Abstract
Chronic lymphocytic leukemia (CLL) is frequently associated with autoimmune diseases directed against constituents of the blood, including hemolytic anemia (AIHA). We hypothesized that CLL cells predispose to hematologic autoimmunity by acting as aberrant antigen-presenting cells (APCs). Initially, it was confirmed that all studied patients with AIHA secondary to CLL harbored activated helper T (TH) cells specific for epitopes on the dominant red blood cell (RBC) autoantigens in primary AIHA, the Rh proteins. Rh-specific TH cells were also detected in a number of patients with CLL who, although they did not have AIHA, had low levels of anti-RBC antibody in their sera. Fractionation of putative APC populations from the peripheral blood of patients by negative selection showed that CD5+ CLL cells are the most effective cell type in processing and presenting purified Rh protein to autoreactive TH cells. This ability was confirmed using positively selected CD5+ CLL cells. Thus, our study provides the first evidence for malignant cells driving an autoimmune response by acting as aberrant APCs.
Introduction
Chronic lymphocytic leukemia (CLL) is a common, low-grade malignancy of CD5-expressing B cells that may be pregerminal or postgerminal center.1,2 The condition is unusual among leukemias because it is frequently associated with immunologically mediated complications that contribute substantially to morbidity and mortality. In particular, autoantibodies specific for red blood cells (RBCs) are detectable by direct antiglobulin test (DAT) in up to 20% of patients with advanced CLL and can cause autoimmune hemolytic anemia (AIHA).3,4 Other common immunologic complications include pure RBC aplasia and immune thrombocytopenic purpura.5-7 In addition, rare autoantibodies can develop against factor VIII, von Willebrand factor, and C1 esterase inhibitor.8-10 A striking feature of autoimmunity in CLL is that it appears to be directed against constituents of the blood with no documented propensity to other targets.11 However, despite the importance of this immune dysregulation in CLL, the causes of autoantibody production remain unclear.
Studies of animal models and human AIHA demonstrate that the activation of autoreactive helper T (TH) cells by antigen-presenting cells (APCs) is a key event in the induction of disease.12-20 The generation of most immunoglobulin G (IgG) antibodies is T-cell dependent, and responses to RBC autoantigens appear to be no exception. Thus, in mice, T-cell depletion blocks the autoantibody response induced by cross-reactive rat RBCs.12 Furthermore, spontaneous anti-RBC autoantibody production in New Zealand Black (NZB) mice can be retarded by anti-CD4 monoclonal antibody14 or CD4 gene deletion,18 and anemia can be modulated by peptides containing the dominant TH cell epitope.19 We have previously demonstrated that patients with primary warm-type AIHA mediated by IgG autoantibodies harbor activated TH cells specific for the Rh proteins,16,17 the dominant human RBC autoantigens,13,20 but there have been no studies of RBC-reactive TH cells in AIHA secondary to CLL. If AIHA associated with CLL is TH dependent, the question that arises is why the autoreactive TH cells are activated.
In autoimmune disease, the B cell is largely studied in the context of pathogenic autoantibody production while other functions, including antigen presentation, have often been ignored.21 RBC- and platelet-reactive autoantibodies identified in patients with CLL are polyclonal and differ in specificity and isotype from the immunoglobulins secreted directly by CLL cells.22 It is, therefore, generally accepted that residual, nonmalignant B cells must produce the pathogenic autoantibodies in most cases.22-24 We wanted to test the hypothesis24 that the malignant B cell may, instead, play an important role in presentation of autoantigen to activate pathogenic TH cells.
In a conventional immune response, B cells can act as “professional” APCs, with the ability to process and present antigen and to express the costimulatory molecules necessary to stimulate TH cells.25,26 In murine models of autoimmune diseases, such as diabetes27 and multiple sclerosis,28 B cells have been shown to be important in presenting autoantigen. A role for CD5+ CLL B cells in autoantigen presentation has seemed unlikely because the malignant cells appear to be inefficient APCs in vitro.29 However, this possibility should not be discounted in the light of further reports showing that costimulatory molecules, such as CD80, are up-regulated and antigen presentation is enhanced after encounter with T cells expressing CD40 ligand.26,30,31 Self-tolerance is maintained in part by the elimination or silencing of autoreactive lymphocytes,32,33 but TH cells specific for many autoantigens, including Rh proteins,34 can escape these censoring mechanisms in healthy persons. There has been considerable interest in the hypothesis that such surviving autoreactive T cells can be activated and can cause autoimmune disease after changes in APC function or type that enhance presentation of the epitopes they recognize.16,17,34,35
As already mentioned, it is striking that autoimmunity in CLL predominantly targets blood constituents. We hypothesize that the large numbers of malignant B cells in CLL can act as an aberrant APC population: when exposed to high concentrations of RBC and platelet breakdown products, there may be sufficient presentation of blood cell autoantigens by CLL cells to overcome self-tolerance. Here, we test this proposal by first establishing whether the peripheral blood of patients with CLL with AIHA contains TH cells specific for a dominant RBC autoantigen and then determining the ability of malignant CLL B cells to present this autoantigen.
Patients, materials, and methods
Patients
AIHA was diagnosed in patients with CLL attending the Aberdeen Royal Infirmary on the basis of clinical and hematologic investigation and positive findings on DAT. The protocol for investigation was approved by the Grampian Health Board and the University of Aberdeen Joint Ethical Committee, and all patients gave informed consent. Details of the cases are summarized in Table 1. Most blood samples were obtained when patients were receiving low to moderate doses of steroid treatment. A group of patients with CLL, but no evidence of clinical hemolysis, was also included (Table 2).
Details of patients with CLL with secondary AIHA (CLL + AIHA)
. | . | . | . | . | . | Autoantibody specificity . | . | Splenomegaly, Rai stage/splenomegaly, length below costal margin . | . | Blood counts at diagnosis and at sampling . | . | . | . | . | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient . | Sex . | Age, y . | RhD +/- . | Coombs IgG . | Coombs C3 . | Rh . | Pan-agglutinin . | . | CLL/AIHA duration, mo . | Hb, g/L . | WBC, 109/L . | Platelets, 109/L . | Previous therapy . | Current therapy, mg/d . | |||
| 1 | M | 66 | + | +++++ | - | Y | N | II/Tip | 24/24 | 50 | 590 | 100 | Chlorambucil, CHOP | Prednisolone, 5 | |||
| 113 | 6.7 | 138 | |||||||||||||||
| 2 | M | 73 | + | +++++ | +++++ | Y | N | III/- | 18/8 | 62 | 5.9 | 193 | Chlorambucil | Prednisolone, 5; Azathioprine, 100 | |||
| 142 | 42 | 90 | |||||||||||||||
| 3 | M | 75 | + | ++++ | ++ | Y | N | I/- | 3/3 | 83 | 159 | 228 | None | Prednisolone, 7.5 | |||
| 114 | 63 | 206 | |||||||||||||||
| 4 | F | 83 | + | +++++ | - | Y | N | 0/- | 1/1 | 90 | 10.7 | 195 | None | Prednisolone, 40 | |||
| 96 | 12.8 | 220 | |||||||||||||||
| 5 | M | 87 | + | + | - | Y | N | II/2 cm | 48/48 | 51 | 35.5 | 53 | None | Prednisolone, 40 | |||
| 84 | 57 | 99 | |||||||||||||||
| 6 | M | 68 | + | + | + | Y | N | IV/6 cm | 4/2 | 68 | 366 | 111 | Chlorambucil | Prednisolone, 20 | |||
| 97 | 166 | 53 | |||||||||||||||
| 7 | M | 69 | - | +++ | ++ | Y | Y | IV/- | 20/16 | 40 | 240 | 331 | Chlorambucil, CHOP, etoposide, 2 courses fludaribine 15/12 presampling | Prednisolone, 80 | |||
| 79 | 339 | 130 | |||||||||||||||
| 8 | F | 82 | - | +++ | ++ | Y | Y | I/- | 98/11 | 78 | 137 | 46 | Chlorambucil | Prednisolone, 5 | |||
| 119 | 15.5 | 213 | |||||||||||||||
| 9 | M | 68 | + | ++ | ++ | UA | UA | II/- | 42/36 | 111 | 98 | 205 | Chlorambucil | Chlorambucil, 2.5 | |||
| 134 | 14.1 | 161 | |||||||||||||||
| 10 | M | 74 | + | ++ | ++ | UA | UA | I/- | 43/1 | 72 | 78.8 | 260 | None | Prednisolone, 30 | |||
| 111 | 83.4 | 312 | |||||||||||||||
| 11 | M | 70 | + | +++ | ++ | UA | UA | IV/6 cm | 74/9 | 48 | 208 | 32 | Chlorambucil Fludarabine 2 courses 2 mo previously | Prednisolone, 60 | |||
| 52 | 204 | 39 | |||||||||||||||
| 12 | F | 78 | + | +++ | - | UA | UA | II/5 cm | 234/140 | 67 | 156 | 201 | Prednisolone Azathioprine | None | |||
| 126 | 30.7 | 104 | |||||||||||||||
. | . | . | . | . | . | Autoantibody specificity . | . | Splenomegaly, Rai stage/splenomegaly, length below costal margin . | . | Blood counts at diagnosis and at sampling . | . | . | . | . | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient . | Sex . | Age, y . | RhD +/- . | Coombs IgG . | Coombs C3 . | Rh . | Pan-agglutinin . | . | CLL/AIHA duration, mo . | Hb, g/L . | WBC, 109/L . | Platelets, 109/L . | Previous therapy . | Current therapy, mg/d . | |||
| 1 | M | 66 | + | +++++ | - | Y | N | II/Tip | 24/24 | 50 | 590 | 100 | Chlorambucil, CHOP | Prednisolone, 5 | |||
| 113 | 6.7 | 138 | |||||||||||||||
| 2 | M | 73 | + | +++++ | +++++ | Y | N | III/- | 18/8 | 62 | 5.9 | 193 | Chlorambucil | Prednisolone, 5; Azathioprine, 100 | |||
| 142 | 42 | 90 | |||||||||||||||
| 3 | M | 75 | + | ++++ | ++ | Y | N | I/- | 3/3 | 83 | 159 | 228 | None | Prednisolone, 7.5 | |||
| 114 | 63 | 206 | |||||||||||||||
| 4 | F | 83 | + | +++++ | - | Y | N | 0/- | 1/1 | 90 | 10.7 | 195 | None | Prednisolone, 40 | |||
| 96 | 12.8 | 220 | |||||||||||||||
| 5 | M | 87 | + | + | - | Y | N | II/2 cm | 48/48 | 51 | 35.5 | 53 | None | Prednisolone, 40 | |||
| 84 | 57 | 99 | |||||||||||||||
| 6 | M | 68 | + | + | + | Y | N | IV/6 cm | 4/2 | 68 | 366 | 111 | Chlorambucil | Prednisolone, 20 | |||
| 97 | 166 | 53 | |||||||||||||||
| 7 | M | 69 | - | +++ | ++ | Y | Y | IV/- | 20/16 | 40 | 240 | 331 | Chlorambucil, CHOP, etoposide, 2 courses fludaribine 15/12 presampling | Prednisolone, 80 | |||
| 79 | 339 | 130 | |||||||||||||||
| 8 | F | 82 | - | +++ | ++ | Y | Y | I/- | 98/11 | 78 | 137 | 46 | Chlorambucil | Prednisolone, 5 | |||
| 119 | 15.5 | 213 | |||||||||||||||
| 9 | M | 68 | + | ++ | ++ | UA | UA | II/- | 42/36 | 111 | 98 | 205 | Chlorambucil | Chlorambucil, 2.5 | |||
| 134 | 14.1 | 161 | |||||||||||||||
| 10 | M | 74 | + | ++ | ++ | UA | UA | I/- | 43/1 | 72 | 78.8 | 260 | None | Prednisolone, 30 | |||
| 111 | 83.4 | 312 | |||||||||||||||
| 11 | M | 70 | + | +++ | ++ | UA | UA | IV/6 cm | 74/9 | 48 | 208 | 32 | Chlorambucil Fludarabine 2 courses 2 mo previously | Prednisolone, 60 | |||
| 52 | 204 | 39 | |||||||||||||||
| 12 | F | 78 | + | +++ | - | UA | UA | II/5 cm | 234/140 | 67 | 156 | 201 | Prednisolone Azathioprine | None | |||
| 126 | 30.7 | 104 | |||||||||||||||
Hb indicates hemoglobin; +, positive; -, negative; Y, yes; N, no; Tip, just palpable; CHOP, cyclophosphamide, adriamycin, vincristine, and prednisolone; and UA, unavailable.
Details of patients with CLL
. | . | . | . | Rai stage splenomegaly . | CLL duration, mo . | Blood counts at sampling . | . | . | Previous therapy . | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient . | Sex . | Age, y . | RhD +/- . | . | . | Hb, g/L . | WBC, 109/L . | Plts, 109/L . | . | ||
| 1 | M | 13 | + | 0 | 85 | 108 | 297 | 86 | None | ||
| 2 | F | 26 | + | I | 26 | 143 | 11.2 | 121 | None | ||
| 3 | M | 58 | + | 0 | 21 | 140 | 71.4 | 97 | None | ||
| 4 | M | 22 | + | II | 59 | 163 | 37.3 | 214 | Chlorambucil | ||
| 4fb | |||||||||||
| 5 | M | 31 | + | I | 42 | 149 | 22 | 136 | None | ||
| 6 | F | 67 | + | 0 | 120 | 128 | 29.6 | 197 | None | ||
| 7 | F | 30 | + | 0 | 30 | 132 | 9.7 | 288 | None | ||
| 8 | M | 62 | + | 0 | 82 | UA | 17.6 | 238 | None | ||
| 9 | F | 65 | + | I | 22 | 133 | 53.2 | 338 | None | ||
| 10 | F | 66 | - | 0 | 84 | 143 | 17.3 | 284 | None | ||
. | . | . | . | Rai stage splenomegaly . | CLL duration, mo . | Blood counts at sampling . | . | . | Previous therapy . | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient . | Sex . | Age, y . | RhD +/- . | . | . | Hb, g/L . | WBC, 109/L . | Plts, 109/L . | . | ||
| 1 | M | 13 | + | 0 | 85 | 108 | 297 | 86 | None | ||
| 2 | F | 26 | + | I | 26 | 143 | 11.2 | 121 | None | ||
| 3 | M | 58 | + | 0 | 21 | 140 | 71.4 | 97 | None | ||
| 4 | M | 22 | + | II | 59 | 163 | 37.3 | 214 | Chlorambucil | ||
| 4fb | |||||||||||
| 5 | M | 31 | + | I | 42 | 149 | 22 | 136 | None | ||
| 6 | F | 67 | + | 0 | 120 | 128 | 29.6 | 197 | None | ||
| 7 | F | 30 | + | 0 | 30 | 132 | 9.7 | 288 | None | ||
| 8 | M | 62 | + | 0 | 82 | UA | 17.6 | 238 | None | ||
| 9 | F | 65 | + | I | 22 | 133 | 53.2 | 338 | None | ||
| 10 | F | 66 | - | 0 | 84 | 143 | 17.3 | 284 | None | ||
None of the patients listed in the table was receiving current therapy; all were Coombs IgG negative.
Plts indicates platelets; other abbreviations are as for Table 1.
Determination of autoantibody specificity
Autoantibody eluted by ether treatment13 from the RBC of AIHA patients, or serum antibody, was screened in hemagglutination assays for the ability to bind a panel of RBC consisting of MKMK, U–, Rhnull,.D., LW(a–b–), Oh, Fy(a–b–), Lu(a–b–), Ko,
Detection of human anti-RBC antibodies by indirect enzyme-linked antiglobulin test
The indirect enzyme-linked antiglobulin test (IELAT) is a sensitive method of detecting RBC-reactive antibodies.36,37 Based on a modification of our published method,38 this test was used to measure the levels of serum anti–human red blood cell IgG in patients with primary and CLL-associated AIHA, patients with CLL with no evidence of AIHA, and healthy donors. Round-bottomed microtiter plates (Nunc, Roskilde, Denmark) were blocked in phosphate-buffered saline (PBS), pH 7.4, containing 0.2% bovine serum albumin (BSA), before the addition of 50 μL 2% vol/vol washed human D-positive RBCs. Test sera were incubated at 50 μL per well with triplicate RBC samples for 1 hour at 37°C and were washed 3 times in PBS-BSA before fixing for 30 minutes in 0.15% glutaraldehyde (Sigma, Dorset, United Kingdom) to prevent lysis of the cells in the alkaline conditions required later in the test. The fixed RBCs were transferred to fresh, preblocked, 96-well plates and were washed before incubation with 50 μL per well 1 μg/mL goat anti–human IgG γ-chain specific antibody (Sigma) for 1 hour at 37°C. After washing, the plates were incubated with 50 μL per well 1 μg/mL rabbit anti–goat IgG alkaline phosphatase (Sigma) for 1 hour at 37°C and washed; 100 μL phosphatase substrate solution was then incubated in each well for 1 hour at 37°C. After pelleting of the RBCs by centrifugation, 50 μL each supernatant was transferred into the wells of fresh, flat-bottomed microtiter plates (Nunc), and absorbance was measured at 405 nm, with 492 nm as a reference, using a Multiscan plate reader (Labsystems, Basingstoke, United Kingdom). Each result is expressed as the mean of triplicate wells. Interassay variation was controlled for by including previously tested serum samples on each plate.
Antigens and mitogens
A complete panel of 68 15-mer peptides, with 5–amino acid overlaps, was synthesized39 (Department of Biochemistry, University of Bristol, United Kingdom), corresponding to the sequences of the 30-kDa Rh protein associated with expression of the D blood group antigen.40 For testing in patients who were D negative, a second panel of 15-mer peptides16,17 corresponding to the ce Rh protein41 was made. To ensure purity, peptides were synthesized by fluorenylmethoxycarbonyl chemistry on resin using a base-labile linker rather than by pin technology and were screened by high-performance liquid chromatography (HPLC) and amino acid analysis. As previously optimized,16,17,39 the peptides were used to stimulate cultures at 20 μg/mL.
As previously described,17 RhD protein was purified from D-positive RBCs by immunoprecipitation using monoclonal antibody T19 (Scottish National Blood Transfusion Service) bound to magnetic beads (Biomag; PerSeptive Biosystems, Framingham, MA) and was added to cultures at an estimated concentration of 5 μg/mL. The control antigen, Mycobacterium tuberculosis purified protein derivative (PPD) (Statens Seruminstitut, Copenhagen, Denmark), was added to cultures at 20 μg/mL. PPD readily provokes recall T-cell responses in vitro16,42 because most United Kingdom citizens have been immunized with Bacillus Calmette-Guérin (BCG).
Isolation of peripheral blood mononuclear cells and preparation of T cells
As described elsewhere,16,17,39 peripheral blood mononuclear cells (PBMCs) were separated from fresh blood samples by density gradient centrifugation. T cells were positively selected from PBMCs using kits of magnetic beads coated with anti-CD2 monoclonal antibody, released, and washed according to the manufacturer's instructions (CELLection; Dynal Biotech, Wirral, United Kingdom). Unbound PBMCs were removed after magnetic separation and were used as a source of APCs.
Preparation of APC fractions by negative selection
Three APC fractions were prepared from T-cell–depleted PBMCs by successive negative-selection steps using antibody-coated magnetic beads (Dynabeads; Dynal Biotech). The cells were sequentially depleted of monocytes with anti-CD14–coated beads, dendritic cells with a mixture of mouse anti–human CD1a and CD11c (both PharMingen, San Diego, CA) immobilized on anti–mouse immunoglobulin G (IgG)–coated beads, and CLL B cells with mouse antihuman CD5 (PharMingen) on anti–mouse IgG-coated beads.
Positive selection of CLL B cells
In some experiments, CLL B cells were isolated from the other potential APC types in T-depleted PBMCs by a single positive isolation step. Kits of magnetic beads (Dynal Biotech) coated with mouse anti–human CD5 (PharMingen) were used, and the CD5+ cells were released, according to the manufacturer's instructions (Dynal Biotech).
Flow cytometry
The purity of negatively and positively selected PBMC populations was screened by flow cytometry using an EPICS XL cytometer (Beckman Coulter, High Wycombe, Buckinghamshire, UK) and Expo version 2 analysis software (Applied Cytometry Systems, Yorks, United Kingdom). Each negatively depleted population contained fewer than 10% cells expressing the respective marker, and more than 95% of the positively selected CLL cells stained CD5+.
Cell culture
Cell culture conditions have previously been described.16,17,39 Briefly, PBMCs were cultured at a concentration of 1.25 × 106 cells/mL in the alpha modification of Eagle medium (Gibco, Paisley, United Kingdom) supplemented with 5% autologous serum, 4 mM l-glutamine (Gibco), 100 U/mL sodium benzylpenicillin G (Sigma), 100 μg/mL streptomycin sulfate (Sigma), and 20 mM HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid) pH 7.2 (Sigma), in a humidified atmosphere of 5% CO2/95% air. Similar conditions were used for T-cell cultures, with APCs supplied by the addition of an equal concentration of one of the different APC fractions.
T-cell stimulation assays
As described elsewhere,16,17,39 PBMCs or T cells plus APCs were cultured with antigen, and T-cell proliferation was estimated from the incorporation of 3H-thymidine in triplicate microtiter wells 5 days after stimulation. Proliferation results are presented either as the mean ± SD counts per minute (cpm) of the triplicate samples or as a stimulation index (SI) expressing the ratio of mean cpm in stimulated compared with unstimulated control cultures. An SI greater than 3 with cpm greater than 1000 is interpreted as representing a significant positive response.43
Inhibition of major histocompatibility complex class II–restricted responses
Results
T-cell responses to the RhD protein and peptide panel
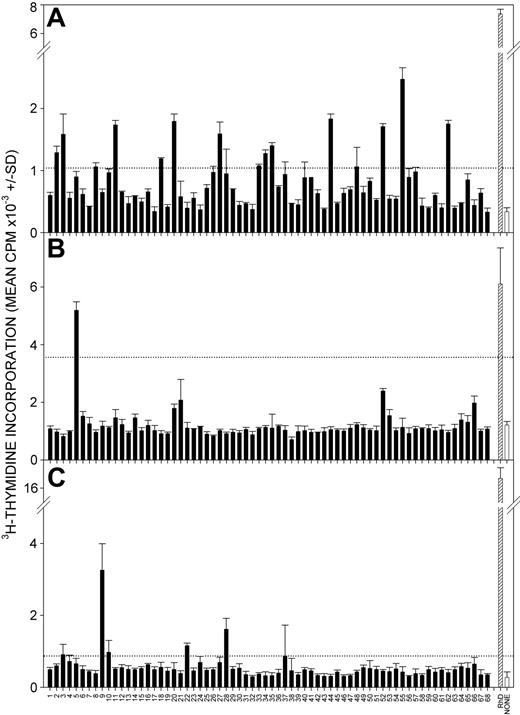
We have previously demonstrated that the blood of patients with primary AIHA contains activated TH cells specific for epitopes on the RhD and RhCcEe proteins.16,17 Experiments were set up to determine whether such TH cells are also present in AIHA secondary to CLL. PBMCs were obtained from 12 patients with CLL with concurrent AIHA (Table 1) and were tested for the ability to proliferate in response to Rh protein or the corresponding panel of peptides. Given the ethical constraints regarding the size of the blood samples, and thus the number of TH cells available, we were only able comprehensively to study responses against either the RhD or the RhCcEe sequences in each case. It was important to ensure that the responses observed were autoimmune and, therefore, that the Rh sequences tested corresponded to “self.” Most of the patients were D-positive; for them, we focused on the RhD rather than the RhCcEe polypeptide. This enabled us to take advantage of our validated method for immunopurifying RhD protein from RBCs,17 allowing a comparison with previous data of TH cell responses in patients with primary AIHA,16,17 and it obviated the need to match the type of the polymorphic RhCcEe protein sequence with each patient. Representative results from 3 of the 10 RhD-positive patients, who were screened with purified RhD protein and the RhD peptide panel, are illustrated in Figure 1, and data from all patients are summarized in Table 3. In all the RhD-positive patients, purified RhD protein induced proliferative responses by PBMCs. Furthermore, it can be seen that 1 or more peptides from the RhD protein elicited significant proliferation by PBMCs from all patients tested, and, typically, multiple peptides were stimulatory. Comparable results were obtained in the 2 RhD-negative patients, whose PBMCs proliferated in response to multiple peptides from the sequence of the Rhce protein autoantigen. As in our previous studies of primary AIHA,16,17 proliferation was assessed on day 5 after stimulation, and cell culture was performed in microtiter plates without the enrichment of TH cells, conditions that strongly favor recall, rather than primary, responses.34,39,42 It was confirmed that the PBMCs proliferating against Rh protein epitopes were helper T cells because the removal of T cells from cultures, or the addition of blocking antibody specific for major histocompatibility complex (MHC) class II molecules, significantly inhibited responses (results not shown).
Responses to RhD autoantigen by PBMCs from patients with CLL with secondary AIHA. Representative experiments showing proliferation by PBMCs from patients CLL+AIHA1 (A), CLL+AIHA2 (B), and CLL+AIHA3 (C) after stimulation with a panel of overlapping 15-mer peptides spanning the sequence of the RhD protein (▪) or purified RhD protein (▨). Control unstimulated cultures were also included (□). Peptides are numbered 1 to 68 from the N-terminus. The dotted line indicates the level of response taken as representing a positive response (SI > 3). Error bars indicate SD.
Responses to RhD autoantigen by PBMCs from patients with CLL with secondary AIHA. Representative experiments showing proliferation by PBMCs from patients CLL+AIHA1 (A), CLL+AIHA2 (B), and CLL+AIHA3 (C) after stimulation with a panel of overlapping 15-mer peptides spanning the sequence of the RhD protein (▪) or purified RhD protein (▨). Control unstimulated cultures were also included (□). Peptides are numbered 1 to 68 from the N-terminus. The dotted line indicates the level of response taken as representing a positive response (SI > 3). Error bars indicate SD.
Summary of proliferative responses to RhD protein and RhD peptides by patients with CLL with secondary AIHA (CLL + AIHA)
Patient . | Purified RhD protein-induced proliferation . | Rh-specific peptides stimulating proliferation* . |
|---|---|---|
| 1 | Y | 2, 3, 8, 11, 18, 20, 27, 33, 34, 35, 44, 46, 48, 50, 52, 55, 62, 67, 68 |
| 2 | Y | 2, 5, 26, 34, 57 |
| 3 | Y | 3, 9, 10, 22, 28, 37 |
| 4 | Y | 1, 23 |
| 5 | Y | 19, 68 |
| 6 | Y | NT |
| 7 | NA | 12, 20, 50, 58, 63 |
| 8 | NA | 2, 3, 4, 20, 21, 23, 44, 52, 54, 63 |
| 9 | Y | NT |
| 10 | Y | 5, 6, 9, 17, 18, 21, 30 |
| 11 | Y | 5, 57 |
| 12 | Y | 21, 46 |
Patient . | Purified RhD protein-induced proliferation . | Rh-specific peptides stimulating proliferation* . |
|---|---|---|
| 1 | Y | 2, 3, 8, 11, 18, 20, 27, 33, 34, 35, 44, 46, 48, 50, 52, 55, 62, 67, 68 |
| 2 | Y | 2, 5, 26, 34, 57 |
| 3 | Y | 3, 9, 10, 22, 28, 37 |
| 4 | Y | 1, 23 |
| 5 | Y | 19, 68 |
| 6 | Y | NT |
| 7 | NA | 12, 20, 50, 58, 63 |
| 8 | NA | 2, 3, 4, 20, 21, 23, 44, 52, 54, 63 |
| 9 | Y | NT |
| 10 | Y | 5, 6, 9, 17, 18, 21, 30 |
| 11 | Y | 5, 57 |
| 12 | Y | 21, 46 |
NA indicates not applicable (RhD-negative patient); NT, not tested.
PBMCs from patients tested with the appropriate panel of synthetic RhD or Rhce peptides to match phenotype (Rhce panel tested in patients CLL + AIHA 7 and 8).
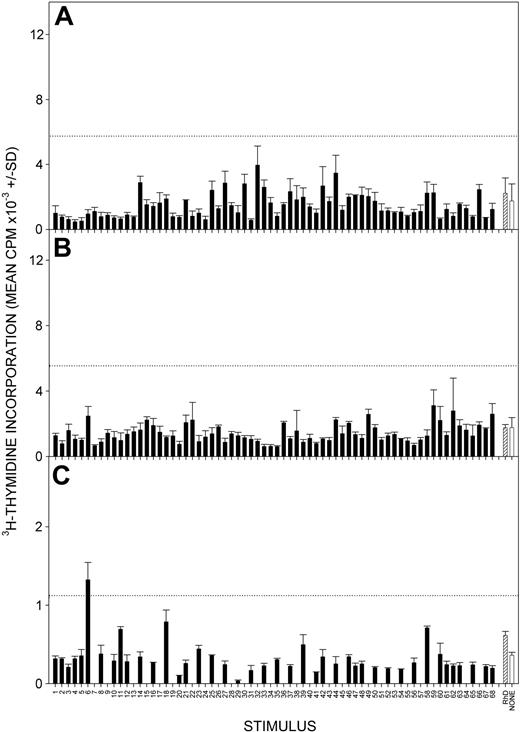
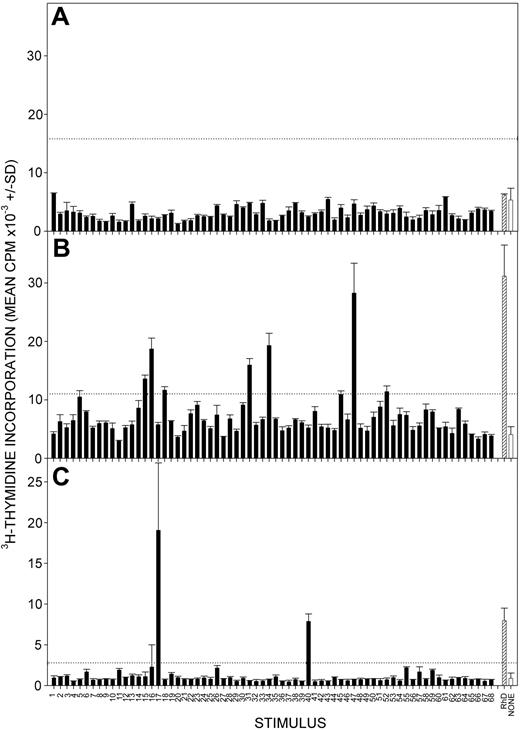
A number of publications16,17,34,35,39 have demonstrated that, in contrast to those in AIHA patients, TH cells capable of recall responses in vitro to epitopes on the Rh proteins are rarely detected in healthy donors or in patients with nonimmunologic causes of anemia, providing support for the view that such cells provide help for the production of anti-RBC autoantibodies. Examples of this lack of responsiveness in healthy donors are illustrated in Figure 2. We now wanted to ascertain whether there may be subclinical anti-RBC autoimmune responses in patients with CLL with no overt signs of AIHA. It was determined whether the PBMCs from 10 such patients with CLL were able to proliferate in response to Rh protein, Rh peptides, or both, and the results are summarized in Table 4, with representative data from 3 patients illustrated in Figure 3. PBMCs from 8 of 10 patients proliferated when they were stimulated with purified RhD protein, and 6 of these 8 patients were also responsive to multiple peptides from the Rh sequence.
Responses to Rh protein and peptides by PBMCs from healthy control donors. Representative experiments showing absent or weak proliferation by PBMCs from healthy D-positive (A-B) and D-negative (C) control donors after stimulation with panels of overlapping 15-mer peptides spanning the sequences of the RhD (A-B) or Rhce (C) proteins (▪) or purified RhD protein (▨). Control unstimulated cultures were also included (□). Peptides are numbered 1 to 68 from the N-terminus: the ce panel lacks numbers 7, 9, 13, 15, 17, 19, 22, 24, 26, 28, 30, 32, 34, 36, 38, 40, 43, 45, 49, 51, 53, 55, 57, 59, 64, and 66. The dotted line indicates the level of response taken as representing a positive response (SI > 3). Error bars indicate SD.
Responses to Rh protein and peptides by PBMCs from healthy control donors. Representative experiments showing absent or weak proliferation by PBMCs from healthy D-positive (A-B) and D-negative (C) control donors after stimulation with panels of overlapping 15-mer peptides spanning the sequences of the RhD (A-B) or Rhce (C) proteins (▪) or purified RhD protein (▨). Control unstimulated cultures were also included (□). Peptides are numbered 1 to 68 from the N-terminus: the ce panel lacks numbers 7, 9, 13, 15, 17, 19, 22, 24, 26, 28, 30, 32, 34, 36, 38, 40, 43, 45, 49, 51, 53, 55, 57, 59, 64, and 66. The dotted line indicates the level of response taken as representing a positive response (SI > 3). Error bars indicate SD.
Summary of proliferative responses to RhD protein and RhD peptides by patients with CLL without overt AIHA (CLL)
Patient . | Purified RhD protein-induced proliferation . | Rh-specific peptides stimulating proliferation* . |
|---|---|---|
| 1 | N | None |
| 2 | Y | 15, 16, 31, 34, 47 |
| 3 | Y | 17, 40 |
| 4 | N | None |
| 5 | Y | 2, 3, 17 |
| 6 | Y | None |
| 7 | Y | 2, 9, 41 |
| 8 | Y | None |
| 9 | Y | 14, 38 |
| 10 | Y | 27, 28, 29, 30, 41, 42, 53, 54, 63, 68 |
Patient . | Purified RhD protein-induced proliferation . | Rh-specific peptides stimulating proliferation* . |
|---|---|---|
| 1 | N | None |
| 2 | Y | 15, 16, 31, 34, 47 |
| 3 | Y | 17, 40 |
| 4 | N | None |
| 5 | Y | 2, 3, 17 |
| 6 | Y | None |
| 7 | Y | 2, 9, 41 |
| 8 | Y | None |
| 9 | Y | 14, 38 |
| 10 | Y | 27, 28, 29, 30, 41, 42, 53, 54, 63, 68 |
PBMCs from patient tested with the panel of synthetic RhD peptides to match phenotype.
Responses to RhD autoantigen by PBMCs from patients with CLL with no clinical AIHA. Representative experiments showing proliferation by PBMCs from patients CLL1 (A), CLL2 (B), and CLL3 (C) after stimulation with a panel of overlapping 15-mer peptides spanning the sequence of the RhD protein (▪) or purified RhD protein (▨). Control unstimulated cultures were also included (□). Peptides are numbered 1 to 68 from the N-terminus. The dotted line indicates the level of response taken as representing a positive response (SI > 3). Error bars indicate SD.
Responses to RhD autoantigen by PBMCs from patients with CLL with no clinical AIHA. Representative experiments showing proliferation by PBMCs from patients CLL1 (A), CLL2 (B), and CLL3 (C) after stimulation with a panel of overlapping 15-mer peptides spanning the sequence of the RhD protein (▪) or purified RhD protein (▨). Control unstimulated cultures were also included (□). Peptides are numbered 1 to 68 from the N-terminus. The dotted line indicates the level of response taken as representing a positive response (SI > 3). Error bars indicate SD.
Antibody levels
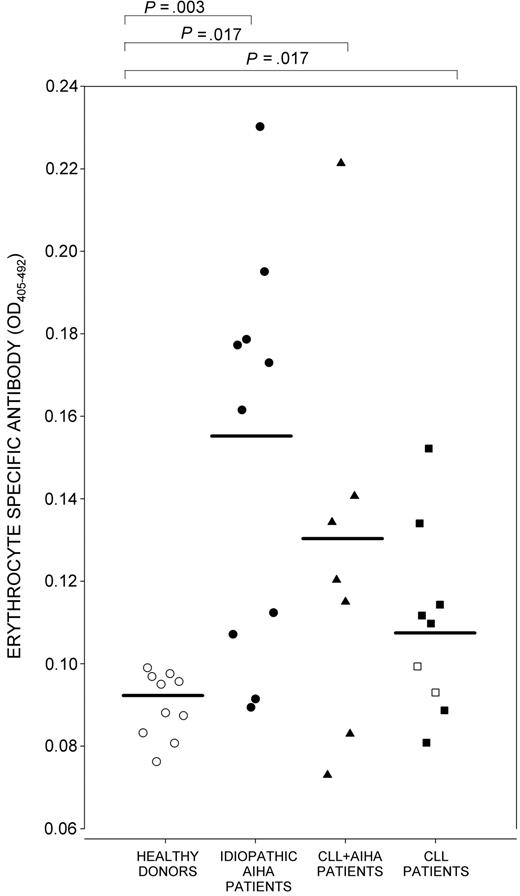
One explanation for the responsiveness of PBMCs from patients with CLL, but not with overt AIHA, is that TH cells in these patients provided help for anti-RBC autoantibodies that are undetectable by DAT and that do not cause clinical hemolysis. Accordingly, a sensitive IELAT was used to compare the levels of RBC-reactive antibodies in the sera of healthy control donors, primary AIHA patients, patients with AIHA secondary to CLL, and patients with CLL but no clinical hemolysis. Figure 4 demonstrates that, as expected, high levels of antibody were present in the sera of most of the patients with primary or secondary AIHA but that antibody levels were also elevated in 6 of the 10 patients with CLL without overt AIHA. All these antibody-positive, but AIHA-negative, patients with CLL showed proliferative responses to the RhD protein.
Anti-RBC antibodies in patients with CLL. IELAT measurements of serum antibodies specific for human RBCs in healthy control donors (n = 10; ○), patients with primary AIHA (n = 10; •), patients with CLL with AIHA (n = 7; ▴), and patients with CLL with no clinical AIHA (n = 10). ▪ indicates Patients with CLL with no clinical AIHA whose PBMCs responded to purified RhD protein, RhD peptides, or both (n = 7); □, patients in this group with no such responses (n = 2). Horizontal bars indicate mean antibody level in each group. Significant differences are marked (Mann-Whitney U test).
Anti-RBC antibodies in patients with CLL. IELAT measurements of serum antibodies specific for human RBCs in healthy control donors (n = 10; ○), patients with primary AIHA (n = 10; •), patients with CLL with AIHA (n = 7; ▴), and patients with CLL with no clinical AIHA (n = 10). ▪ indicates Patients with CLL with no clinical AIHA whose PBMCs responded to purified RhD protein, RhD peptides, or both (n = 7); □, patients in this group with no such responses (n = 2). Horizontal bars indicate mean antibody level in each group. Significant differences are marked (Mann-Whitney U test).
Comparison of responses to Rh protein epitopes in primary and CLL-associated AIHA
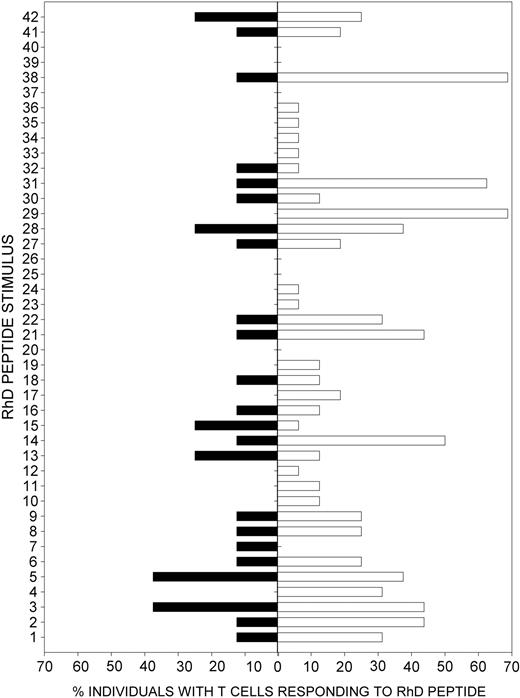
The question arose as to how the fine specificities of the Rh-specific TH cells in patients with CLL with secondary AIHA compared with those previously identified in patients with primary AIHA.16,17 Figure 5 demonstrates a significant correlation (Rs = 0.55; P < .0001) between the Rh peptides that most commonly induce proliferative responses in patients with primary AIHA and those that are dominant in patients with CLL with AIHA.
Summary of proliferative responses to RhD autoantigen peptides by PBMCs from Patients with CLL with AIHA. ▪ indicates proportion of D-positive Patients with CLL with secondary AIHA (n = 8) whose PBMCs proliferate in response to peptides spanning the RhD protein. □ indicates results of parallel studies17 to map RhD peptides that stimulate PBMCs from patients with patients with primary AIHA patients (n = 11). The peptide panel is reduced to the 42 sequences with less overlap used in the previous work17 and is numbered accordingly. There is a significant correlation (Rs = 0.55; P < .0001) between the abilities of each RhD peptide to elicit proliferative responses in patients with primary and secondary AIHA.
Summary of proliferative responses to RhD autoantigen peptides by PBMCs from Patients with CLL with AIHA. ▪ indicates proportion of D-positive Patients with CLL with secondary AIHA (n = 8) whose PBMCs proliferate in response to peptides spanning the RhD protein. □ indicates results of parallel studies17 to map RhD peptides that stimulate PBMCs from patients with patients with primary AIHA patients (n = 11). The peptide panel is reduced to the 42 sequences with less overlap used in the previous work17 and is numbered accordingly. There is a significant correlation (Rs = 0.55; P < .0001) between the abilities of each RhD peptide to elicit proliferative responses in patients with primary and secondary AIHA.
Capacity of different APC types to stimulate TH cells responsive to Rh protein
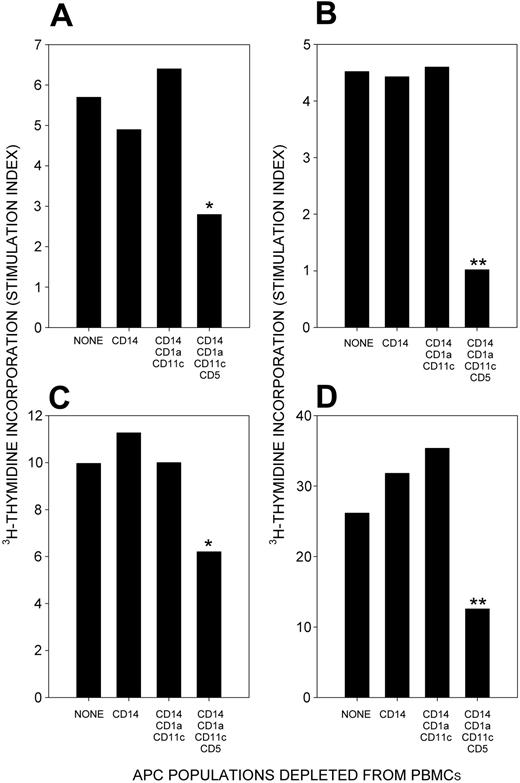
Having shown that CLL is associated with responsiveness of Rh-reactive TH cells, we next tested our primary hypothesis that CLL B cells are effective in presenting Rh autoantigen to activate TH cells. T cells were purified from the PBMCs of patients with CLL with AIHA and were stimulated with RhD protein presented by different putative APC populations from the remaining PBMCs. The first approach to obtaining APC fractions was negative selection, by sequentially depleting PBMCs. The PBMCs of 3 AIHA-positive patients with CLL were fractionated in this way, and experiments illustrating the antigen-presenting capacity of the fractions are depicted in Figure 6. The proliferative responses against purified RhD protein by T cells from all patients were maintained when CD14+ and then CD1a+/CD11c+ cells were removed from cultures, but responses decreased significantly after CD5+ cells were depleted. Neither purified T cells nor T-depleted APC fractions proliferated when stimulated with Rh protein or peptides. These results suggest that CLL B cells, rather than monocytes, dendritic cells, or B2 cells, play an important role in processing and presenting the RhD protein in vitro.
Responses to RhD protein by T cells from patients with CLL with AIHA when different PBMC populations are used as APCs. T cells were purified from PBMCs, and the remaining cells were fractionated by negative selection into putative APC types. Sequential depletion of CD14+, CD1a+/11c+, and CD5+ cells removes monocytes, dendritic cells, and CLL cells, respectively. The APC fractions were tested for the ability to stimulate T-cell proliferation in response to RhD protein. Proliferative responses in patients CLL+AIHA1 (A), CLL+AIHA4 (B-C), and CLL+AIHA5 (D) are inhibited by the depletion of CLL B cells, but they remain unaffected by the removal of other APC fractions. Significant differences between cultures stimulated using unfractionated and fractionated PBMCs as APCs are indicated (Student t test: *P < .05; **P < .01).
Responses to RhD protein by T cells from patients with CLL with AIHA when different PBMC populations are used as APCs. T cells were purified from PBMCs, and the remaining cells were fractionated by negative selection into putative APC types. Sequential depletion of CD14+, CD1a+/11c+, and CD5+ cells removes monocytes, dendritic cells, and CLL cells, respectively. The APC fractions were tested for the ability to stimulate T-cell proliferation in response to RhD protein. Proliferative responses in patients CLL+AIHA1 (A), CLL+AIHA4 (B-C), and CLL+AIHA5 (D) are inhibited by the depletion of CLL B cells, but they remain unaffected by the removal of other APC fractions. Significant differences between cultures stimulated using unfractionated and fractionated PBMCs as APCs are indicated (Student t test: *P < .05; **P < .01).
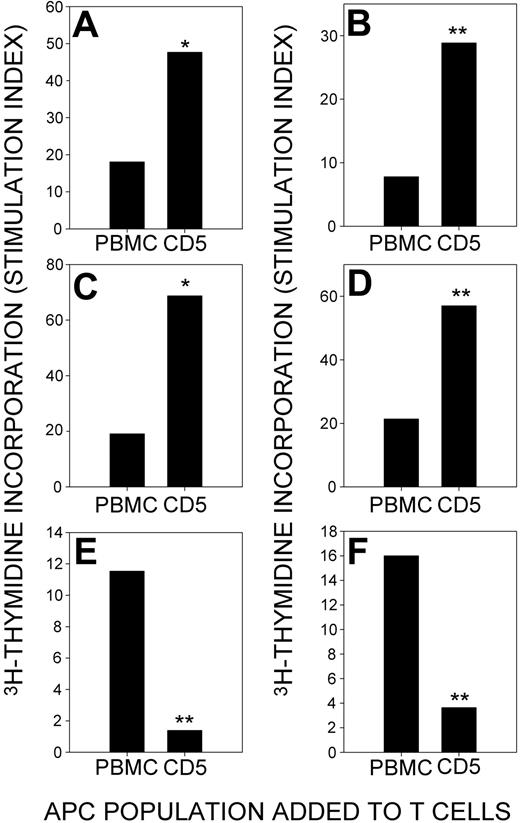
To confirm that CLL B cells were able to present Rh protein effectively, T cells were stimulated with purified autoantigen in the presence of freshly isolated CD5+ cells that had been positively selected from T-depleted PBMCs. Results are summarized in Table 5, and representative data are shown in Figure 7A-D. In 13 experiments on cells from 9 different patients, positively selected CD5+ cells presented RhD protein to stimulate TH cell proliferation, and, on 12 of 13 occasions, these responses were higher than those supported by APCs from unfractionated PBMCs. This ability to stimulate RhD protein–reactive TH cells contrasts with the lack of responses elicited by the control recall antigen PPD when APCs were provided by the CD5+ fraction (Figure 7E-F). Thus, although, as previously reported,29 CLL cells are inefficient APCs for conventional antigens, they are effective in presenting the RhD autoantigen to T cells.
Responses to purified RhD protein by T cells from patients with CLL with secondary AIHA when CD5+ CLL cells are used as APCs
No. CLL + AIHA patients tested . | 2 . | 2 . | 3 . | 3 . | 4 . | 4 . | 5 . | 6 . | 9 . | 10 . | 11 . | 11 . | 12 . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Change in response, % | 245 | 818 | 201 | 160 | 151 | 85 | 333 | 356 | 173 | 140 | 167 | 239 | 119 |
No. CLL + AIHA patients tested . | 2 . | 2 . | 3 . | 3 . | 4 . | 4 . | 5 . | 6 . | 9 . | 10 . | 11 . | 11 . | 12 . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Change in response, % | 245 | 818 | 201 | 160 | 151 | 85 | 333 | 356 | 173 | 140 | 167 | 239 | 119 |
Responses are shown relative to proliferation stimulated when unfractionated PBMCs act as APCs.
RhD protein, but not the control recall antigen PPD, is presented to T cells by CLL B cells. T cells were purified from PBMCs, and CD5+ CLL B cells were positively selected from the remaining cells. The ability of the T cell–depleted PBMCs and the CD5+ CLL B cell fraction to stimulate T cell proliferation in response to RhD protein was compared. CLL B cells are more effective than PBMCs in presenting RhD protein to T cells from patients CLL+AIHA2 (A), CLL+AIHA5 (B), CLL+AIHA6 (C), and CLL+AIHA10 (D). In contrast, CLL B cells fail to stimulate proliferative responses against PPD by T cells from patients CLL+AIHA1 (E) and CLL+AIHA7 (F). Significant differences between cultures stimulated using unfractionated PBMCs and CD5+ CLL B cells as APCs are indicated (Student t test: *P < .05; **P < .01).
RhD protein, but not the control recall antigen PPD, is presented to T cells by CLL B cells. T cells were purified from PBMCs, and CD5+ CLL B cells were positively selected from the remaining cells. The ability of the T cell–depleted PBMCs and the CD5+ CLL B cell fraction to stimulate T cell proliferation in response to RhD protein was compared. CLL B cells are more effective than PBMCs in presenting RhD protein to T cells from patients CLL+AIHA2 (A), CLL+AIHA5 (B), CLL+AIHA6 (C), and CLL+AIHA10 (D). In contrast, CLL B cells fail to stimulate proliferative responses against PPD by T cells from patients CLL+AIHA1 (E) and CLL+AIHA7 (F). Significant differences between cultures stimulated using unfractionated PBMCs and CD5+ CLL B cells as APCs are indicated (Student t test: *P < .05; **P < .01).
Capacity of different APC types to stimulate TH cells responsive to Rh peptides
There are at least 2 possible explanations for the ability of CLL B cells to present RhD autoantigen. One is that tumor cells may take up and process the antigen more effectively than other APC types. The other is that they may preferentially present to autoreactive T cells. To address this, we determined whether the different APC fractions from 4 patients with CLL could stimulate T cells specific for the respective dominant peptides that had been identified earlier. Results are summarized in Table 6 and show that CD5+ cells can present effectively to particular peptide-specific T cells. However, compared with responses to the purified RhD protein, the bias toward peptide presentation by this fraction is less clear, and other APC populations that include dendritic cells or B2 B cells can be equally or more effective.
Responses to Rh peptides by T cells from patients with CLL with secondary AIHA when different PBMC populations are used as APCs
. | . | Proportion of responses when PBMCs were used as APCs, % . | . | . | . | |||
|---|---|---|---|---|---|---|---|---|
| . | . | Populations depleted from PBMCs . | . | . | Positively selected population . | |||
| CLL patient . | Rh peptide tested . | CD14+ . | CD14+, CD1a+/11c+ . | CD14+, CD1a+/11c+, CD5+ . | CD5+ . | |||
| 1 | D33 | NT | NT | 140 | 176 | |||
| 1 | D46 | NT | NT | 64 | 46 | |||
| 1 | D62 | 65 | 54 | 43 | 39 | |||
| 1 | D20 | 117 | 25 | 32 | NT | |||
| 1 | D27 | 116 | 63 | 39 | NT | |||
| 4 | D23 | 25 | 22 | 21 | 46 | |||
| 7 | ce20 | NT | NT | 47 | 59 | |||
| 8 | ce2 | 12 | NT | 64 | 114 | |||
| 8 | ce21 | NT | NT | 166 | 124 | |||
. | . | Proportion of responses when PBMCs were used as APCs, % . | . | . | . | |||
|---|---|---|---|---|---|---|---|---|
| . | . | Populations depleted from PBMCs . | . | . | Positively selected population . | |||
| CLL patient . | Rh peptide tested . | CD14+ . | CD14+, CD1a+/11c+ . | CD14+, CD1a+/11c+, CD5+ . | CD5+ . | |||
| 1 | D33 | NT | NT | 140 | 176 | |||
| 1 | D46 | NT | NT | 64 | 46 | |||
| 1 | D62 | 65 | 54 | 43 | 39 | |||
| 1 | D20 | 117 | 25 | 32 | NT | |||
| 1 | D27 | 116 | 63 | 39 | NT | |||
| 4 | D23 | 25 | 22 | 21 | 46 | |||
| 7 | ce20 | NT | NT | 47 | 59 | |||
| 8 | ce2 | 12 | NT | 64 | 114 | |||
| 8 | ce21 | NT | NT | 166 | 124 | |||
PBMCs remaining after T-cell purification were fractionated by negative or positive selection into putative APC types, and all results are expressed as percentages of the response when the unfractionated PBMCs act as APCs. Sequential depletion of CD14+, CD1a+/11c+, and CD5+ cells removes monocytes, dendritic cells, and CLL cells, respectively.
NT indicates not tested.
Discussion
We here describe a novel role for malignant B cells in the autoimmune pathologic process associated with CLL. The results demonstrate that AIHA secondary to CLL is associated with the activation of TH cells specific for epitopes on the dominant RBC autoantigens, the Rh proteins. Thus, as in primary AIHA, the production of pathogenic anti-RBC autoantibodies in CLL appears to be helper dependent. Cell fractionation experiments showed that the CLL B cells were the major APC type that process and present the Rh proteins to stimulate the autoreactive TH cells. This is the first example of malignant cells driving an autoimmune response by acting as aberrant APCs.
Murine studies12,14-19 have demonstrated that IgG autoantibodies against RBC proteins are dependent on the activation of autoreactive TH cells specific for these antigens, and an Rh-reactive helper response has previously been identified in human patients with primary AIHA.16,17 The characterization of patients with AIHA secondary to CLL reveals similar TH responsiveness to the Rh proteins. Thus, TH cells from the peripheral blood of all such patients proliferated in response to purified Rh protein and, typically, also responded to multiple synthetic peptides derived from the sequence. There are further parallels between the TH responses in primary and CLL-associated AIHA. The patterns of Rh peptides that elicit TH cell proliferation vary among the patients with CLL, but particular sequences are commonly stimulatory, and these correspond to the peptides that are dominant in primary AIHA.17 Together, these data lead us to conclude that AIHA secondary to CLL is TH driven, and they suggest that, as in primary AIHA, the breakdown of self-tolerance may be attributed to changes in autoantigen presentation.35
The results also demonstrate that anti-RBC autoimmune responses are more common in patients with CLL than the incidence of overt AIHA suggests. The view that latent autoimmunity is underestimated in CLL is supported by previous studies using a mitogen-stimulated DAT.44 TH cells from a number of our patients with CLL with no clinical evidence of hemolysis exhibited reactivity to Rh epitopes, and a sensitive enzyme-linked assay revealed that this responsiveness is often associated with elevated levels of anti-RBC antibodies compared with healthy individuals. Although titers are typically lower than in patients diagnosed with AIHA, either primary or secondary to CLL, it is probable that the TH reactivity reflects help provided for the production of antibodies that do not result in overt hemolysis. It remains to be determined whether these antibodies appear to be nonpathogenic solely because of their low titer because many other factors, including autoantibody subclass, galactosylation, and the activation state of phagocytes within the reticulo-endothelial system, also influence the rate of hemolyis.36-38,45,46 In the remaining patients with CLL with Rh-specific TH cells in the absence of hemolysis, but no detectable anti-RBC antibodies, it could be argued that TH activation precedes the antibody response or that it may represent the occasional cross-reactivity to environmental antigens seen in healthy controls.16,17,34,35,39
Although CLL B cells can secrete polyreactive autoantibodies,47 these are not the cause of hemolysis in AIHA associated with the tumor.22 Autoreactive B and T cells form part of the normal immune repertoire, remaining quiescent in healthy persons, but are capable of driving autoimmune disease if activated.34,35 It has previously been proposed34,35 that autoimmune diseases, including AIHA, are initiated by changes in autoantigen presentation such as the recruitment of new APC types, leading to the activation of autoreactive TH cells. We therefore sought to determine whether malignant CLL cells, which could represent a large, abnormal APC population, were capable of presenting the Rh autoantigens to TH cells in patients with secondary AIHA. Although it is well documented that CLL cells are poor APCs for conventional antigens29 —and this is supported in the current work—we demonstrated that they are effective in stimulating the proliferation of TH cells specific for Rh protein. This ability was shown by experiments to fractionate different APC types by negative selection and was confirmed by positively isolated CD5+ B cells from patients with CLL, stimulating significantly higher responses than the other APC populations in PBMCs.
There are precedents for B cells acting as APCs to drive autoimmune pathologic processes. For example, myelin oligodendrocyte protein is immunogenic and can induce EAE in wild-type, but not in B cell–deficient, mice.48 Furthermore, B cells can effectively process and present protein antigens to prime naive T cells,49 and they are particularly efficient as APCs for conformational immunogens.50 In the context of AIHA, the ability of B cells to form a synapse and to internalize membrane-bound or integral antigens51 provides a mechanism for the uptake and presentation of the Rh proteins from RBCs.
There are a number of possible explanations, which are not mutually exclusive, for the preferential presentation of Rh autoantigen by the malignant cells. First, CLL cells may be particularly effective in taking up or processing the Rh protein. Second, CLL cells may stimulate autoreactive TH cells more efficiently than conventional responses. Both these factors may be relevant because CLL cells were able to present many synthetic Rh-derived peptides to TH cells, but not so strikingly as the purified Rh protein. An alternative explanation, which we favor, is that CLL cells process Rh protein in such a way as to skew presentation away from epitopes to which the TH cell repertoire is normally tolerant. It is widely believed that such presentation of previously cryptic epitopes can initiate and drive autoimmune diseases,51-57 including AIHA.16,17,34,35,58 Whatever the reasons for the efficacy of CLL cells as APCs for Rh protein, the uncontrolled expansion of these malignant cells in vivo, combined with their localization with blood breakdown products, provides the opportunity to activate autoreactive TH cells specific for RBCs, platelets, and other blood-derived antigens. It is yet to be determined where the TH cell activity occurs, but the spleen is likely to be important, given its role as a secondary lymphoid organ and a major site where blood cell breakdown products are available. Indeed, 5 of our 12 patients with CLL and overt hemolysis exhibited palpable splenomegaly. The observation that the presence of CLL cells does not inevitably lead to AIHA reflects the complex, multifactorial etiology of autoimmune conditions and indicate that other, as yet unidentified, genetic and environmental factors contribute to the development of the disease.
The recognition that AIHA secondary to CLL is helper dependent and that the malignant B cells may drive activation of the autoreactive TH cells by acting as APCs has implications for the development of future treatments. For example, specific peptide immunotherapy, based on the induction of tolerance to dominant TH cell epitopes, is under development for primary AIHA17 and for the prevention of allo-responses to the RhD blood group antigen.39 This approach can now be extended to patients with CLL. Such therapy would remove one important complication of this leukemia.
Prepublished online as Blood First Edition Paper, July 29, 2004; DOI 10.1182/blood-2003-10-3563.
Supported by grants from the Wellcome Trust (United Kingdom) and the Grampian University Hospitals Trust (United Kingdom).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Drs D. Culligan, J. Tighe, and H. Watson (Aberdeen Royal Infirmary) for help with patient recruitment.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal