosa prevents immune responses to RhD protein. The production of serum antibodies, detectable by IELAT (A-D) and IAT (E-H), specific for RhD-positive RBC, is inhibited by the nasal administration of RhD peptides p6 (RhD52-66), p13 (RhD97-111), p17 (RhD117-131), and p28 (RhD177-191) before the immunization of HLA-DR15 transgenic mice with RhD protein. A representative experiment (n = 12) is shown for each peptide. Splenocyte in vitro proliferative responses (I-L) to purified RhD protein (black bars) above the unstimulated background (white bars) are inhibited when HLA-DR15 transgenic mice are given RhD peptides p6 (RhD52-66), p13 (RhD97-111), p17 (RhD117-131), or p28 (RhD177-191) intranasally before RhD immunization. Significant differences are shown (Student t test). Error bars indicate SD of triplicates.
Administration of dominant RhD peptides through the nasal mucosa prevents immune responses to RhD protein. The production of serum antibodies, detectable by IELAT (A-D) and IAT (E-H), specific for RhD-positive RBC, is inhibited by the nasal administration of RhD peptides p6 (RhD52-66), p13 (RhD97-111), p17 (RhD117-131), and p28 (RhD177-191) before the immunization of HLA-DR15 transgenic mice with RhD protein. A representative experiment (n = 12) is shown for each peptide. Splenocyte in vitro proliferative responses (I-L) to purified RhD protein (black bars) above the unstimulated background (white bars) are inhibited when HLA-DR15 transgenic mice are given RhD peptides p6 (RhD52-66), p13 (RhD97-111), p17 (RhD117-131), or p28 (RhD177-191) intranasally before RhD immunization. Significant differences are shown (Student t test). Error bars indicate SD of triplicates.
Nasal tolerance is associated with inhibition of helper T cells
To confirm that the prevention of anti-RhD protein antibody production by nasal peptide therapy is associated with the inhibition of Th cells, the proliferative responses of splenocytes isolated from treated mice were tested. Nasal administration of each of the dominant peptides before immunization was found to reduce significantly the proliferative response to RhD protein (Figure 3). Two peptides—p6 (RhD52-66) and p17 (RhD117-131)—were consistently the most effective in tolerizing antibody and Th cell responses to RhD protein.
The main results reported here are that humanized mice transgenic for an HLA-DR molecule can make IgG antibodies specific for the RhD protein and that these responses can be prevented by nasal administration of synthetic peptides containing dominant Th epitopes. The demonstration of a murine model of immune responses to RhD protein using mice transgenic for a major restricting element, the HLA-DR15 molecule, is an important step in the development of specific immunotherapy for HDN.
It was important to develop a consistent model of immune responses to the RhD protein given that wild-type mice have proven particularly resistant to specific RhD immunization, perhaps because their T-cell repertoire does not recognize the same epitopes as humans have. We previously demonstrated that HLA-DR15 is a major restricting element for human Th responses to the RhD protein.10 The importance of this MHC class II molecule in presenting RhD epitopes is confirmed by the finding that DR15 transgene expression conferred on mice the ability to mount strong T-cell responses to the RhD protein and to make specific antibody. These results also indicate that the resistance of wild-type mice to mounting antibody responses to the RhD protein is attributed to a lack of T-cell help, associated with inappropriate MHC class 2 expression rather than with the absence of specific B cells from the repertoire.
In HDN, the pathogenic antibody is of the IgG isotype, and measurement of this isotype was the primary focus in our animal model. In humans, an early IgM component is observed after first exposure to the RhD protein preparation, together with IgG antibodies of low affinity, and a brief autoantibody response may occasionally be seen on secondary immunization.20,21 The immune response observed in the transgenic mice shows a number of similarities with the human response to RhD. Notably, the murine IgG antibody response generated after RhD immunization was mainly RhD specific and could not be removed by adsorption with RhD-negative RBCs. Although sera from some immunized mice showed weak reactivity with RhD-negative cells, this could have reflected the generation of antibodies that cross-reacted with the homologous Rhce proteins, analogous to the early autoantibody response occasionally seen in RhD-negative humans after exposure to RhD.21 This might be expected given that homologous sequences on the RhD and Rhce proteins, in addition to immunodominant RhD-specific epitopes, could potentially be recognized by the mice as foreign.
The ability of one mucosally delivered peptide containing a dominant Th epitope to induce tolerance to the entire antigen from which it is derived,10 such as the blocking of RhD responses observed here, can be explained by the activation of regulatory T cells.2,5,6,8,22 This mechanism of active suppression, therefore, differs from the effects of passive RhD immune globulin on which HDN prophylaxis is currently reliant, and it offers the ability to overcome a number of its limitations.23 These disadvantages include the transient nature of the protective effects conferred by passive antibody, the necessity to treat after each exposure to incompatible RBCs, and the need for the antibody dose to be matched to the size of RBC challenge.
It is well established that the timing and route of administration of peptides is crucial to the induction of tolerance.4,5 We used the intranasal route because that offers convenient delivery to a mucosal site and avoids degradation in the digestive tract with the oral route. Our approach was based on successful nasal peptide therapy used to suppress autoimmune pathology in animal models.4,5 We also chose to administer a single dose of each candidate RhD peptide 2 weeks before the first RhD immunization. We avoided the use of multiple treatments given over several days, though such regimens are reportedly efficacious in other mouse models,10 because it was judged that a single dose would ultimately be more practical for human subjects.
In addition to the advantages implicit in its mechanism of action, tolerance induction by mucosal delivery of synthetic RhD peptides is free from the health risks associated with blood products derived from human plasma. There are increasing concerns about the potential contamination of RhD immune globulin by infectious agents, heightened by evidence that variant Creutzfeldt-Jacob disease (CJD) may be transmitted by blood products.24,25 Human plasma from countries in which bovine spongiform encephalopathy (BSE) has been prevalent has been excluded from the manufacture of blood products on a precautionary principle, and Rh-immune globulin in the United Kingdom is now made from United States–derived plasma.23 The recent confirmation of BSE in North American cattle26 and the diagnosis of variant CJD in the United States may further threaten supplies of RhD immune globulin. In the absence of evidence that monoclonal antibody replacements for RhD immune globulin will be safe, efficacious, or affordable,1,27 synthetic peptide immunotherapy represents an important alternative approach to HDN prophylaxis.
Although the only human MHC class II molecule expressed by the mice was DR15, the RhD peptides tested for the ability to induce mucosal tolerance were selected because they are promiscuous and contain dominant epitopes recognized by Th cells restricted by a wide range of HLA-DR molecules.3 The success of this particular selection of peptides at inducing tolerance in the humanized mouse model forms the basis for further studies into the mechanism of tolerance and into phase 1 trials of peptide immunotherapy to prevent anti-RhD responses in humans.
Prepublished online as Blood First Edition Paper, September 21, 2004; DOI 10.1182/blood-2004-04-1554.
Supported by grants from the Scottish Enterprise Proof of Concept Fund (United Kingdom) and the Scottish National Blood Transfusion Service.
A.M.H. and L.S.C. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Mr J. Duncan (Scottish National Blood Transfusion Service) for technical assistance with the indirect antiglobulin test.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal