Abstract
Chronic lymphocytic leukemia (CLL) B cells that express unmutated immunoglobulin heavy-chain variable region genes (IgVH) generally express ZAP-70, in contrast to normal B cells or most CLL cases with mutated IgVH. Following IgM ligation, ZAP-70+ CLL cells had significantly higher levels of phosphorylated p72Syk, BLNK, and phospholipase-Cγ (PLCγ) and had greater[Ca2+]i flux than did ZAP-70–negative CLL cases, including unusual ZAP-70–negative cases with unmutated IgVH. IgM ligation of ZAP-70–negative CLL B cells infected with an adenovirus vector encoding ZAP-70 induced significantly greater levels of phosphorylated p72Syk, BLNK, and PLCγ and had greater[Ca2+]i flux than did similarly stimulated, noninfected CLL cells or CLL cells infected with a control adenovirus vector. We conclude that expression of ZAP-70 in CLL allows for more effective IgM signaling in CLL B cells, a feature that could contribute to the relatively aggressive clinical behavior generally associated with CLL cells that express unmutated IgVH.
Introduction
The B cells of patients with chronic lymphocytic leukemia (CLL) can be segregated into one of at least 2 major subsets based upon the mutational status of the expressed immunoglobulin (Ig) heavy-chain variable region genes (IgVH).1 Patients with CLL cells that express unmutated IgVH tend to have a relatively aggressive clinical course when compared with patients who have CLL cells that express IgVH with somatic mutations.2,3 Although these 2 types share a common gene expression profile, isolated CLL B cells of these 2 subgroups can be distinguished from each other by the differential expression of a relatively small subset of genes.4 One of these genes encodes the zeta-associated protein of 70 kDa (ZAP-70), a receptor-associated protein tyrosine kinase (PTK) expressed by T lymphocytes and natural killer cells (NK cells) but not by normal B cells or most cases of CLL with mutated IgVH.
The functional significance of ZAP-70 gene expression in this subset of CLL B cells is unknown. Irrespective of the expression of ZAP-70, CLL cells generally express similar levels of p72Syk, a related PTK.5,6 B cells use p72Syk for signal transduction via the B-cell receptor (BCR) complex.7 Following BCR ligation, p72Syk is recruited to the phosphorylated immunoreceptor tyrosine-based activation motifs (ITAM) of the activated BCR complex, where it subsequently becomes phosphorylated and activated.8 In normal B cells, activated p72Syk phosphorylates several proteins, including BLNK (for B-cell linker protein, also known as SLP-65, BASH, or BCA).9-11 BLNK serves as a docking site for a number of signaling molecules, including Btk, Vav, and phospholipase C-gamma (PLCγ). Phosphorylation and activation of PLCγ lead to hydrolysis of the polyphosphoinositides and subsequent production of inositol 1,4,5-trisphosphate (IP3) and diacylglycerol (DAG), which serve to increase intracellular calcium (Ca2+) and activate protein kinase C (PKC) and Ras, respectively.
Recent studies indicate that CLL cells that express ZAP-70 mRNA have levels of ZAP-70 protein that are similar to those of normal blood T cells.6,12,13 Furthermore, treatment of ZAP-70+ CLL cells with anti-μ induced significantly greater tyrosine phosphorylation of cytosolic proteins, including p72Syk, than CLL cells that lacked ZAP-70.6 Moreover, following treatment with anti-μ, ZAP-70 underwent tyrosine phosphorylation and became associated with surface μ and CD79b, arguing that this PTK might enhance BCR receptor signaling in CLL B cells. However, the contributions of ZAP-70 to the activation of downstream adaptor proteins, such as BLNK or PLCγ, or to changes in intracellular Ca2+ in response to Ig receptor signaling in CLL have not been resolved.
Resolution of the role that ZAP-70 plays in Ig receptor signaling in CLL is important, as this protein tyrosine kinase is not an obvious candidate to enhance BCR signaling, given that CLL cells generally also express p72Syk.6 Indeed, p72Syk has an approximately 100-fold greater intrinsic PTK activity than does ZAP-70.14 Furthermore, normal B cells that do not express ZAP-70 can respond to BCR ligation better than the leukemia B cells of most patients with CLL. Finally, in contrast to ZAP-70, p72Syk can undergo stimulation in the absence of src-related kinases, initiate immunoreceptor signaling, and promote tyrosine phosphorylation of immunoreceptor tyrosine-based activation motif (ITAM) in the BCR complex.15 As such, it is not certain whether ZAP-70 enhances actual BCR signaling in CLL cells or merely is associated with other factor(s) that are found in CLL with unmutated IgVH that enhance BCR-induced phosphorylation of p72Syk.
In this study, we determined the levels of phosphorylated p72Syk, BLNK, and PLCγ and measured the intracellular flux of calcium ([Ca2+]i) following BCR ligation on CLL cells that did or did not express ZAP-70. Moreover, to test the hypothesis that ZAP-70 is involved in BCR receptor signaling, we infected CLL cells lacking ZAP-70 with an adenovirus vector encoding this PTK and then examined for changes in intensity of BCR signaling. These studies allowed us to determine whether ZAP-70 directly enhances BCR signaling in CLL cells independent of IgVH mutation status.
Materials and methods
Cells and sample preparation
Blood samples were collected from consenting patients who satisfied diagnostic and immunophenotypic criteria for common B-cell CLL.16 The patients were not previously treated and had not received recombinant growth factors or exogenous cytokines. Blood mononuclear cells were isolated by density-gradient centrifugation over Ficoll-Hypaque (Pharmacia, Uppsala, Sweden). ZAP-70 protein was detected by immunoblot analysis of cell lysates prepared from purified CLL B-cell populations containing less than 0.1% non-CD19+ cells.6 In addition we performed flow cytometry on the blood mononuclear cells to evaluate for expression of ZAP-70, as described.17 Cases were considered as positive for ZAP-70 if they had detectable ZAP-70 protein by immunoblot analysis. In all such cases, more than 20% of the leukemia cells had a fluorescence intensity greater than a set threshold when labeled with a fluorescent anti–ZAP-70 antibody, as described.17 We examined the Ig VH genes used by CLL B cells as described.6 Leukemia cells were stimulated with biotinylated goat anti–human IgM F(ab)2, as described.6 Before and after stimulation, cell pellets were prepared and then lysed in ice-cold 1% Nonidet P-40 (NP-40) lysis buffer for 20 minutes on ice. Cell lysates were clarified by centrifugation at 20 000g for 15 minutes. The protein concentration of each cell lysate was determined using the Bio-Rad Protein Assay (Bio-Rad Laboratories, Hercules, CA).
Adenovirus transduction of CLL B cells
We subcloned ZAP-70 cDNA into the multiple cloning sites of shuttle plasmid MCS(SK)pXCX2 containing the expression cassette. The ZAP-70 pXCX2 plasmid was cotransfected with pJM17 into 293 cells. High-titer adenovirus was determined by infecting 293 cells with serial dilutions of the purified adenovirus. Viral titers ranged from 1 × 1010 to 2 × 1010 plaque-forming units per milliliter. 2 × 106 CLL B cells in 100 μL of RPMI-1640 medium supplemented with 10% fetal bovine serum was infected with 100 μL control adenovirus vectors or Ad-ZAP70 and cultured for 2 days at 37°C prior to analysis.18
Multiplexed bead assay
Sodium dodecyl sulfate (SDS) was added to each cell lysate for a final concentration of 1%. The lysates were heated to boiling temperature for 5 minutes prior to being used for the cytometric bead array (CBA) assay. A BD CBA assay (BD Biosciences, San Diego, CA) was used to measure tyrosine-phosphorylated p72Syk, BLNK, and PLCγ. Briefly, 3 μg cell lysate was incubated with a 3-specificity mixture of CBA beads, each coated with antibodies specific for p72Syk, BLNK, or PLCγ. After 3 hours at room temperature, the beads were washed, and phycoerythrin (PE)–conjugated antiphosphotyrosine detector antibody was added for another hour. The fluorescence intensity of the bead-bound antiphosphotyrosine detector antibody was assessed using a dual-laser FACS-Calibur (BD Biosciences, San Diego, CA). Lysate from pervanadate-activated EBI cells (lymphoma B cell) was used as a standard in all assays. Cell lysates were denatured by adding SDS to a final concentration of 1% and boiling for 5 minutes. One thousand nanograms of lysate gave a good signal that was arbitrarily called 1000 units. A serial dilution of the lysate was made, and the mean fluorescence intensity (MFI) of each standard versus the number of units was plotted as a standard curve using BD CBA software. The signal from 3-μg samples was compared to the standard curve, and the readings reflected relative units of phosphorylated protein. To report the change in phosphorylated protein following stimulation, we subtracted 1 from the ratio of result for the stimulated sample over that of the same sample before stimulation and multiplied this number by 100 to derive the percent change in protein phosphorylation.
Immune precipitation and immunoblot analyses
Immune precipitation and immunoblot analyses were performed as described.6 Size-separated proteins were transferred to membranes and were blotted with primary antibody and secondary antibodies that were conjugated with horseradish peroxidase. Blots then were prepared for enhanced chemiluminescence and subsequent autoradiography. For immune precipitation studies, bound proteins were eluted by boiling the samples in SDS sample buffer prior to polyacrylamide gel electrophoresis.
Measurement of intracellular calcium
2 × 106 CLL cells were loaded with 2 μM Fluo-4AM (Molecular Probes, Eugene, OR) in Hanks balanced salt solution (HBSS) without Ca++ and Mg++ and incubated for 30 minutes at 37°C. Cells were washed 2 times with HBSS and then suspended in 1 mL of deficient RPMI. Fluorescence of the cellular suspension was observed with a flow cytometer (Becton Dickinson, San Jose, CA). Ionomycin was added to release all calcium. Cells were kept at 37°C for IgM stimulation. To report the change in calcium influx following stimulation, we calculated the fluorescence intensity peak increase (FIPI) (fluorescence intensity peak value after stimulation minus fluorescence intensity peak value before stimulation), as described in the “Results” section of the text.
Statistical analysis
Differences between 2 groups of CLL samples were determined by unpaired t tests, with P < .05 considered significant. The relevance of ZAP-70 and mutation status to increased protein phosphorylation was analyzed by software R (R Foundation for Statistical Computing).
Results
IgM signaling in CLL cells with or without ZAP-70
We developed a multiplexed bead assay that allowed for simultaneous, quantitative assessment of tyrosine-phosphorylated p72Syk, BLNK, and PLCγ in small amounts of cell lysate. Each of 3 different fluorescent microspheres were coated with monoclonal antibody (mAb) specific for p72Syk, BLNK, or PLCγ and subsequently incubated with a denatured cell lysate. The beads were washed and then incubated with phycoerythrin-conjugated antiphosphotyrosine mAb. The fluorescence intensities of the treated microspheres then were assessed via flow cytometry.
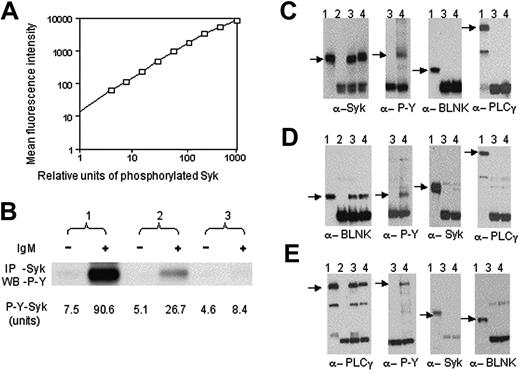
For each assay we developed a standard curve using serial dilutions of a lysate prepared from the human B-cell lymphoma line EBI, which contains high levels of phosphorylated p72Syk, BLNK, and PLCγ after incubation with pervanadate. Serial dilutions of this lysate provided for proportionately less mean fluorescence intensity, demonstrating the linearity of this assay through nearly 3 log orders of lysate concentrations (Figure 1A). Figure 1B compares the results of measuring for phosphorylated p72Syk in lysates using the CBA assay versus those obtained from standard p72Syk-immune precipitation and immunoblot analysis probed with antiphosphotyrosine mAb. The band intensity by densitometry for the anti–IgM-stimulated sample no. 1 was 3 times that noted for the anti–IgM-stimulated sample no. 2 in Figure 1B, providing a ratio of signal intensity that was similar to that noted for anti–IgM-stimulated samples no. 1 versus no. 2 using the CBA assay (eg, 3.3) (Figure 1B).
Multiplex bead assay to detect phosphorylated proteins. (A) Standard curve of phosphorylated p72Syk for CBA assay. The bar size corresponds to the mean fluorescence intensity, the horizontal axis represents relative units of phosphorylated p72Syk in activated EBI cells. The open squares indicate the data from analyses of serial dilutions of the activated EBI cell lysate. (B) Comparison of CBA assay with immunoblot analysis to detect phosphorylated p72Syk. Phosphorylated p72Syk was determined by immunoblot analysis (upper panel) and CBA assay (low panel) for 3 CLL before (–) or after (+) anti-μ stimulation. p72Syk expression was similar among the 3 samples (data not shown). In panels C, D, and E, we present the immunoblot analyses of lane 1, whole EBI cell lysate; lane 2, protein stripped from CBA beads; lane 3, protein stripped from beads after they were incubated with lysate from resting EBI cells; lane 4, protein stripped from beads after they were incubated with lysate from activated EBI cells. Immunoblots were probed with antibodies specific for α-p72Syk (α-Syk), α-tyrosine-phosphorylated protein (α-P-Y), α-BLNK, and α-PLCγ as indicated at the bottom of each immunoblot. The beads used in these studies were coated with antibodies specific for p72Syk (panel C), BLNK (panel D), or PLCγ (panel E), respectively.
Multiplex bead assay to detect phosphorylated proteins. (A) Standard curve of phosphorylated p72Syk for CBA assay. The bar size corresponds to the mean fluorescence intensity, the horizontal axis represents relative units of phosphorylated p72Syk in activated EBI cells. The open squares indicate the data from analyses of serial dilutions of the activated EBI cell lysate. (B) Comparison of CBA assay with immunoblot analysis to detect phosphorylated p72Syk. Phosphorylated p72Syk was determined by immunoblot analysis (upper panel) and CBA assay (low panel) for 3 CLL before (–) or after (+) anti-μ stimulation. p72Syk expression was similar among the 3 samples (data not shown). In panels C, D, and E, we present the immunoblot analyses of lane 1, whole EBI cell lysate; lane 2, protein stripped from CBA beads; lane 3, protein stripped from beads after they were incubated with lysate from resting EBI cells; lane 4, protein stripped from beads after they were incubated with lysate from activated EBI cells. Immunoblots were probed with antibodies specific for α-p72Syk (α-Syk), α-tyrosine-phosphorylated protein (α-P-Y), α-BLNK, and α-PLCγ as indicated at the bottom of each immunoblot. The beads used in these studies were coated with antibodies specific for p72Syk (panel C), BLNK (panel D), or PLCγ (panel E), respectively.
Control studies were performed to evaluate whether each of the beads captured proteins other than that targeted by the bead-bound mAb. For this, we stripped the protein(s) bound to each of the 3 types of beads following their incubation with a denatured cell lysate. Immunoblot analyses of the stripped proteins revealed that each of the microspheres bound only to the protein targeted by the bead-bound mAb under the conditions used (Figure 1C-E for beads coated with mAb specific for p72Syk, BLNK, or PLCγ, respectively).
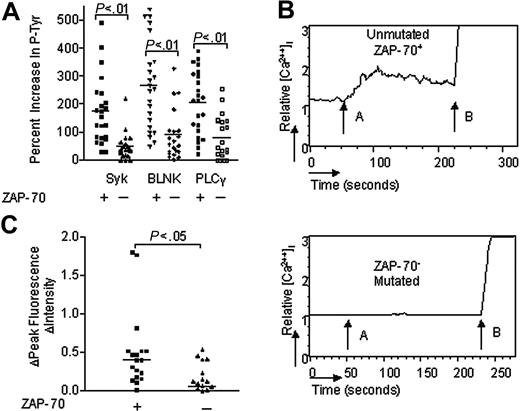
We studied the relationship between expression of ZAP-70 and magnitude of anti-μ signaling in 23 ZAP-70+ CLL cases and 21 ZAP-70–negative cases. Each of the CLL samples had comparable expression of surface IgM (data not shown), allowing us to use anti-μ F(ab)2 (abbreviated as “anti-μ”) as an agonist to effect BCR signaling. Two samples were stimulated with anti-μ for 30 seconds, 1 minute, 5 minutes, 10 minutes, 15 minutes, or 30 minutes. We detected increased levels of phosphorylated p72Syk in the leukemia cell sample that expressed ZAP-70 as early as 30 seconds after treatment. However, the level of detected phosphorylated p72Syk increased over time, reaching a maximum plateau by 10 minutes after treatment. On the other hand, we did not detect increased levels of phosphorylated p72Syk in lysates of anti-μ–treated leukemia cells lacking ZAP-70, even at 30 minutes after anti-μ stimulation. Therefore, to examine multiple samples, we chose to measure the levels of tyrosine-phosphorylated proteins in lysates of CLL cells before and at 10 minutes after treatment with the anti-μ antibody. The same amounts of cell lysate were used in each assay. Using the CBA assay we found that lysates prepared from ZAP-70+ and ZAP-70–negative CLL cells prior to anti-μ stimulation had similar average amounts of tyrosine-phosphorylated p72Syk (11.6 ± 15.6 SD for the ZAP-70+ cases versus 9.3 ± 5.3 SD for the ZAP-70–negative cases), phosphorylated BLNK (6.2 ± 6.2 SD versus 5.5 ± 3.3 SD), or phosphorylated PLCγ (5.1 ± 4.0 S.D vs 6.7 ± 5.2 S.D). Following treatment with anti-μ, however, we found that the ZAP-70+ CLL cases had average increases of 175% (± 121% SD) in tyrosine-phosphorylated p72Syk, 267% (± 156% SD) in tyrosine-phosphorylated BLNK, and 207% (± 112% SD) in tyrosine-phosphorylated PLCγ. The increase in tyrosine-phosphorylated p72Syk was comparable to noted increase in tyrosine-phosphorylated p72Syk detected in normal B cells following treatment with anti-μ (data not shown). However, these increases in the levels of these tyrosine-phosphorylated proteins following treatment with anti-μ were significantly greater than the 49% increase (± 48% SD) in phosphorylated p72Syk, 89% increase (± 88% SD) in phosphorylated BLNK, or 81% increase (± 78% SD) in phosphorylated PLCγ observed in anti-μ–treated ZAP-70–negative cases (P < .0001, Student t test) (Figure 2A).
IgM-induced p72Syk, BLNK, and PLCγ phosphorylation and calcium mobilization in CLL B cells with or without ZAP-70. (A) IgM-induced phosphorylation of p72Syk (Syk), BLNK, and PLCγ in CLL B cells with or without ZAP-70. The bar size corresponds to the percent increase in phosphorylated proteins after anti-μ stimulation by CBA assay. (B) IgM-induced calcium mobilization in CLL B cells with or without ZAP-70. The relative mean fluorescence intensity in intracellular calcium is plotted as a function of time: arrow A indicates IgM stimulation; arrow B, ionomycin stimulation. (C) IgM-induced calcium fluorescence intensity increase in CLL B cells with or without ZAP-70. The bar size corresponds to the increase of fluorescence intensity value after anti-μ stimulation. + indicates CLL B cells with ZAP-70 expression; –, CLL B cells without ZAP-70 expression. The horizontal line drawn among the samples of each group represents the median increase in signal intensity for that group.
IgM-induced p72Syk, BLNK, and PLCγ phosphorylation and calcium mobilization in CLL B cells with or without ZAP-70. (A) IgM-induced phosphorylation of p72Syk (Syk), BLNK, and PLCγ in CLL B cells with or without ZAP-70. The bar size corresponds to the percent increase in phosphorylated proteins after anti-μ stimulation by CBA assay. (B) IgM-induced calcium mobilization in CLL B cells with or without ZAP-70. The relative mean fluorescence intensity in intracellular calcium is plotted as a function of time: arrow A indicates IgM stimulation; arrow B, ionomycin stimulation. (C) IgM-induced calcium fluorescence intensity increase in CLL B cells with or without ZAP-70. The bar size corresponds to the increase of fluorescence intensity value after anti-μ stimulation. + indicates CLL B cells with ZAP-70 expression; –, CLL B cells without ZAP-70 expression. The horizontal line drawn among the samples of each group represents the median increase in signal intensity for that group.
Activation of PLCγ induces mobilization of intracellular calcium that can be monitored via flow cytometry11,19 . Following treatment with anti-μ, we typically observed increases in[Ca2+]I in ZAP-70+ CLL cells, but not in ZAP-70–negative CLL cells (Figure 2B). Nevertheless, both types of CLL cells still could mobilize intracellular calcium in response to ionomycin. To compare samples, we determined the average fluorescence intensity peak value after anti-μ stimulation and subtracted the background fluorescence intensity observed prior to anti-μ stimulation, which we designate “FIPI.” The average FIPI following IgM ligation was significantly greater in ZAP-70+ CLL B cells (0.51 units ± 0.51 SD, n = 18) than it was in ZAP-70–negative CLL B cells (0.08 units ± 0.26 S.D, n = 20, P = .002, Student t test) (Figure 2C).
IgM signaling is associated with expression of ZAP-70
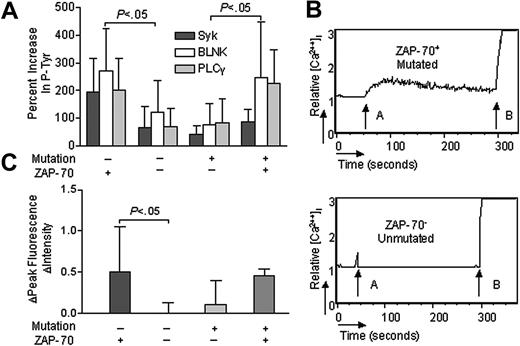
Conceivably, the relative effectiveness of IgM signaling in CLL could be enhanced by factors other than ZAP-70 that also are found associated with CLL cells that express unmutated IgVH. To address this issue, we focused attention on unusual cases of CLL in which the leukemia cells expressed mutated IgVH and ZAP-70 or unmutated IgVH without ZAP-70. Of the 44 cases examined, 4 of the ZAP-70+ cases also had mutated IgVH, each having less than 96% homology to any known germ line IgVH. On the other hand, 6 of the ZAP-70–negative cases expressed unmutated IgVH with more than 98% homology to a known germ line IgVH, indicating that the association between expression unmutated IgVH and ZAP-70 is not absolute. Following IgM ligation, the 6 ZAP-70–negative CLL samples that had unmutated IgVH had average increases in the levels of tyrosine-phosphorylated p72Syk of 65% (± 80% SD), BLNK of 121% (± 115% SD), or PLCγ of 70% (± 67% SD). These average increases were significantly less than that noted for 19 anti-μ–treated cases that expressed ZAP-70 and unmutated IgVH, namely 193% (± 124% SD), 271% (± 152% SD) or 203% (± 113% SD) for phosphorylated p72Syk, BLNK, or PLCγ, respectively (P < .05; Student t test) (Figure 3A). On the other hand, the increases in the levels of phosphorylated p72Syk (87% ± 46% SD), BLNK (246% ± 201% SD) or PLCγ (224% ± 124% SD) following anti-μ treatment of the 4 ZAP-70+ CLL cell samples with mutated IgVH were significantly greater than that observed for the 15 ZAP-70–negative CLL with mutated IgVH, namely 42% (± 32% SD), 76% (± 76% SD), or 85% (± 84% SD) for phosphorylated p72Syk, BLNK, or PLCγ, respectively (P < .05; Student t test) (Figure 3A). As such, the relative proficiency of IgM signaling in CLL appears more tightly associated with the expression of ZAP-70 than with the Ig mutation status.
p72Syk, BLNK, and PLCγ phosphorylation and calcium mobilization in CLL B cells with discordant expression of Ig genes and ZAP-70. (A) IgM-induced phosphorylation of p72Syk (dark gray bars), BLNK (open bars), and PLCγ (light gray bars) in CLL B cells with discordant expression of Ig genes and ZAP-70. The bar size corresponds to the percent increase in phosphorylated proteins after anti-μ stimulation by CBA assay. (B) IgM-induced calcium mobilization in CLL B cells with discordant expression of Ig genes and ZAP-70. The relative mean fluorescence intensity in intracellular calcium is plotted as a function of time: arrow A indicates IgM stimulation; arrow B, ionomycin stimulation. (C) IgM ligation–induced calcium fluorescence intensity increase in CLL B cells with discordant expression of Ig genes and ZAP-70. The bar size corresponds to the increase of fluorescence intensity value after anti-μ stimulation. + indicates CLL B cells with mutated Ig genes or ZAP-70 expression; –, CLL B cells with unmutated Ig genes or without ZAP-70 expression. Error bars indicate mean ± SD.
p72Syk, BLNK, and PLCγ phosphorylation and calcium mobilization in CLL B cells with discordant expression of Ig genes and ZAP-70. (A) IgM-induced phosphorylation of p72Syk (dark gray bars), BLNK (open bars), and PLCγ (light gray bars) in CLL B cells with discordant expression of Ig genes and ZAP-70. The bar size corresponds to the percent increase in phosphorylated proteins after anti-μ stimulation by CBA assay. (B) IgM-induced calcium mobilization in CLL B cells with discordant expression of Ig genes and ZAP-70. The relative mean fluorescence intensity in intracellular calcium is plotted as a function of time: arrow A indicates IgM stimulation; arrow B, ionomycin stimulation. (C) IgM ligation–induced calcium fluorescence intensity increase in CLL B cells with discordant expression of Ig genes and ZAP-70. The bar size corresponds to the increase of fluorescence intensity value after anti-μ stimulation. + indicates CLL B cells with mutated Ig genes or ZAP-70 expression; –, CLL B cells with unmutated Ig genes or without ZAP-70 expression. Error bars indicate mean ± SD.
Consistent with the phosphorylation results, anti-μ treatment induced a robust increase in[Ca2+]I in CLL cells that expressed mutated IgVH and ZAP-70 (Figure 3B). Moreover, the average FIPI for anti-μ–treated CLL cases that expressed mutated IgVH and ZAP-70 (0.46 ± 0.08 SD, n = 2) appeared greater than that observed for similarly treated CLL cells with mutated IgVH that lacked ZAP-70 (0.11 ± 0.30 SD, n = 14) (Figure 3C). On the other hand, [Ca2+]I generally was not increased following IgM ligation on CLL cases with unmutated IgVH that lacked ZAP-70 (Figure 3B). For these unusual cases, the average FIPI (0.01 ± 0.13 SD, n = 6) following anti-μ treatment was significantly lower than that observed in similarly treated CLL cases that expressed unmutated IgVH and ZAP-70 (0.51 ± 0.54 SD, n = 16) (Figure 3C).
CLL cells transduced with Ad-ZAP70 have enhanced IgM signaling
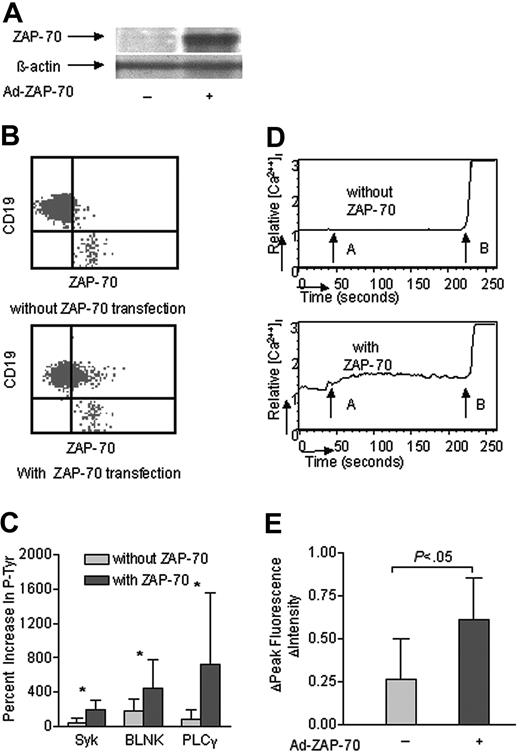
We infected CLL cells that lacked ZAP-70 with an adenovirus vector encoding this PTK (Ad-ZAP70). Expression of ZAP-70 was monitored in the infected CLL cells via immunoblot analyses (Figure 4A) and by flow cytometry (Figure 4B). We determined the IgM-induced phosphorylation of p72Syk, BLNK, and PLCγ in cells that had or had not been transduced with Ad-ZAP70 using the CBA assay. CLL B cells made to express ZAP-70 had a respective average increase of 200% ± 100% (SD), 480% ± 380%, or 750% ± 100% in the levels of phosphorylated p72Syk, BLNK, or PLCγ following treatment with anti-μ. These values were significantly greater than the 50% ± 50%, 200% ± 160%, and 70% ± 120% respective increases noted in the levels of phosphorylated p72Syk, BLNK, and PLCγ of mock-infected CLL cells following IgM ligation (Figure 4C). Moreover, anti-μ treatment induced a rapid and sustained calcium influx in CLL B cells made to express ZAP-70, but not in mock-infected CLL B cells that did not express ZAP-70 (Figure 4D). The FIPI following IgM ligation also was greater in cells made to express ZAP-70 (0.61 ± 0.24 SD) than that in mock-infected CLL cells (0.26 ± 0.24 SD) (P < .05, n = 11) (Figure 4E).
Transduction of ZAP-negative CLL B cells with Ad-ZAP70 enhanced p72Syk, BLNK, and PLCγ phosphorylation and calcium mobilization. (A, B) Intracellular ZAP-70 in ZAP-70– CLL B cells following transduction with a control adenovirus vector (–) or Ad-ZAP70 (+) by immunoblot analysis and flow cytometry. (C) IgM-induced phosphorylation of p72Syk, BLNK and PLCγ in ZAP-70– CLL B cells following transduction with a control adenovirus vector (light gray bars) or Ad-ZAP70 (dark gray bars). The bar size corresponds to the percent increase in phosphorylated proteins after anti-μ stimulation by CBA assay. *P < .01 between the cells transduced with a control adenovirus vector versus Ad-ZAP70. (D) IgM-induced calcium mobilization in ZAP-70– CLL B cells following transduction with a control adenovirus vector (–) or Ad-ZAP70 (+). The relative mean fluorescence intensity in intracellular calcium is plotted as a function of time: arrow A indicates IgM stimulation; arrow B, ionomycin stimulation. (E) IgM ligation–induced calcium fluorescence intensity increase in ZAP-70– CLL B cells following transduction with a control adenovirus vector (–) or Ad-ZAP70 (+). The bar size corresponds to the increase of fluorescence intensity value after anti-μ stimulation. + indicates CLL B cells transduced with Ad-ZAP70; –, CLL B cells transduced with control adenovirus vector. Error bars indicate mean ± SD.
Transduction of ZAP-negative CLL B cells with Ad-ZAP70 enhanced p72Syk, BLNK, and PLCγ phosphorylation and calcium mobilization. (A, B) Intracellular ZAP-70 in ZAP-70– CLL B cells following transduction with a control adenovirus vector (–) or Ad-ZAP70 (+) by immunoblot analysis and flow cytometry. (C) IgM-induced phosphorylation of p72Syk, BLNK and PLCγ in ZAP-70– CLL B cells following transduction with a control adenovirus vector (light gray bars) or Ad-ZAP70 (dark gray bars). The bar size corresponds to the percent increase in phosphorylated proteins after anti-μ stimulation by CBA assay. *P < .01 between the cells transduced with a control adenovirus vector versus Ad-ZAP70. (D) IgM-induced calcium mobilization in ZAP-70– CLL B cells following transduction with a control adenovirus vector (–) or Ad-ZAP70 (+). The relative mean fluorescence intensity in intracellular calcium is plotted as a function of time: arrow A indicates IgM stimulation; arrow B, ionomycin stimulation. (E) IgM ligation–induced calcium fluorescence intensity increase in ZAP-70– CLL B cells following transduction with a control adenovirus vector (–) or Ad-ZAP70 (+). The bar size corresponds to the increase of fluorescence intensity value after anti-μ stimulation. + indicates CLL B cells transduced with Ad-ZAP70; –, CLL B cells transduced with control adenovirus vector. Error bars indicate mean ± SD.
Discussion
Prior studies had indicated that CLL B cells varied in their capacity to respond to IgM ligation.20-22 Semiquantitative studies found that CLL cells with unmutated Ig genes had greater increases in the levels of tyrosine-phosphorylated proteins following IgM cross-linking than did CLL cells that expressed mutated Ig receptors.6,23 Although we noted that increased IgM signaling capacity also was associated with expression of ZAP-70,6 this could have been secondary to factors other than ZAP-70 that might also have been associated with leukemia cells that express unmutated Ig genes.
In the present study, we developed a CBA assay that allowed for quantitative and simultaneous measurement of the levels of tyrosine-phosphorylated p72Syk, BLNK, and PLCγ in relatively small amounts of cell lysate compared to that required for standard immune precipitation and immunoblot analyses. We examined samples that expressed unmutated genes without ZAP-70 or mutated Ig genes with ZAP-70, allowing us to examine the relative importance of ZAP-70 versus the IgVH mutational status in influencing the relative intensity of IgM signaling. In such cases, the induced increases in phosphorylated forms of p72Syk, BLNK, or PLCγ and[Ca2++]I after IgM ligation appeared more closely associated with the expression of ZAP-70 than with the expression of unmutated IgVH.
We found that ZAP-70+ CLL cells experienced greater increases in the levels of tyrosine-phosphorylated BLNK following IgM ligation than did leukemia cells lacking ZAP-70. BLNK represents a central linker protein that bridges the B-cell receptor–associated kinases with a multitude of signaling pathways that regulate the biologic outcome of BCR ligation9,10,24 . Following BCR ligation on normal B cells, phosphorylated BLNK brings PLCγ into close proximity with activated p72Syk, thereby facilitating tyrosine-phosphorylation of PLCγ and subsequent calcium mobilization.9,10,25 Consistent with this also being the case in CLL B cells we noted that those cases that experienced high-level BLNK phosphorylation following treatment with anti-μ also were best able to mobilize intracellular calcium, a characteristic we found also associated with expression of ZAP-70. Although prior studies also observed heterogeneity among CLL cases in the capacity of BCR ligation to induce tyrosine phosphorylation of cytosolic proteins associated with increases in [Ca2+]i26,27 this is the first study to demonstrate that the capacity of anti-μ ligation to induce an increase in[Ca2+]i is associated with BCR-induced phosphorylation of BLNK and PLCγ and expression of ZAP-70.
Transduction studies on ZAP-70–negative CLL cells with Ad-ZAP70 revealed that expression of ZAP-70 was sufficient to enhance IgM signaling in these CLL B cells. The enhanced signaling noted in transduced cells was not a consequence of adenovirus infection per se, as ZAP-70–negative CLL cells transduced with an adenovirus vector encoding an irrelevant transgene (eg, lacZ) did not have augmented Ig-receptor signaling relative to that of mock-infected cells (Figure 4, and data not shown). Also, Ad-ZAP70–transduced CLL cells still required IgM ligation to induce tyrosine phosphorylation of p72Syk, BLNK, and PLCγ. Moreover, transduction of ZAP-70–negative CLL cells with Ad-ZAP70, but not a control adenovirus, enhanced the capacity of anti-μ to induce mobilization of intracellular calcium.
These results appear similar to those of p72Syk-deficient chicken B cells.28 Transduction of p72Syk-deficient B cells with vectors encoding ZAP-70 restored the capacity of these cells to signal via the BCR complex. However, in contrast to these studies, the ZAP-70–negative CLL cells studied in this and prior studies expressed levels of p72Syk that were similar to that of ZAP-70+ CLL cells. Moreover, transduction of ZAP-70–negative CLL cells with Ad-ZAP70 revealed that such cells could experience p72Syk phosphorylation following IgM ligation, thereby suggesting that p72Syk can be activated in most, if not all, CLL B cells.
A recent study indicated that the response to ligation of IgD on CLL cells may differ from that induced by ligation of IgM.23 Although the capacity of p72Syk to undergo phosphorylation in this study was associated with the expression of unmutated Ig genes (and conceivably also ZAP-70), ligation of IgD on CLL cells could induce phosphorylation of p72Syk also in many CLL cell samples that expressed mutated Ig genes. This study implies that signaling induced by anti-IgD may be more robust or different from that induced by ligation of IgM. Conceivably, there may be less dependency on ZAP-70 for phosphorylation of p72Syk following ligation of IgD in CLL B cells. Alternatively, stimulation with anti-IgD may affect a superphysiologic stimulus that exceeds the stimulation provided by ligation of surface IgM and possibly also by exposure to antigen that does not cross-link all surface Ig receptor. Further studies are required to resolve this issue.
The reason(s) CLL cells that lack ZAP-70 do not appear to be sensitive to treatment with anti-μ is unclear. Conceivably, the p72Syk in CLL cells might be impaired, precluding it from associating with the ITAMs of the BCR accessory molecules or from undergoing phosphorylation following IgM ligation without ZAP-70. Alternatively, p72Syk might be less able than ZAP-70 to overcome the inhibition imposed by overexpression of the alternative transcript of CD79b in CLL, which encodes a truncated form of CD79b that lacks the extracellular Ig-like domain and the Cys residues necessary to form stable heterodimers with CD79a in the BCR complex.29 In any case, our studies demonstrate that expression of ZAP-70, either constitutively or following transduction with Ad-ZAP70, is sufficient to allow for phosphorylation following IgM ligation of downstream adapter proteins that are required for IgM-signaling.
It appears that a certain level of ZAP-70 protein is required to affect proficient anti-IgM signaling. Moreover, levels of ZAP-70 exceeding this threshold do not apparently enhance the capacity of anti-IgM to affect phosphorylation of p72Syk or downstream adaptor/signaling proteins. For example, linear regression analysis comparing the levels of phosphorylated p72Syk detected following anti-μ treatment with the levels of ZAP-70 detected by flow cytometry in the different ZAP-70–positive leukemia cell samples did not reveal a significant relationship (R2 = 0.03) (data not shown). This mimics the situation observed in the different tendencies for disease progression noted among patients with this disease. In a recent study we observed that patients who had CLL cells with intermediate levels of ZAP-70 had a risk for early disease progression that was similar to that of patients with CLL cells that had high levels of ZAP-70 protein.17 However, these patients had a risk for early disease progression that was significantly greater than that of patients with CLL cells that had little or no ZAP-70 protein. Conceivably, the increased risk for early disease progression in cases that expressed ZAP-70 may be due to the Ig signaling capacity of the CLL cells, which also demonstrated no significant differences between CLL cells that express intermediate or high levels of ZAP-70 protein.
Conceivably, the qualitative expression of ZAP-70 may enhance Ig-receptor signaling in vivo, which in turn could enhance the survival and/or proliferation of CLL B cells.30,31 This could account in part for the more aggressive clinical behavior noted for patients with CLL cells that express unmutated IgV genes, which typically also express ZAP-70.
Prepublished online as Blood First Edition Paper, October 28, 2004; DOI 10.1182/blood-2004-05-1715.
Supported in part by National Institutes of Health grants R37 CA49870 and P01 CA81534 of the CLL Research Consortium and P01 AI45865 (A.W.).
J.A. and T.G.-M. are employed by BD PharMingen, whose potential product was studied in the present work.
An Inside Blood analysis of this article appears in the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors appreciate the advice and skilled assistance of Ms Esther Avery with the ZAP-70 assays and of Christina Wu with calcium influx assay.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal