Abstract
Endothelial cell-cell junctional proteins and cortical actin are of central importance for regulating vascular permeability. Rap1, a member of the Ras family of GTPases, is enriched at endothelial cell-cell contacts and activated by cyclic AMP (cAMP) through a PKA-independent pathway. Activation of a cAMP-inducible guanine-exchange factor for Rap, Epac, results in markedly enhanced basal endothelial barrier function by increasing cortical actin and subsequent redistribution of adherens and tight junctional molecules to cell-cell contacts. Activation of Epac also counteracts thrombin-induced hyperpermeability through down-regulation of Rho GTPase activation, suggesting cross-talk between Rap and Rho GT-Pases. Thus, Epac/Rap activation represents a new pathway for regulating endothelial cell barrier function.
Introduction
The endothelium of the vascular system forms a barrier between blood and the extravascular space that controls the extravasation of solutes, macromolecules, and white blood cells. In homeostasis, a high barrier function of the cerebral microcirculation is required to maintain the blood brain barrier. In the peripheral vasculature during inflammation, local increases in permeability and the accompanying leakage of plasma proteins play a critical role in counteracting infections and assisting in tissue repair. Microvascular hyperpermeability is associated with morbidity and mortality in several acute and chronic diseases such as acute respiratory distress syndrome and diabetes. Increased permeability to low-density lipoprotein (LDL) particles is linked to the development of atherosclerosis, and leakiness of tumor vessels has implications for cancer cell growth and metastasis.1 Thus, the selective regulation of vascular permeability is critical for maintaining vascular integrity in homeostasis and disease.
Endothelial barrier function is dynamically regulated by secondary messengers such as cAMP. cAMP elevation by beta-adrenergic agents stimulating Gs protein–coupled receptors reduces vascular leakage. Furthermore, cAMP-elevating agonists (phosphodiesterase inhibitors and adenylate cyclase activators) decrease basal permeability and reverse vascular leakage induced by inflammatory mediators both in vitro and in vivo. cAMP exerts its effect on physiologic processes primarily through direct activation of cAMP-dependent protein kinase A (PKA). However, pharmacological inhibitors of PKA do not consistently reverse cAMP-enhanced endothelial cell barrier function, suggesting the existence of PKA-independent pathways.2-6 Endothelial permeability to macromolecules occurs via the formation of small gaps between (paracellular) or through (transcellular) cells. Paracellular permeability in response to vasoactive amines occurs as a result of cytoskeletal-based contractile forces combined with a reduction or redistribution of junctional molecules at adherens junctions (AJs) (eg, VE-cadherin linked to the cytoskeleton through catenins) and tight junctions (TJs) (eg, JAM-A, claudins, ZO-1 and 2).4,7,8 Formation and reclosure of such gaps require dynamic reorganization of the actin cytoskeleton, which is primarily orchestrated by the small GTPases of the Rho family.9,10 Recently, Rap1, a member of the Ras family of GTPases known to promote integrin activation in several cell types,11 was shown in Drosophila to localize at AJ in epithelial cells and play a role in AJ positioning after cell division in the developing wing disc.12 Furthermore, cAMP was shown to activate Rap through Epac1 and 2, a newly described group of guanine nucleotide exchange factors (GEFs) specific for Rap GTPases that contain cAMP-binding domain(s).13 Thus, we sought to test the hypothesis that Rap1 activation by the cAMP-responsive GEF, Epacs plays a role in endothelial cell junction biology, particularly in vascular permeability and the associated remodeling of junctional proteins and the actin cytoskeleton.
Materials and methods
Reagents
Reagents used were anti-Rap1, anti-Rap2, anti-RhoA, anti-Epac1 and 2 (Santa Cruz Biotechnology, Santa Cruz, CA); Rhotekin-RBD beads (Cytoskeleton, Denver, CO); anti-cAMP response element-binding transcription factor (CREB) and anti–phospho-CREB (Ser 133) (Cell Signaling Technology, Beverly, MA); anti-AF-6 (BD Transduction Labs, clone 35, San Diego, CA); TEA 1/31 (Beckman Coulter, Miami, FL), a nonblocking antibody recognizing the extracellular domain of vascular endothelial (VE)–cadherin; fluorescein isothiocyanate (FITC)–phalloidin (Sigma Chemicals, St Louis, MO); 8-pCPT-2′O-Me-cAMP (Biolog Life Science Institute, Germany). Thrombin (Sigma) was used at 2 U/mL. Forskolin and rolipram (Calbiochem, La Jolla, CA) were used at 10 μM and 20 μM, respectively.
Plasmids
Spa1, ΔGRD-Spa1 (deleted of GTPase-activating protein [GAP]–related domain, residues 195-535) (gifts from N. Minato, Kyoto University, Japan) and Rap1 (a gift from VA Boussiotis, Dana-Farber Cancer Institute, Boston, MA) cDNAs were cloned into the vector pLEGFP-C1 (BD Biosciences) to express green fluorescence protein (GFP)–tagged fusion proteins. Glutathione S-transferase (GST)–RBD (a GST fusion protein containing the Rap1-binding domain of Ral-GDS) cDNA was a gift from J. de Gunzburg (Institut National de la Santé et de la Recherche Médicale, France).
Virus production
pLEGFP-C1-Spa1,-ΔGRD-Spa1 and -Rap1 plasmids were cotransfected with pVSV-G (BD Biosciences), a plasmid expressing the envelope glycoprotein VSV-G from the vesicular stomatatis virus into the ecotropic Phoenix cell line using lipofectamine 2000 (Invitrogen, Carlsbad, CA). Forty-eight hours later, supernatants were collected, filtered, concentrated by high-speed centrifugation, and resuspended in TNE (20 mM Tris [tris(hydroxymethyl)aminomethane]-HCl at pH 7.5, 100 mM NaCl, and 1 mM EDTA [ethylenediaminetetraacetic acid]). Cells were seeded in regular culture medium, infected after 24 hours, and analyzed 4 to 5 days later.
Cell culture
Human pulmonary aortic endothelial cells (HPAECs) (Cambrex, Walkersville, MD) were cultured in EBM-2 supplemented with EGM-2MV Singlequots (Cambrex, Walkersville, MD). For low calcium experiments, cells were cultured for 8 hours in Eagle minimum essential medium without calcium chloride (Cambrex) supplemented with dialyzed fetal bovine serum (FBS) (Invitrogen). Human umbilical vein endothelial cells (HUVECs) were isolated as previously described14 and cultured in M199 medium containing 20% fetal calf serum (FCS), l-glutamine, penicillin-streptomycin, and growth factors on gelatin-coated dishes. Experiments were performed with subculture 2 cells, grown to postconfluence for 2 to 4 days in M199, 10% FCS, glutamax (l-Alanyl-l-glutamine, 2 mM Invitrogen), penicillin-streptomycin, 100 nM hydrocortisone, and 100 μM ascorbic acid (these last 2 ingredients were supplemented to physiologic plasma levels and improve endothelial barrier function in vitro, S.K.S., unpublished observations, December 2003).
Rap and Rho activation assays
For Rap1 pull-downs, cells were lysed in buffer (25 mM Tris-HCl at pH 7.5, 150 mM NaCl, 5 mM MgCl2, 1% NP-40, 1 mM DTT (dithiothreitol), 5% glycerol, 1 μg/mL aprotinin, 1 μg/mL leupeptin, and 1 mM phenyl methylsulphonyl fluoride [PMSF]) and clarified by centrifugation. An aliquot of the lysate was used to determine the amount of Rap protein in the experimental samples by Western blot. Clarified lysates were incubated with 50 μg GST fusion protein containing the Rap1-binding domain of Ral-GDS coupled to glutathione-sepharose beads for 1 hour at 4°C. Proteins bound to beads were extracted in Laemmli buffer. Rap-GTP samples and total lysates (40 μg) were separated on 4% to 20% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) gels and subjected to Western blot analysis using anti-Rap1 or anti-Rap2 antibodies. For Rho analysis, cell lysis was in 50 mM Tris-HCl at pH 7.5, 500 mM NaCl, 10 mM MgCl2, 1% Triton X-100, 1 μg/mL aprotinin, 1 μg/mL leupeptin, and 1 mM PMSF, and clarified lysates were incubated with 50 μg Rhotekin-RBD beads. Rho was detected by Western blot using anti-RhoA antibody.
Western blot analysis
After stimulation, cells were washed once with cold phosphate-buffered saline (PBS) and lysed in radioimmunoprecipitation assay (RIPA) buffer (50 mM Tris-HCl at pH 7.5, 500 mM NaCl, 20 mM MgCl2, 0.5% deoxycholic acid, 0.1% SDS, 1% Triton X-100, 1 mM PMSF, 10 μg/mL leupeptin, 10 μg/mL aprotinin, 1 mM EDTA, 1 mM Na3VO4, 1 mM NaF), clarified by centrifugation, resolved by SDS-PAGE, followed by Western blot. Densitometric analysis of the Western blots for Rap and Rho activation was performed, and the data were analyzed to determine the fold induction in GTPase activation compared to untreated controls.
Confocal microscopy
HUVECs were grown in fibronectin-coated glass-bottom microwell dishes (MatTek, Ashland, MA), transduced with GFP-Rap1 or GFP retroviral vectors, and allowed to grow to confluence. Live cells were stained with Alexa-568 (Invitrogen)–conjugated anti–VE-cadherin (TEA 1/31). Cells were imaged with a Nikon TE-2000 inverted microscope (Melville, NY), using a 60 × oil-immersion PlanApo lens 1.4NA and a BioRad Radiance 2100 Laser Scanning Confocal unit (Hercules, CA). Images were deconvolved by AutoQuant software (AutoQuant Imaging, Watervliet, NY).
Immunofluorescence microscopy
HUVECs were plated on fibronectin-coated glass coverslips (Fisher Scientific, Pittsburgh, PA). Confluent monolayers were treated with F + R, O-Me-cAMP, and/or thrombin in the absence of serum for 30 minutes. Monolayers were then washed in Dulbecco's phosphate buffered saline (DPBS) and fixed with ethanol at –20°C for 20 minutes. Cells were stained by indirect immunofluorescence and imaged using a Nikon TE-2000 inverted microscope and an Orca-ER–cooled CCD camera (Hamamatsu, Bridgewater, NY) controlled by MetaMorph software (v.4.6r8, Universal Imaging, Downingtown, PA).
Neutrophil transendothelial cell migration assay
HUVECs were used at confluence on gelatin-coated transwell inserts (Corning, NY). The bottom chamber was coated with polyheme to prevent adherence of neutrophils to plastic. HUVECs were treated with human tumor necrosis factor (TNF)-α (50 ng/mL) for 4 hours. Prior to neutrophil transmigration, endothelial cells were pretreated with or without O-Me-cAMP (100 μM) for 30 minutes. Cells were washed and 2 × 106 human neutrophils, isolated from healthy donors and resuspended in M199 + 0.5% BSA, were added to the top chamber in the presence or absence of O-Me-cAMP. After 45 minutes at 37°C neutrophils transmigrated into the lower chamber were collected and counted.
Permeability assays
Assays were performed as previously described.15 Briefly, transwells inserts (Costar, Corning, NY) were coated with 0.1% gelatin, and HUVECs were plated at 2.5 × 105 cells/well. Fluorescein-dextran (70-kDa molecular mass; Invitrogen) was added 4 to 6 days later, at 500 μg/mL in Hanks balanced salt solution (HBSS) without phenol red, and the bottom chamber replaced with HBSS. All endothelial treatments were added along with the fluorescein-dextran. Fluorescence in the bottom chamber was read after 5, 15, or 60 minutes at 37°C.
Statistical analysis
The results of the Spa1 and SpaΔGRD–expressing cells were compared in 3 independent experiments using a Mann Whitney U test, which tests the medians between 2 independent samples and is the nonparametric equivalent of a Student t test. For comparing differences in fold GTPase activation versus untreated cells and differences in permeability of treated versus untreated samples, repeated measures analysis of variance (ANOVA) followed by the Dunnett multiple comparison test was used. Significance was set at P less than .05.
Results and discussion
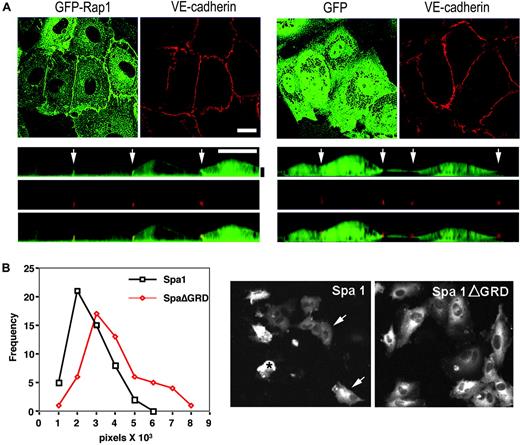
The distribution of Rap1 in HUVECs was examined by retroviral expression of GFP-Rap1 fusion protein. This was followed by live-cell confocal imaging of cells costained with a fluorescently tagged antibody to VE-cadherin to delineate cellular junctions. GFP-Rap1 was consistently enriched at cell-cell contacts (Figure 1A), suggesting that a stable pool of Rap is present at junctional sites. Inactivation of endogenous Rap in HUVECs and HPAECs by overexpression of the Rap-specific GAP, Spa-116 (that inactivated Rap by more than 90%, data not shown), resulted in significantly reduced cell spreading and rounding up of the cells. Similar over expression of Spa-1 lacking its GAP domain (Spa▵GRD) had no effect (Figure 1B). The integrity of the endothelial monolayer is dependent upon the coordinated regulation of cell-cell junctions, integrin-dependent cell-matrix interactions, and the cytoskeleton.7 Thus, the altered phenotype observed upon efficient inactivation of endogenous Rap suggests a role for Rap in one or more of these functions.
Rap localization and function in human umbilical vein endothelial cell monolayers. (A) HUVECs were retrovirally transduced with GFP-Rap1 or GFP vector and stained with an fluorochrome-conjugated VE-cadherin antibody. Live cells were imaged by confocal microscopy for GFP (green) or VE-cadherin (red). The upper panels depict a single 0.5-μm confocal slice showing GFP-Rap enriched in the lateral junctions as costained by VE-cadherin. Control GFP alone was evenly distributed throughout the cytoplasm. Bottom panels depict an orthogonal (xz) slice from the confocal stack above. GFP-Rap enrichment is likewise observed at junctions (arrows). The vertical (z) dimension has been exaggerated for clarity. Horizontal white scale bar = 10 μm. Vertical black scale bar = 2 μm. (B) HUVECs were retrovirally transduced with GFP-Spa1 or GFP-Spa1ΔGRD and cultured for 4 days. GFP was visualized by live-cell imaging and pictures taken and analyzed for the area (pixels) of the GFP-positive cells, the results of which were plotted. One of three representative experiments is shown for which more than 50 cells were analyzed per group. The frequency of the analyzed cells represents the percentage of cells with the indicated number of pixels in the x-axis. The difference in the median size of cells between the 2 groups, from 3 independent experiments, was statistically significant (median Spa1ΔGRD, 26 501 (n = 155); median Spa1, 20 592 (n = 154), P < .001). Representative pictures of Spa1 and Spa1ΔGRD-expressing cells are shown. For the analysis of monolayers expressing Spa1, only cells with discernable cellular structures were evaluated (arrows), while those that were completely round (*) were excluded to avoid inclusion of debris in the analysis.
Rap localization and function in human umbilical vein endothelial cell monolayers. (A) HUVECs were retrovirally transduced with GFP-Rap1 or GFP vector and stained with an fluorochrome-conjugated VE-cadherin antibody. Live cells were imaged by confocal microscopy for GFP (green) or VE-cadherin (red). The upper panels depict a single 0.5-μm confocal slice showing GFP-Rap enriched in the lateral junctions as costained by VE-cadherin. Control GFP alone was evenly distributed throughout the cytoplasm. Bottom panels depict an orthogonal (xz) slice from the confocal stack above. GFP-Rap enrichment is likewise observed at junctions (arrows). The vertical (z) dimension has been exaggerated for clarity. Horizontal white scale bar = 10 μm. Vertical black scale bar = 2 μm. (B) HUVECs were retrovirally transduced with GFP-Spa1 or GFP-Spa1ΔGRD and cultured for 4 days. GFP was visualized by live-cell imaging and pictures taken and analyzed for the area (pixels) of the GFP-positive cells, the results of which were plotted. One of three representative experiments is shown for which more than 50 cells were analyzed per group. The frequency of the analyzed cells represents the percentage of cells with the indicated number of pixels in the x-axis. The difference in the median size of cells between the 2 groups, from 3 independent experiments, was statistically significant (median Spa1ΔGRD, 26 501 (n = 155); median Spa1, 20 592 (n = 154), P < .001). Representative pictures of Spa1 and Spa1ΔGRD-expressing cells are shown. For the analysis of monolayers expressing Spa1, only cells with discernable cellular structures were evaluated (arrows), while those that were completely round (*) were excluded to avoid inclusion of debris in the analysis.
cAMP is a known regulator of barrier function in endothelial cells. Furthermore, Epacs have been described as a family of cAMP-responsive GEFs for Rap. Thus, we sought to examine the potential contribution of the Epac/Rap pathway in endothelial barrier properties. Epac1 and 2 were present in HUVECs as detected by Western blot analysis (Figure 2A). cAMP-elevating agents rolipram and forskolin significantly increased GTP loading of endogenous Rap1 (Rap-GTP) (Figure 2B) as well as retrovirally expressed GFP-Rap1 (as used in Figure 1, data not shown) in endothelial cells. Active Rap2 was present at relatively higher levels in untreated HUVECs, and cAMP induced a more modest increase in activation (Figure 2B). To test whether Epac/Rap signaling represents a new pathway for cAMP-dependent modulation of endothelial barrier function, we exploited the recently described cAMP analog, 8-pCPT-2′-O-Me-cAMP (O-Me-cAMP) with demonstrated selectivity for Epacs and no detectable effect on PKA activation.13 Treatment of HUVECs with O-Me-cAMP resulted in rapid and sustained activation of Rap1 (Rap1-GTP) and a modest increase in Rap2-GTP. Furthermore, O-Me-cAMP treatment did not result in PKA activation as assessed by phosphorylation of the transcription factor CREB (cAMP-response element-binding protein) (Figure 2C). These results provided the framework to understand the functional role of Epac/Rap-dependent and PKA-independent pathways in endothelial cell barrier function. Vascular permeability in HUVECs was measured in an in vitro assay of leakage to fluorescein-dextran at 60 minutes after addition of the dye, a time point at which leakage is prominent. Treatment with O-Me-cAMP led to significantly decreased dextran leakage that was comparable to that observed after cAMP treatment (Figure 2D). The effect of O-Me-cAMP was dose dependent, and pretreatment of the monolayer prior to addition of dye was not necessary, suggesting that Epac activation rapidly modulates endothelial barrier function. Fluorescein-dextran leakage also is detectable at 5 and 15 minutes, and increased barrier function by O-Me-cAMP and cAMP at these times was again observed (Figure 2E). Rap1 activation also was detected at these early time points (Figure 2B). This suggests a functional correlation of activation of this GTPase and regulation of vascular permeability.
Rap activation and function in barrier integrity in endothelial cells through Epac. (A) Western blot analysis of cell lysates from HUVECs using an Epac1 or Epac2 antibody revealed a specific band at 110 kDa for Epac1, and 120 kDa and 79 kDa for Epac2, consistent with their predicted molecular weight and a described short isoform of Epac2 that has been shown to retain GEF activity toward Rap1.22 (B) HUVECs were treated with forskolin and rolipram (F + R, 10 μM, and 20 μM, respectively) for 25 minutes or O-Me-cAMP (100 μM) for the times indicated. Pull-down assays to detect active Rap1 (Rap1-GTP) and Rap2 (Rap2-GTP) were then performed. Western blot for total Rap shows equal amount of Rap protein in all samples. The “fold induction” of Rap activation following the indicated treatments (numbers below Rap1-GTP and Rap2-GTP blots) was statistically significant for Rap1 at all time points (P < .05) and for Rap2 only at 15 minutes following O-Me-cAMP treatment (P < .05). (C) Analysis of PKA activation. HUVECs were treated with F + R, as in panel C, or O-Me-cAMP (500 μM) for the times indicated. Western blots for phospho-CREB (pCREB) or total CREB are shown and are representative of 4 independent experiments. (D) Permeability assays show HUVEC response with F + R or O-Me-cAMP at the doses indicated. Results are the normalized mean and standard error of 4 independent experiments (*P < .01 as compared to control). (E) Permeability was measured at 5 and 15 minutes following addition of F + R or O-Me-cAMP. cAMP elevation (F + R; □) and 100 μM (▦) and 500 μM of O-Me-cAMP (▪) significantly increased barrier function at both 5 and 15 minutes after their addition. Results are the normalized mean and standard error of 3 independent experiments (*P < .05, **P < .01 as compared to control [set at 1.0] for each time point).
Rap activation and function in barrier integrity in endothelial cells through Epac. (A) Western blot analysis of cell lysates from HUVECs using an Epac1 or Epac2 antibody revealed a specific band at 110 kDa for Epac1, and 120 kDa and 79 kDa for Epac2, consistent with their predicted molecular weight and a described short isoform of Epac2 that has been shown to retain GEF activity toward Rap1.22 (B) HUVECs were treated with forskolin and rolipram (F + R, 10 μM, and 20 μM, respectively) for 25 minutes or O-Me-cAMP (100 μM) for the times indicated. Pull-down assays to detect active Rap1 (Rap1-GTP) and Rap2 (Rap2-GTP) were then performed. Western blot for total Rap shows equal amount of Rap protein in all samples. The “fold induction” of Rap activation following the indicated treatments (numbers below Rap1-GTP and Rap2-GTP blots) was statistically significant for Rap1 at all time points (P < .05) and for Rap2 only at 15 minutes following O-Me-cAMP treatment (P < .05). (C) Analysis of PKA activation. HUVECs were treated with F + R, as in panel C, or O-Me-cAMP (500 μM) for the times indicated. Western blots for phospho-CREB (pCREB) or total CREB are shown and are representative of 4 independent experiments. (D) Permeability assays show HUVEC response with F + R or O-Me-cAMP at the doses indicated. Results are the normalized mean and standard error of 4 independent experiments (*P < .01 as compared to control). (E) Permeability was measured at 5 and 15 minutes following addition of F + R or O-Me-cAMP. cAMP elevation (F + R; □) and 100 μM (▦) and 500 μM of O-Me-cAMP (▪) significantly increased barrier function at both 5 and 15 minutes after their addition. Results are the normalized mean and standard error of 3 independent experiments (*P < .05, **P < .01 as compared to control [set at 1.0] for each time point).
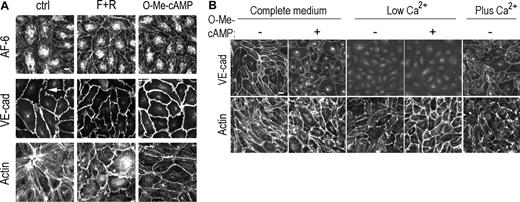
Immunofluorescence analysis of HUVEC revealed that both cAMP and O-Me-cAMP increased continuity of staining of the TJ molecule and cytoskeletal linker proteinAF-6 along cell-cell contacts (Figure 3A). Rap1, shown previously to interact withAF-6,16 bound endothelialAF-6 in an in vitro assay (data not shown). Thus AF-6 could serve as a molecular link between Epac/Rap1 and increased junction integrity. We also observed a change in staining for VE-cadherin (Figure 3A) and other AJ and TJ molecules (ZO-1, ZO-2, occludin, claudin 5, β-catenin) (data not shown) from a discontinuous, zipperlike (Figure 3A, arrow) to a more linear pattern at junctions. Both agonists induced a relative increase in cortical versus stress fiber actin (Figure 3A). Thus, Epac activation may directly promote actin reorganization. Alternatively, Epac-induced changes in junctional VE-cadherin might result in the observed changes in actin. To distinguish between these 2 possibilities, a low calcium culturing condition was developed for endothelial cells to eliminate Ca2+-dependent, homotypic interactions of junctional VE-cadherin. Incubation of confluent HPAEC in low calcium for 8 hours resulted in the loss of VE-cadherin expression at junctional sites (Figure 3B) and an increase in vascular permeability (data not shown). Junctional VE-cadherin reappeared within 45 minutes of calcium supplementation, confirming that the effect of low Ca2+ was reversible. HPAECs cultured in low calcium and treated with O-Me-cAMP exhibited notably increased density of cortical actin despite the absence of junctional VE-cadherin (Figure 3B). Thus, active Epac exerts its effects on cortical actin prior to VE-cadherin–mediated actin rearrangement. In addition to paracellular permeability, leukocyte transmigration requires junctional remodeling.7,15 However, the total number of transmigrated human neutrophils was unaffected in O-Me-cAMP versus vehicle-treated TNF-α–stimulated endothelial monolayers (Vehicle: 14.3 ± 4.1; + O-Me-cAMP: 13.0 ± 5.4; + TNF + Veh: 30.6 ± 1.9; + TNF + O-Me-cAMP: 26.4 ± 2.7, average × 103 ± SD). Together these data suggest that Epac activation reduces basal permeability in endothelial cells through enhanced cortical cytoskeleton and subsequent actin-driven recruitment of junctional molecules. Despite these significant Epac-stimulated changes, Epac activation had no effect on endothelial leukocyte transmigration, thus suggesting these functions are independently regulated.
Mechanism of Epac/Rap-induced increase in barrier function. (A) Immunofluorescence of fixed HUVECs treated for 30 minutes with F + R or O-Me-cAMP (100 μM) or control untreated cells (ctrl) using antibodies to AF-6 (top row), VE-cadherin (middle row), or FITC-phalloidin to detect actin filaments (bottom row). Scale bar = 10 μm. (B) Immunofluorescence of fixed HPAECs cultured in complete medium, low calcium medium, or after calcium replenishment (Plus Ca2+). Cells were treated with O-Me-cAMP for 30 minutes (+) or vehicle control (–) and fixed and stained for VE-cadherin (top row) or FITC-phalloidin to detect actin filaments (bottom row). Scale bar = 20 μm.
Mechanism of Epac/Rap-induced increase in barrier function. (A) Immunofluorescence of fixed HUVECs treated for 30 minutes with F + R or O-Me-cAMP (100 μM) or control untreated cells (ctrl) using antibodies to AF-6 (top row), VE-cadherin (middle row), or FITC-phalloidin to detect actin filaments (bottom row). Scale bar = 10 μm. (B) Immunofluorescence of fixed HPAECs cultured in complete medium, low calcium medium, or after calcium replenishment (Plus Ca2+). Cells were treated with O-Me-cAMP for 30 minutes (+) or vehicle control (–) and fixed and stained for VE-cadherin (top row) or FITC-phalloidin to detect actin filaments (bottom row). Scale bar = 20 μm.
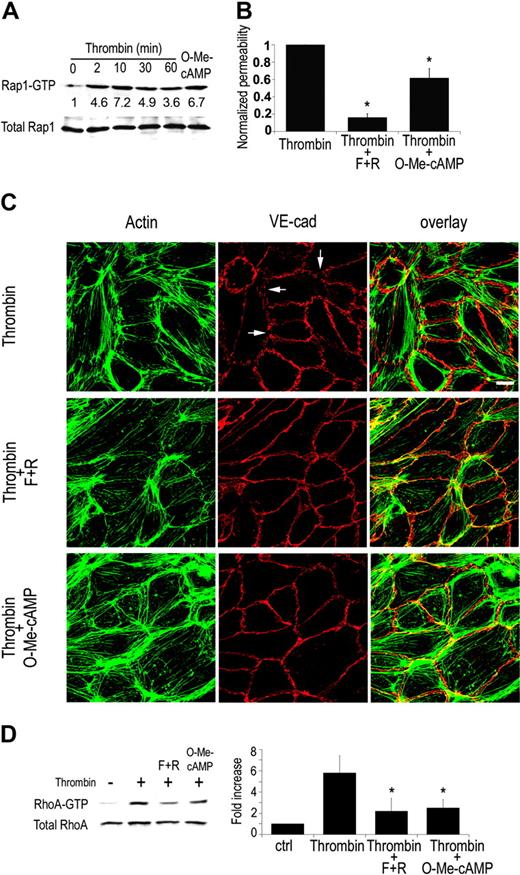
Vascular permeability in vivo is induced by various pro-inflammatory mediators including thrombin, histamine, and platelet-activating factor (PAF). In vitro, thrombin engagement of its receptor PAR1 promotes Rho GTPase-dependent actin cytoskeletal changes. This results in myosin light chain (MLC) phosphorylation and actin-myosin–driven cell contraction, which contributes to thrombin-induced barrier dysfunction.17,18 cAMP has been reported to reverse thrombin-induced permeability in HUVECs largely through PKA-dependent mechanisms.5 We sought to examine the contribution of Epac/Rap and PKA-independent pathways in counteracting thrombin-mediated hyperpermeability. Thrombin activation of endothelial cells resulted in Rap1 activation (Figure 4A). Thrombin-treated HUVECs showed an average 3-fold increase in permeability. Thrombin-enhanced permeability was significantly attenuated by cAMP treatment as previously reported17 and partially but reproducibly inhibited by 100 μM O-Me-cAMP treatment (by approximately 40%) (Figure 4B). Higher concentrations of Epac (500 μM and 1000 μM) resulted in a trend toward increased barrier function compared to 100 μM, although not statistically significant (P > .05). F-actin immunostaining of thrombin-stimulated endothelial cells revealed a reorganization of actin into stress fibers that spanned the cells, contraction of cells resulting in gaps in the monolayer and the partial dissociation of VE-cadherin from cell-cell contacts (Figure 4C). cAMP and O-Me-cAMP treatment attenuated these effects and increased cortical actin. Thus, our studies suggest that Rap activation can counteract thrombin-mediated contraction and distribution of VE-cadherin and imply that thrombin-mediated Rap activation may exist as a homeostatic mechanism for modulating thrombin-induced permeability. To further explore the observed protective effect of Epac/Rap activation, thrombin-induced Rho activation was analyzed following O-Me-cAMP treatment. O-Me-cAMP decreased thrombin-induced Rho activation, which was similar in magnitude to that observed following treatment with cAMP-elevating agents (Figure 4D). Others have reported that PKA plays a role in cAMP-dependent inhibition of Rho. That is, overexpression of the PKA inhibitor PKI in endothelial cells results in a 30% inhibition of thrombin-induced Rho activation.19 Our results demonstrating that the PKA-independent Epac pathway leads to a reduction in RhoA activation, similar to that observed following cAMP treatment, suggest that the effect of cAMP on RhoA may be largely through Epac/Rap. Our studies suggest a cross-talk between Rho and Rap. Rap regulation of other Rho family members has been shown previously; Rap stimulated cdc42 in yeast16 and Rac in a mammalian cell line.20 It is noteworthy that O-Me-cAMP had no significant effect on basal Rho activity in untreated endothelial cells (data not shown). Therefore, the down-modulatory effect of Rap on Rho activation requires a component of the thrombin-signaling pathway. Despite the comparable decrease in Rho activation by cAMP and O-Me-cAMP, cAMP-elevating agonists are significantly more potent in decreasing vascular permeability than O-Me-cAMP. This may be because Rho or Rho-kinase inhibition reduces thrombin-induced permeability by only 50% with the remaining permeability being dependent on Ca2+/calmodulin pathways.4 This suggests a Rho-independent pathway that may be regulated by cAMP/PKA but not Epac/Rap-dependent signaling. Indeed, PKA promotes inactivation of the Ca2+/calmodulin-dependent actin-myosin contraction.21 Thus, activation of Rap through Epac triggers pathways that compete for thrombin-elicited Rho-dependent mechanisms of vascular permeability, while efficient down-regulation of Rho-independent pathways likely require PKA.
Role of EPAC/Rap in thrombin-induced permeability. (A) Pull-down assay for active Rap in HUVECs treated with thrombin (2 U/mL) for the times indicated or O-Me-cAMP for 30 minutes. Rap1 activation by thrombin was reproducibly observed at different time points ranging from 2 to 60 minutes (n = 6), a representative blot of which is shown. Densitometric analysis of experiments in which activation of Epac was included as a positive control was undertaken. Thrombin led to a 2- to 3-fold activation of Rap1 (P < .05 for all time points except 60 minutes). On the other hand, only a modest increase in Rap2 was observed under the same conditions (P > .05) (data not shown). (B) Permeability assay for HUVEC response to F + Ror O-Me-cAMP (100 μM) in the presence of thrombin (2 U/mL). Results are the normalized mean and standard error of 6 independent experiments (asterisks indicate P < .01 as compared to thrombin control). (C) Immunofluorescence of HUVECs treated with thrombin alone or in combination with F + R or O-Me-cAMP (100 μM) for 30 minutes. Cells were fixed and stained for actin with FITC-phalloidin (green) and VE-cadherin (red). Right column shows the overlay of actin and VE-cadherin. Thrombin-induced stress fibers and gaps in VE-cadherin (arrows), which were partially reversed by F + R or O-Me-cAMP. The number of gaps were quantitated in 3 independent experiments. Gaps were identified as significant discontinuities in VE-cadherin staining as indicated by the white arrows. The results, expressed as percent compared to thrombin (Thr)–treated cells, are as follows: Thr, 100%; Thr plus F + R, 22.2% ± 6.7%; Thr plus O-Me-cAMP, 37% ± 22%. (D) Pull-down assay for active RhoA (RhoA-GTP) in HUVECs. Rho assays were performed on endothelial monolayers that were stimultaneously treated with vehicle (–, ctrl), thrombin (2 U/mL) alone, thrombin plus O-Me-cAMP, or thrombin plus cAMP for 30 minutes. Results of densitometric analysis of 3 independent experiments are shown in right panel. Asterisk indicates P < .05.
Role of EPAC/Rap in thrombin-induced permeability. (A) Pull-down assay for active Rap in HUVECs treated with thrombin (2 U/mL) for the times indicated or O-Me-cAMP for 30 minutes. Rap1 activation by thrombin was reproducibly observed at different time points ranging from 2 to 60 minutes (n = 6), a representative blot of which is shown. Densitometric analysis of experiments in which activation of Epac was included as a positive control was undertaken. Thrombin led to a 2- to 3-fold activation of Rap1 (P < .05 for all time points except 60 minutes). On the other hand, only a modest increase in Rap2 was observed under the same conditions (P > .05) (data not shown). (B) Permeability assay for HUVEC response to F + Ror O-Me-cAMP (100 μM) in the presence of thrombin (2 U/mL). Results are the normalized mean and standard error of 6 independent experiments (asterisks indicate P < .01 as compared to thrombin control). (C) Immunofluorescence of HUVECs treated with thrombin alone or in combination with F + R or O-Me-cAMP (100 μM) for 30 minutes. Cells were fixed and stained for actin with FITC-phalloidin (green) and VE-cadherin (red). Right column shows the overlay of actin and VE-cadherin. Thrombin-induced stress fibers and gaps in VE-cadherin (arrows), which were partially reversed by F + R or O-Me-cAMP. The number of gaps were quantitated in 3 independent experiments. Gaps were identified as significant discontinuities in VE-cadherin staining as indicated by the white arrows. The results, expressed as percent compared to thrombin (Thr)–treated cells, are as follows: Thr, 100%; Thr plus F + R, 22.2% ± 6.7%; Thr plus O-Me-cAMP, 37% ± 22%. (D) Pull-down assay for active RhoA (RhoA-GTP) in HUVECs. Rho assays were performed on endothelial monolayers that were stimultaneously treated with vehicle (–, ctrl), thrombin (2 U/mL) alone, thrombin plus O-Me-cAMP, or thrombin plus cAMP for 30 minutes. Results of densitometric analysis of 3 independent experiments are shown in right panel. Asterisk indicates P < .05.
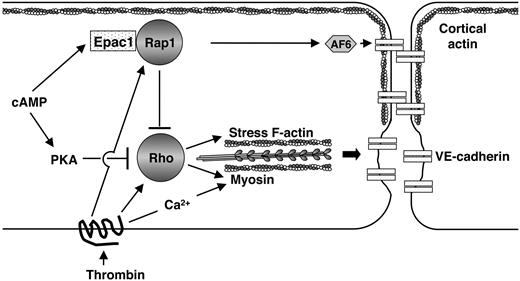
In summary, our data show 3 important findings. First, Rap1 is present at cell-cell contacts in confluent cultured human endothelial cell monolayers. Inactivation of endogenous Rap by expression of a specific RapGAP, Spa1 disrupts the endothelial cell monolayer, providing evidence for a role for Rap in cell-cell and/or cell-matrix interactions. Second, Epac-regulated Rap activation is important for maintaining barrier integrity by reorganizing actin and subsequently AJ and TJ components via a PKA-independent mechanism. Third, Epac/Rap GTPase down-regulates thrombin-induced Rho activation and Rho-induced stress fibers, demonstrating a previously unappreciated cross-talk between these 2 GTPases (Figure 5). These studies shed new light on signaling pathways that regulate the integrity of the endothelial barrier function. Vascular leakage leading to excessive tissue edema is a hallmark of chronic inflammation and often has severe pathologic consequences in patients, yet current therapies to counteract this process often fail.1 Our studies show that Rap/Epac figures prominently in regulating permeability independent of PKA, and therefore may represent a new therapeutic target for modulating vascular permeability.
Model for the role of Epac/Rap in endothelial barrier function. An en face schematic of 2 endothelial cells is illustrated. cAMP activates Epac, resulting in Rap activation at junctions. This induces reorganization of cortical actin and subsequent redistribution of VE-cadherin and other AJ and TJ molecules, to cell-cell contacts, enhancing macromolecular barrier function. AF-6, an effector of Rap, may direct Epac/Rap effects in cytoskeletal rearrangement. Thrombin promotes cytoskeletal changes by both Rho-dependent and -independent (calcium/calmodulin mediated) pathways, resulting in increase in stress fibers and actin-myosin contraction. Rap stimulated by thrombin may serve as a negative feedback to down-regulate Rho and may be required for reigning in thrombin/Rho-induced changes in permeability. Thrombin modulation of endothelial permeability results from the integration of positive and negative signals through Rap and Rho altering junctional and stress fiber components.
Model for the role of Epac/Rap in endothelial barrier function. An en face schematic of 2 endothelial cells is illustrated. cAMP activates Epac, resulting in Rap activation at junctions. This induces reorganization of cortical actin and subsequent redistribution of VE-cadherin and other AJ and TJ molecules, to cell-cell contacts, enhancing macromolecular barrier function. AF-6, an effector of Rap, may direct Epac/Rap effects in cytoskeletal rearrangement. Thrombin promotes cytoskeletal changes by both Rho-dependent and -independent (calcium/calmodulin mediated) pathways, resulting in increase in stress fibers and actin-myosin contraction. Rap stimulated by thrombin may serve as a negative feedback to down-regulate Rho and may be required for reigning in thrombin/Rho-induced changes in permeability. Thrombin modulation of endothelial permeability results from the integration of positive and negative signals through Rap and Rho altering junctional and stress fiber components.
Prepublished online as Blood First Edition Paper, September 16, 2004; DOI 10.1182/blood-2004-05-1987.
X.C. and S.K.S. contributed equally to this study.
Supported by National Institutes of Health grants PO1 HL036028 and K01 DK002798.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Dr Seema Sehrawat for Western blot analysis of Epac2.

![Figure 2. Rap activation and function in barrier integrity in endothelial cells through Epac. (A) Western blot analysis of cell lysates from HUVECs using an Epac1 or Epac2 antibody revealed a specific band at 110 kDa for Epac1, and 120 kDa and 79 kDa for Epac2, consistent with their predicted molecular weight and a described short isoform of Epac2 that has been shown to retain GEF activity toward Rap1.22 (B) HUVECs were treated with forskolin and rolipram (F + R, 10 μM, and 20 μM, respectively) for 25 minutes or O-Me-cAMP (100 μM) for the times indicated. Pull-down assays to detect active Rap1 (Rap1-GTP) and Rap2 (Rap2-GTP) were then performed. Western blot for total Rap shows equal amount of Rap protein in all samples. The “fold induction” of Rap activation following the indicated treatments (numbers below Rap1-GTP and Rap2-GTP blots) was statistically significant for Rap1 at all time points (P < .05) and for Rap2 only at 15 minutes following O-Me-cAMP treatment (P < .05). (C) Analysis of PKA activation. HUVECs were treated with F + R, as in panel C, or O-Me-cAMP (500 μM) for the times indicated. Western blots for phospho-CREB (pCREB) or total CREB are shown and are representative of 4 independent experiments. (D) Permeability assays show HUVEC response with F + R or O-Me-cAMP at the doses indicated. Results are the normalized mean and standard error of 4 independent experiments (*P < .01 as compared to control). (E) Permeability was measured at 5 and 15 minutes following addition of F + R or O-Me-cAMP. cAMP elevation (F + R; □) and 100 μM (▦) and 500 μM of O-Me-cAMP (▪) significantly increased barrier function at both 5 and 15 minutes after their addition. Results are the normalized mean and standard error of 3 independent experiments (*P < .05, **P < .01 as compared to control [set at 1.0] for each time point).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/105/5/10.1182_blood-2004-05-1987/6/m_zh80050574780002.jpeg?Expires=1769084867&Signature=kUBhYj~f6~8ph5SYdZJFd3gkxYIJjZM8T8L4oNoItLgV4ooLLydNSyrHcnZjJ9musMhm~f5opgLVF6VEjHKeK3jIGF1JT-YZqH45Wp5rOYPaTBbTNU7bcdanuDeJ4RvCSdPWuEYkGxLcsUzc2QOYuRiVfdFcW-LTbXVdfUyqDbQIoLEtr8A4XYOw~g417MjZyyt9kNAh7KwEf~V1JwUrqw934nQvEuCGwdbCBvLfWaBx~UFGxtXpsvWh3uXF8gr1UMSg8PDLs8WM9odYXAVuPREBvYviPhCMvNo5NaBaRjf4GKT-B-BasUwJ1Y~7eOcq6S-OOPmoUCFozTXMKTud5g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal