Comment on Guthrie et al, page 1916
The nitric oxide pathway plays an important role in modulating endothelial progenitor cell function, playing a critical role in blood vessel repair in response to injury.
Avariety of studies in the murine system provide substantial evidence that blood and endothelial progenitor cells (EPCs) arise from a common progenitor, the hemangioblast, during embryogenesis.1 Recent evidence that adult murine bone marrow hematopoietic stem cells (HSCs) possess hemangioblastic activity has also been reviewed.2 From this work, one would predict that the hemangioblasts give rise to circulating EPCs that participate in vessel homeostasis throughout murine life. Understanding the role for hemangioblasts and EPCs in the repair of damaged vessels or in pathogenic tumor neoangiogenesis has been complicated by apparent differences in the involvement of these cells depending upon the type of host injury or tumor model used.
Ischemic or traumatic injury alone is not sufficient to induce retinal neovascularization in the adult mouse. However, ischemic retinal injury in mice exposed to high concentrations of intraocular vascular endothelial growth factor (VEGF) develop a robust proliferative retinopathy similar to that seen in human diabetic retinopathy. Using such a model, Grant et al3 previously reported that adult murine bone marrow HSCs possess functional hemangioblastic activity in the injured murine retina.FIG1
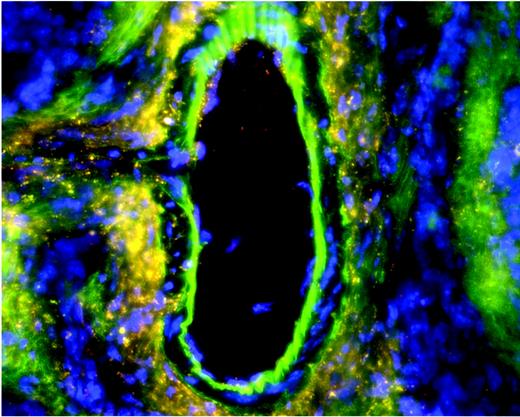
Chronic vascular injury in eNOSgfpchimeras induces widespread hemangioblast activity from adult HSCs. See the complete figure in the article beginning on page 1916.
Chronic vascular injury in eNOSgfpchimeras induces widespread hemangioblast activity from adult HSCs. See the complete figure in the article beginning on page 1916.
In this issue of Blood, Guthrie and colleagues examined the role of the nitric oxide (NO) pathway in modulating vessel repair by transplanted populations of green fluorescence protein–expressing (gfp+) bone marrow cells. Recipient mice deficient in inducible nitric oxide synthase (iNOS–/–) or endothelial nitric oxide synthase (eNOS–/–) received transplants of congenic marrow gfp+ cells enriched for HSCs. At 3 months following transplantation, an adeno-associated viral vector expressing VEGF was injected into the vitreous of the test eye of each host. One month later, laser photocoagulation of the venous vessels juxtaposed to the optic nerve was performed leading to an ischemic injury to nearly one half of the treated retina. The non–laser-treated eye served as a control for each subject. Consistent with prior observations, the gfp+ HSC-enriched cells contributed to new vessel formation in the laser-treated, but not the control, eye of normal congenic hosts. Similarly, gfp+ HSC-enriched cells contributed to new vessel formation in the iNOS–/– hosts only in the laser-injured retina, with little contribution in the contralateral non–laser-treated retina. Surprisingly, gfp+ HSC-enriched cells robustly contributed to neovascularization in both test and control eyes of eNOS–/– hosts, and gfp+ cells populated large and small vessels in all tissues examined. This result was in stark contrast to the paucity of gfp+ cell contributions to systemic vascular tissues in the wild-type and iNOS–/– hosts. Thus, eNOS–/– mice appear to display a systemic vascular dysfunction where marrow-derived progenitor cells are extensively recruited and incorporated into the vessels.
Though the mechanism remains to be elucidated, Guthrie and colleagues propose that eNOS deficiency may lead to a compensatory overexpression of vascular iNOS leading to pathologic vascular endothelial cell turnover. This is an interesting and testable hypothesis that may lead to novel insights into the mechanism of EPC mobilization and engraftment in certain neoangiogenic sites. Further studies defining the ontogeny of the vascular dysfunction, kinetics of EPC turnover, and types of cells that rescue eNOS–/– vessels will be challenging and informative. ▪

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal