Abstract
We developed an approach that increases CD4+CD25+ regulatory T-cell potency by antigen-specifically redirecting them against pathologic T lymphocytes. The regulatory cells are transgenically modified with chimeric receptors that link antigen–major histocompatibility complex (MHC) extracellular and transmembrane domains with the cytoplasmic signaling tail of T-cell receptor ζ (TCR-ζ). The receptors' antigen-MHC recognizes the TCR of cognate T lymphocytes. Receptor engagement stimulates the receptor-modified T cell (RMTC) through the linked ζ chain. CD4+CD25+ RMTCs expressing a myelin basic protein (MBP) 89-101-IAs-ζ receptor, unlike unmodified CD4+CD25+ T cells or CD4+CD25- RMTCs, prevented and treated experimental allergic encephalomyelitis (EAE) induced with MBP89-101. The RMTCs were effective even after the autoreactive T-cell repertoire had diversified to include specificities not directly targeted by the chimeric receptor. Remissions were sustained and mortality was decreased from more than 50% to 0%. These results provide proof of principal for a novel approach to enforce the interaction of regulatory and pathologic T lymphocytes, thereby facilitating the treatment of autoimmune disease.
Introduction
CD4+CD25+ regulatory T cells potently suppress immune responses.1 When therapeutically administered, even antigen nonspecific CD4+CD25+ T cells can prevent or treat immunopathology in several disease models.2-8 Yet, infusions of nonspecific regulatory cells would be expected to have poor specificity and low potency. Indeed, some studies have found that the administration of antigen-specific regulatory T cells substantially increases therapeutic activity.9,10
Considering the low precursor frequency of regulatory T cells, identifying, isolating, and expanding antigen-specific cells for immunotherapy would be technically challenging. As an alternative, we have developed a gene-therapeutic approach that specifically redirects regulatory T cells against pathologic T cells. Therapeutic cells are transgenically modified with a chimeric antigen–major histocompatibility complex (MHC)–ζ receptor.11-13 The extracellular antigen-MHC serves as a bait, engaging the T-cell receptor (TCR) of potentially pathologic antigen-specific T cells. This engagement generates an immunoreceptor tyrosine-based activation motif (ITAM)–mediated signal through the chimeric receptors'cytoplasmic ζ chain.
We hypothesized that the chimeric receptors, by enforcing the interaction between a CD4+CD25+ regulatory T cell and its target, should antigen-specifically focus CD4+CD25+ T-regulatory activity against autoreactive T cells. We previously showed that T cells modified with a chimeric receptor that genetically links the 89-101 epitope of the myelin basic protein (MBP) with its IAs-restricting MHC and the cytoplasmic domain of TCR-ζ (receptor-modified T cells [RMTCs]) specifically recognize MBP89-101–reactive T cells in vitro and in vivo.12 We test here whether the MBP89-101-IAs-ζ receptor can redirect CD4+CD25+ T cells against autoreactive MBP-specific T cells and thereby treat experimental allergic encephalomyelitis (EAE).
Study design
Mice and cells
Peptides
MBP89-101 (VHFFKNIVTPRTP) was synthesized and purified to more than 90% purity by the St Jude Hartwell Center.
Antibodies and flow cytometry
Anti-CD4 (RM4-5), anti-CD25 (7D4), and anti-CD28 antibodies were obtained from Pharmingen (San Diego, CA). 2C11 (anti-CD3ϵ; gift of M. Blackman, Trudeau Institute, Saranac Lake, NY) was purified from hybridoma supernatants. Sorting was performed with a Mo-Flo hi-speed cell sorter (DAKO, Fort Collins, CO).
EAE induction and clinical evaluation
T-cell proliferation and cytokine production
Proliferation was measured after a 72-hour culture by 3H-thymidine (3H-TdR) incorporation. Cytokines were measured after a 48-hour culture by enzyme-linked immunosorbent assay (ELISA) or Bio-Plex (Bio-Rad, Hercules, CA).
Results and discussion
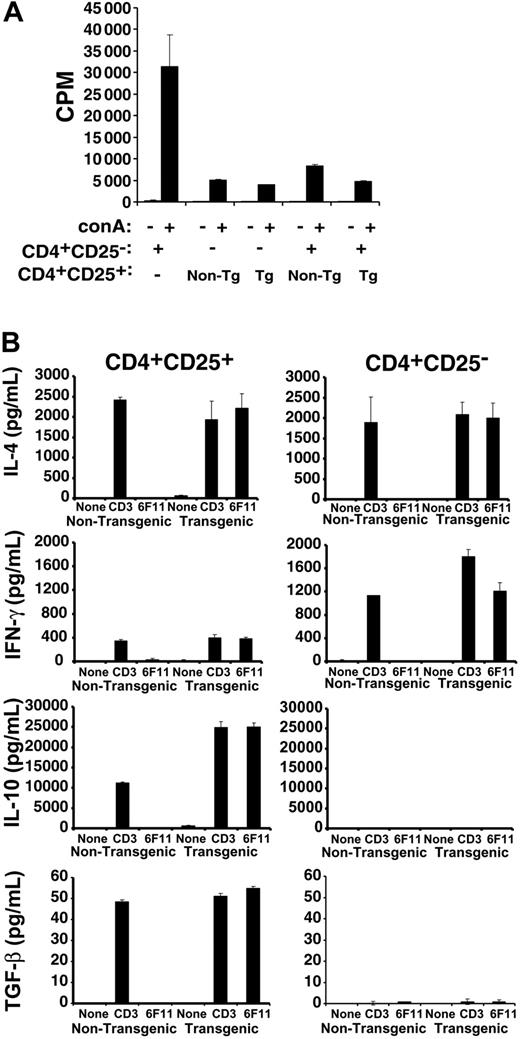
Approximately 5% of splenic and lymph node (LN) CD4+ T cells from MBP89-101-IAs-ζ Tg mice12,14 express the CD25 activation marker (data not shown). Both Tg and non-Tg CD4+CD25+ T cells showed limited proliferation themselves in response to stimulation, a characteristic of regulatory T cells (Figure 1A). The Tg and non-Tg cells also equivalently suppressed the proliferation of naive T cells. Therefore, the suppressive activity of CD4+CD25+ T cells is unaffected by the chimeric receptor transgene.
Activity and redirection of transgenic CD4+CD25+ T cells. (A) 5 × 104 flow-cytometrically purified CD4+CD25- SJL T cells were cultured in the presence or absence of concanavalin A (conA) with or without an equal number of nontransgenic or MBP89-101-IAs-ζ receptor transgenic CD4+CD25+ T cells, and with 2.5 × 105 irradiated splenocyte feeders. Cultures were pulsed with 3H-thymidine 72 hours later and proliferation was measured by scintillation counting. (B) Tg or non-Tg CD4+CD25+ or CD4+CD25- T cells were purified by flow cytometric sorting and stimulated for 4 days with anti-CD3 and anti-CD28 antibodies. The cells were then washed and recultured in the absence of stimulation, or restimulated with anti-CD3 antibody or 200 Gy irradiated 6F11 MBP89-101–specific T-cell hybridoma cells for 48 hours prior to measuring cytokine production by Bio-Plex assay or ELISA. Coculture of the 6F11 T cells with RMTCs, which express the MBP89-101-IAs ligand on their chimeric receptor, would be expected to stimulate both the hybridoma and the RMTCs. To control for 6F11-produced cytokines, irradiated 6F11 cells were also stimulated with anti-CD3 antibody. No IFN-γ, IL-10, or TGF-β was produced, while IL-4 levels were approximately 10% of that produced when RMTCs were cocultured with 6F11 cells (data not shown). Error bars indicate 1 SD.
Activity and redirection of transgenic CD4+CD25+ T cells. (A) 5 × 104 flow-cytometrically purified CD4+CD25- SJL T cells were cultured in the presence or absence of concanavalin A (conA) with or without an equal number of nontransgenic or MBP89-101-IAs-ζ receptor transgenic CD4+CD25+ T cells, and with 2.5 × 105 irradiated splenocyte feeders. Cultures were pulsed with 3H-thymidine 72 hours later and proliferation was measured by scintillation counting. (B) Tg or non-Tg CD4+CD25+ or CD4+CD25- T cells were purified by flow cytometric sorting and stimulated for 4 days with anti-CD3 and anti-CD28 antibodies. The cells were then washed and recultured in the absence of stimulation, or restimulated with anti-CD3 antibody or 200 Gy irradiated 6F11 MBP89-101–specific T-cell hybridoma cells for 48 hours prior to measuring cytokine production by Bio-Plex assay or ELISA. Coculture of the 6F11 T cells with RMTCs, which express the MBP89-101-IAs ligand on their chimeric receptor, would be expected to stimulate both the hybridoma and the RMTCs. To control for 6F11-produced cytokines, irradiated 6F11 cells were also stimulated with anti-CD3 antibody. No IFN-γ, IL-10, or TGF-β was produced, while IL-4 levels were approximately 10% of that produced when RMTCs were cocultured with 6F11 cells (data not shown). Error bars indicate 1 SD.
We previously showed that unsorted MBP89-101-IAs-ζ RMTCs are specifically stimulated by MBP89-101–reactive 6F11 T cells.12 To verify that cognate TCR could similarly stimulate sorted CD4+CD25+ RMTCs, we measured cytokine production by them or CD4+CD25- RMTCs in response to 6F11 T cells or control anti-CD3ϵ antibody (Figure 1B). The pattern of cytokines produced by Tg or non-Tg CD4+CD25+ T cells to control anti-CD3ϵ was identical, as was that produced by Tg or non-Tg CD4+CD25- T cells. Therefore, the chimeric receptor did not significantly impact the T-cell cytokine profile. As expected, cytokine production differed between CD4+CD25+ and CD4+CD25- T cells, with CD4+CD25+ cells producing similar amounts of interleukin 4 (IL-4), reduced interferon γ (IFN-γ), and increased amounts of IL-10 and transforming growth factor β (TGF-β) compared with CD4+CD25- cells. Most importantly, whereas non-Tg cells failed to produce cytokines in response to 6F11 stimulation, Tg cells produced quantities of each cytokine comparable to that induced by anti-CD3ϵ. This demonstrates that the chimeric receptor serves as an effective surrogate for the TCR in CD4+CD25+ T cells, can engage MBP89-101–specific T cells, and can redirect RMTC function.
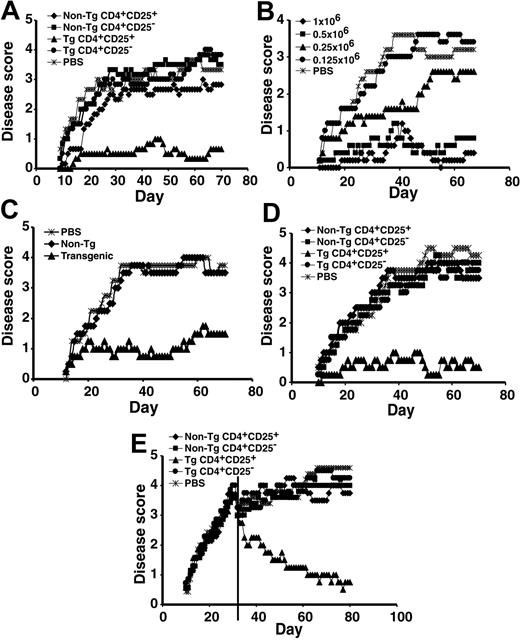
To define the clinical efficacy of the CD4+CD25+ RMTCs, we tested their ability to prevent EAE induced by MBP89-101 peptide. We found limited and inconsistent effects of doses of up to 3 × 106 non-Tg CD4+CD25+ T cells, which would be expected to roughly double to triple the total circulating regulatory T-cell number (data not shown). Partial disease suppression has been observed in 2 other EAE models using similar regulatory cell doses, suggesting that this represents an approximate threshold for disease abatement.2,3 In contrast to the limited effects of nonspecific regulatory T cells, 1 × 106 CD4+CD25+ RMTCs consistently and potently suppressed EAE (Figure 2A).
Prevention and treatment of EAE with CD4+CD25+ RMTCs. (A) Prophylaxis of EAE. EAE was induced using a dual immunization regimen with MBP89-101 followed by the administration of pertussis toxin.15 To determine the clinical impact of the CD4+CD25+ RMTCs on encephalomyelitis, 1 × 106 flow-cytometrically purified CD4+CD25+ RMTCs (▴), control cells, including CD4+CD25- RMTCs (⬡), CD4+CD25+ non-Tg T cells ( ), or CD4+CD25- non-Tg T cells (▪), or saline (PBS;
), or CD4+CD25- non-Tg T cells (▪), or saline (PBS;  ) was intravenously administered into SJL/J mice at the time of disease induction. Clinical course was monitored. (B) Dose-response relationship of CD4+CD25+ RMTCs;
) was intravenously administered into SJL/J mice at the time of disease induction. Clinical course was monitored. (B) Dose-response relationship of CD4+CD25+ RMTCs;  indicates 1 × 106 cells; ▪, 0.5 × 106; ▴, 0.25 × 106; and ⬡, 0.125 × 106.
indicates 1 × 106 cells; ▪, 0.5 × 106; ▴, 0.25 × 106; and ⬡, 0.125 × 106.  indicates PBS. (C) Delayed treatment of EAE. EAE was induced as above and 1 × 106 Tg (▴) or non-Tg CD4+CD25+ T cells (
indicates PBS. (C) Delayed treatment of EAE. EAE was induced as above and 1 × 106 Tg (▴) or non-Tg CD4+CD25+ T cells ( ) were administered 11 days later to measure the activity of RMTCs at the time of early disease development.
) were administered 11 days later to measure the activity of RMTCs at the time of early disease development.  indicates PBS. (D) Treatment of adoptive transfer EAE. Draining LN cells from MBP89-101–immunized mice were briefly cultured in vitro and 25 × 106 cells intravenously transferred into naive mice. On the same day, 1 × 106 CD4+CD25+ RMTCs or control cells were administered intravenously through a separate site and disease was monitored clinically. Symbols indicate same information as in panel A. (E) Treatment of EAE after epitope spread. Treatment with 1 × 106 Tg or non-Tg cells was delayed until 31 days after disease induction (vertical line). At this time, T-cell response was detected not only against the initiating MBP89-101 epitope, but also against pathologic PLP139-151 and PLP178-191 epitopes.12 Mean score for each arm was adjusted at the time of treatment to exclude animals not treated due to a moribund status or death. Symbols indicate same information as in panel A. Plots A-E show mean clinical score from 4 to 6 animals per treatment group. Each plot is representative of 2 to 3 independent experiments. A 2-sided t-test analysis of mean daily clinical score from the time of first disease symptoms (A-D) or treatment (E) to the end of observation showed a statistically significant beneficial effect of the CD4+CD25+ RMTCs compared with each control group in the experiments shown in panels A and C-E (P < .05). In the experiment shown in panel B, a statistically significant benefit (P < .05) was observed for doses equal to or greater than 5 × 105. At a dose of 2.5 × 105, a beneficial effect was observed from days 30 to 45 only (P < .05).
indicates PBS. (D) Treatment of adoptive transfer EAE. Draining LN cells from MBP89-101–immunized mice were briefly cultured in vitro and 25 × 106 cells intravenously transferred into naive mice. On the same day, 1 × 106 CD4+CD25+ RMTCs or control cells were administered intravenously through a separate site and disease was monitored clinically. Symbols indicate same information as in panel A. (E) Treatment of EAE after epitope spread. Treatment with 1 × 106 Tg or non-Tg cells was delayed until 31 days after disease induction (vertical line). At this time, T-cell response was detected not only against the initiating MBP89-101 epitope, but also against pathologic PLP139-151 and PLP178-191 epitopes.12 Mean score for each arm was adjusted at the time of treatment to exclude animals not treated due to a moribund status or death. Symbols indicate same information as in panel A. Plots A-E show mean clinical score from 4 to 6 animals per treatment group. Each plot is representative of 2 to 3 independent experiments. A 2-sided t-test analysis of mean daily clinical score from the time of first disease symptoms (A-D) or treatment (E) to the end of observation showed a statistically significant beneficial effect of the CD4+CD25+ RMTCs compared with each control group in the experiments shown in panels A and C-E (P < .05). In the experiment shown in panel B, a statistically significant benefit (P < .05) was observed for doses equal to or greater than 5 × 105. At a dose of 2.5 × 105, a beneficial effect was observed from days 30 to 45 only (P < .05).
Prevention and treatment of EAE with CD4+CD25+ RMTCs. (A) Prophylaxis of EAE. EAE was induced using a dual immunization regimen with MBP89-101 followed by the administration of pertussis toxin.15 To determine the clinical impact of the CD4+CD25+ RMTCs on encephalomyelitis, 1 × 106 flow-cytometrically purified CD4+CD25+ RMTCs (▴), control cells, including CD4+CD25- RMTCs (⬡), CD4+CD25+ non-Tg T cells ( ), or CD4+CD25- non-Tg T cells (▪), or saline (PBS;
), or CD4+CD25- non-Tg T cells (▪), or saline (PBS;  ) was intravenously administered into SJL/J mice at the time of disease induction. Clinical course was monitored. (B) Dose-response relationship of CD4+CD25+ RMTCs;
) was intravenously administered into SJL/J mice at the time of disease induction. Clinical course was monitored. (B) Dose-response relationship of CD4+CD25+ RMTCs;  indicates 1 × 106 cells; ▪, 0.5 × 106; ▴, 0.25 × 106; and ⬡, 0.125 × 106.
indicates 1 × 106 cells; ▪, 0.5 × 106; ▴, 0.25 × 106; and ⬡, 0.125 × 106.  indicates PBS. (C) Delayed treatment of EAE. EAE was induced as above and 1 × 106 Tg (▴) or non-Tg CD4+CD25+ T cells (
indicates PBS. (C) Delayed treatment of EAE. EAE was induced as above and 1 × 106 Tg (▴) or non-Tg CD4+CD25+ T cells ( ) were administered 11 days later to measure the activity of RMTCs at the time of early disease development.
) were administered 11 days later to measure the activity of RMTCs at the time of early disease development.  indicates PBS. (D) Treatment of adoptive transfer EAE. Draining LN cells from MBP89-101–immunized mice were briefly cultured in vitro and 25 × 106 cells intravenously transferred into naive mice. On the same day, 1 × 106 CD4+CD25+ RMTCs or control cells were administered intravenously through a separate site and disease was monitored clinically. Symbols indicate same information as in panel A. (E) Treatment of EAE after epitope spread. Treatment with 1 × 106 Tg or non-Tg cells was delayed until 31 days after disease induction (vertical line). At this time, T-cell response was detected not only against the initiating MBP89-101 epitope, but also against pathologic PLP139-151 and PLP178-191 epitopes.12 Mean score for each arm was adjusted at the time of treatment to exclude animals not treated due to a moribund status or death. Symbols indicate same information as in panel A. Plots A-E show mean clinical score from 4 to 6 animals per treatment group. Each plot is representative of 2 to 3 independent experiments. A 2-sided t-test analysis of mean daily clinical score from the time of first disease symptoms (A-D) or treatment (E) to the end of observation showed a statistically significant beneficial effect of the CD4+CD25+ RMTCs compared with each control group in the experiments shown in panels A and C-E (P < .05). In the experiment shown in panel B, a statistically significant benefit (P < .05) was observed for doses equal to or greater than 5 × 105. At a dose of 2.5 × 105, a beneficial effect was observed from days 30 to 45 only (P < .05).
indicates PBS. (D) Treatment of adoptive transfer EAE. Draining LN cells from MBP89-101–immunized mice were briefly cultured in vitro and 25 × 106 cells intravenously transferred into naive mice. On the same day, 1 × 106 CD4+CD25+ RMTCs or control cells were administered intravenously through a separate site and disease was monitored clinically. Symbols indicate same information as in panel A. (E) Treatment of EAE after epitope spread. Treatment with 1 × 106 Tg or non-Tg cells was delayed until 31 days after disease induction (vertical line). At this time, T-cell response was detected not only against the initiating MBP89-101 epitope, but also against pathologic PLP139-151 and PLP178-191 epitopes.12 Mean score for each arm was adjusted at the time of treatment to exclude animals not treated due to a moribund status or death. Symbols indicate same information as in panel A. Plots A-E show mean clinical score from 4 to 6 animals per treatment group. Each plot is representative of 2 to 3 independent experiments. A 2-sided t-test analysis of mean daily clinical score from the time of first disease symptoms (A-D) or treatment (E) to the end of observation showed a statistically significant beneficial effect of the CD4+CD25+ RMTCs compared with each control group in the experiments shown in panels A and C-E (P < .05). In the experiment shown in panel B, a statistically significant benefit (P < .05) was observed for doses equal to or greater than 5 × 105. At a dose of 2.5 × 105, a beneficial effect was observed from days 30 to 45 only (P < .05).
Suppression did not result from MBP89-101-IAs-ζ chimeric receptor expression alone. Adoptive transfer of as many as 1 × 107 CD4+CD25- RMTCs failed to influence disease outcome (data not shown). In contrast, as few as 2.5 × 105 CD4+CD25+ RMTCs diminished EAE (P < .05 d30-45 vs saline), and disease suppression was nearly complete at doses of 5 × 105 or more cells (P < .05 vs saline, Figure 2B). Suppression was antigen specific and prophylaxis with MBP89-101-IAs-ζ CD4+CD25+ RMTCs could not prevent EAE induced with a different autoantigen, proteolipid protein (PLP) 139-151 (Rajshekar Alli and T.L.G., unpublished observations, September 2004).
Unlike disease prevention, in which RMTCs may act against naive T cells, disease treatment requires effectiveness after T-cell activation. We therefore treated at day 11, corresponding with the development of early disease symptoms (Figure 2C). The CD4+CD25+ RMTCs, in contrast to antigen-nonspecific CD4+CD25+ T cells, efficiently suppressed the incipient autoreactive T-cell response at this time.
We hypothesized that the RMTCs acted directly on differentiated effector T cells, rather than by influencing their priming and development. To test this, we induced EAE by the adoptive transfer of mature pathologic effector T cells. CD4+CD25+ RMTCs, but not control cell populations, were highly effective in blocking adoptively transferred EAE (Figure 2D) and can therefore directly suppress pathologic T-cell function.
The specificity of pathologic T cells in EAE diversifies with time from the inducing MBP89-101 antigen to include additional autoantigenic epitopes, particularly PLP139-151 and PLP178-191.16 This epitope spread helps sustain the autoimmune pathology. Responses to both of these PLP epitopes were detected 31 days after immunization.12 We delayed RMTC treatment until day 31 to test their effectiveness after epitope spread. Whereas non-Tg CD4+CD25+ T cells and CD4+CD25- RMTCs remained ineffective at this late time, CD4+CD25+ RMTCs ameliorated disease (Figure 2E). Clinical disease scores returned toward baseline. Mortality decreased; 5 of 9 mice receiving saline treatment and 5 of 9 receiving control non-Tg CD4+CD25+ cells succumbed to disease, whereas 8 of 8 receiving CD4+CD25+ RMTCs survived. Remission was sustained for more than 80 days (Figure 2E and data not shown).
CD4+CD25+ T cells require close contact with their targets and must be activated to suppress. Our chimeric receptors facilitate this, luring self-specific target cells and stimulating the CD4+CD25+ RMTCs. Clinical translation of RMTCs will be challenging and require an improved understanding of the epitope dynamics and pathogenesis of human autoimmune diseases, and of how regulatory RMTCs may best be applied. Nevertheless, acquiring requisite numbers of immunotherapeutic RMTCs for clinical application should be achievable. We have transduced small numbers of CD4+CD25+ T cells with retroviral vectors, and have shown that they remain functional when expanded in vitro17 (Christine Duthoit and T.L.G., unpublished data, August 2001). Cotransduction of chimeric receptors and FoxP3, which has been shown to confer regulatory activity, into CD4+CD25- T cells may generate similar regulatory cell populations.4,8
Critically, the RMTCs ameliorated EAE even after the pathologic T-cell response disseminated to include specificities not directly targeted by the CD4+CD25+ RMTCs. Due to the complexity of the pathologic T-cell repertoire at the time of intervention in autoimmune diseases, this capacity for bystander T-cell inhibition will be essential for this or similar approaches to ultimately achieve clinical success.
Prepublished online as Blood First Edition Paper, November 4, 2004; DOI 10.1182/blood-2004-09-3579.
Supported by the National Institutes of Health grants R21 AI49872 and R01 AI056153 (T.L.G.) and by the American Lebanese Syrian Associated Charities/St Jude Children's Research Hospital (T.L.G. and D.J.M.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Richard Cross, Jennifer Hoffrage, and Dick Ashmun for assistance with flow cytometric sorting, and Janet Gatewood for assistance with Bio-Plex cytokine analysis.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal