Abstract
Studies in an in vitro model of cutaneous T-cell lymphoma (CTCL) demonstrated that CTCL cell proliferation is stimulated by direct contact with autologous, immature dendritic cells (DCs), suggesting that CD4+ CTCL cell division is driven by antigens presented by DC major histocompatibility complex (MHC) class 2. We now report that the T-cell receptor (TCR) of the CD4+ CTCL cells is triggered after interaction with DCs loaded with apoptotic CTCL cells, as shown by reduced membrane expression of CD3 and the TCR, up-regulation of cytotoxic T lymphocyte antigen-4 (CTLA-4), and calcium mobilization. CTCL cells adopt a T-regulatory (Treg) phenotype expressing CD25/CTLA-4 and FoxP3 and secreting interleukin-10 (IL-10) and transforming growth factor-β (TGF-β). Treg CTCL cells suppress normal T-cell antigen-driven secretion of IL-2 and interferon-γ (IFN-γ). Blocking DC MHC class 2 expression or transport inhibited CTCL cell adoption of a Treg phenotype. Allogeneic CTCL cells or normal CD4 T cells served as sources of apoptotic material for CTCL cell conversion to a Treg phenotype. Conversion of CTCL cells to Treg cells may explain the anergic, immunosuppressive nature of the malignancy. (Blood. 2005;105:1640-1647)
Introduction
Cutaneous T-cell lymphoma (CTCL) encompasses a spectrum of clinical presentations unified by the immunologic features of neoplastic T cells. CTCL is a clonal malignancy1 of CD4+ 2 helper T cells3,4 expressing a memory phenotype5 and with a propensity to accumulate in the epidermis in proximity to Langerhans cells6 (LCs), which are immature dendritic cells (DCs).7 Epidermal association of CTCL cells with LCs is so distinctive that it is considered a diagnostic hallmark of the malignancy known as Pautrier microabscesses.6 Proliferation of CTCL cells in the epidermis, in association with LCs, has suggested that the tumor cells' growth is stimulated by LC-mediated antigen presentation and has led to the hypothesis that CTCL is a disease of chronic antigen stimulation whose growth is promoted by LC presentation of virally, bacterially, or chemically modified peptides.8,9
Despite the presence in CTCL patients of circulating populations of CD8 T cells10 that recognize major histocompatibility complex (MHC) class 1-displayed tumor-specific peptides derived from the malignant T cell clonotypic T-cell receptor (TCR),11,12 the disease persists and disseminates, indicating that the malignancy commonly evades immunosurveillance. We developed a culture system that allowed assessment of the factors that promote the growth of CTCL cells13 and clarified their interaction with immature DCs.
In the cultures, proliferation of CTCL cells and autologous myeloid DCs, requires direct membrane contact between the 2 cell populations, suggesting a dynamic “cross-talk” between them. Only immature autologous DCs support CTCL cell replication, and CTCL cell production of interleukin-10 (IL-10) maintains longterm DC immaturity.13 After 3 months of culture, the DCs finally progress to maturity, revealed by increased expression of surface MHC class 2 and cytoplasmic DC-LAMP (lysosomal membrane glycoprotein).7 DC maturation abruptly truncates the synergy between DCs and CTCL cells, leading to the degranulation and death of the DCs, followed shortly thereafter by the death of the CTCL cells.
In the current studies, we determined that after exposure to immature DCs loaded with apoptotic CTCL cells, the TCR of the malignant T cell is engaged, and the CTCL cells respond by proliferating, up-regulating cytoplasmic cytotoxic T lymphocyte antigen-4 (CTLA-4), FoxP3, and membrane CD25, and secreting the immunosuppressive cytokines IL-10 and transforming growth factor-β (TGF-β), all features of T-regulatory (Treg) cells.14 Interference with access to DC MHC class 2 molecules diminishes the capacity of the tumor-loaded DCs to trigger CTLA-4 upregulation in CTCL cells.
Treg cells comprise 5% to 10% of the peripheral blood pool and are so named because they regulate the activities of T-cell subsets responding to self-antigens,15 infectious agents,16 and transplantation antigens.17 Treg cells directly suppress T-cell activation, possibly through membrane expression of molecules such as CTLA-4,18 and they may mediate distal antigen nonspecific suppression through the secretion of cytokines such as IL-10 and TGF-β1.19,20
Our studies demonstrate that when CTCL cells adopt a Treg phenotype, they function as suppressors when added to normal cells responding to antigen stimulation. The source of apoptotic material that drives CTCL cells to become Treg cells is unrestricted and can be obtained from CTCL cells derived from different patients and normal CD4 T cells. Adoption of a Treg profile by CTCL cells explains clinical features of the malignancy and identifies new avenues for therapeutic intervention.
Materials and methods
Cell culture
Cultures of CTCL cells and myeloid DCs were established as described.13 Peripheral leukocytes were isolated from a leukapheresis harvest from CTCL patients, from whom informed consent was obtained according to the Declaration of Helsinki and following Yale Human Investigational Review Board guidelines. After harvest, the leukocytes were cultured with granulocyte macrophage-colony-stimulating factor (GM-CSF; 800 U/mL), IL-4 (1000 U/mL), IL-2 (10 U/mL), and IL-7 (10 ng/mL; R&D Systems, Minneapolis, MN) in RPMI 1640/10% AB serum, and 5% fetal calf serum (FCS; Gibco, Gaithersburg, MD). On initial isolation, most T cells were CTLA-4-. Episodic identification of CTLA-4+ CTCL cells was related to high numbers of apoptotic cells in cultures, and CTLA-4 expression reverted to background when CTCL cells were recultured overnight in the absence of apoptotic cells (Supplemental Figure 1; see the Supplemental Figures link at the top of the online article on the Blood Web site). Induction of Treg CTCL cells was replicated in more than 10 cultures, and neither the source of the CTCL cells nor the length of the culture had an impact on the up-regulation of a Treg phenotype.
Cell purification
CTCL cells were purified away from DCs by incubation with CD4 antibody conjugated to magnetic beads (Miltenyi Biotech, Auburn, CA) and passage over a column in a magnetic field, according to the manufacturer's instructions. Immature DCs were weakly CD4+ and did not copurify. CD4 magnetic bead separation resulted in 98% ± 0.5% CD4+ T-cell purity and 91% ± 2% DC enrichment in the column effluent.
Immunophenotyping
Monoclonal antibodies were directly conjugated to fluorescein isothiocyanate (FITC) or phycoerythrin (PE) or were stained with a secondary antibody (goat antimurine immunoglobulin). Antibodies included CTCL cells CD3, CD4, and CD25; family-specific antibodies to the variable region of the α- and β-chains of the TCR; CTLA-4 (Coulter Immunotech, Hialeah, FL); and FoxP3 identified by a secondary antibody to goat immunoglobulin raised in donkeys (Abcam, Cambridge, MA). DCs were identified through staining for MHC class 2 molecules and for CD83. Cells were gated on the lymphocyte or monocyte population, as defined by scatter parameters, and 10 × 103 cells were analyzed by flow cytometry (Coulter Epics). Two-color staining was assessed after the addition of a second antibody conjugated to FITC or PE. Isotype-matched controls established positive regions on 1- or 2-color histograms.
Membrane and cytoplasmic antigen coexpression was examined with the use of a fixation and permeabilization kit (Coulter Immunotech), with antibodies conjugated to FITC or PE. Background staining was determined with isotype controls conjugated to FITC or PE.
Apoptosis and DC loading
Purified CTCL cells (1-6 × 106/mL; 100 μL/well) were rendered apoptotic by CD3 antibody (3.5 μg/0.5 mL purified CD3, 30 minutes, 23°C; Coulter). Apoptosis was monitored with the use of annexin V-FITC staining. CD4 antibody of the same isotype and concentration that binds to the CTCL cells but does not cause apoptosis was used as a negative control. Unbound antibody was removed by extensive washing.
A CD3 dose-response curve revealed that maximal apoptosis of CTCL cells was achieved at a CD3 dose of 33 μg/mL and 1-hour incubation (Supplemental Figure 2A). All concentrations of CD3 tested mediated sufficient apoptosis for Treg-cell induction. In addition, no concentration of CD3 alone, in the absence of DCs, caused induction of a Treg phenotype (Supplemental Figure 2B).
In general, apoptotic tumor cells (1 × 106 cells) were added to purified DCs (1 × 105) and were cocultivated overnight to permit engulfment of the apoptotic tumor. Control CD4 T cells were rendered apoptotic by treatment with CD3 (3.5 μg/0.5 mL) and γ-irradiation (30 Gy; cesium source) and were added to purified DCs. In some experiments, allogeneic CTCL cells were rendered apoptotic by CD3 binding and were used to load DCs autologous to responding CTCL cells. Responding CTCL cells (1 × 106/well) were freshly purified, added to DCs, and coincubated overnight.
Dose response of CTLA-4 expression
To determine the effect of increasing numbers of tumor-loaded DCs on the expression of CTLA-4, CTCL cells and DCs were purified, as described. CTCL cells (2 × 105) were rendered apoptotic by incubation with CD3 or, as a control, with an equivalent dose of CD4 isotype-matched antibody. Cells were washed and added to 103, 104, or 105 purified DCs overnight. Equivalent numbers of purified DCs were not pulsed and were used to stimulate CTCL cells. A constant number of freshly purified CTCL cells (4 × 105 cells) were added to DCs, and the cultures were incubated overnight. Cells were analyzed for the coexpression of membrane T-cell markers (CD3, CD4, CD25, FITC conjugated) and cytoplasmic CTLA-4 PE.
A similar dose-response curve was established by titering the number of apoptotic or viable CTCL cells added to purified DCs (6 × 104 to 6 × 106 CTCL cells pulsed onto 1 × 106 DCs). Loaded or nonloaded DCs were used to stimulate a constant number of responding CTCL cells (1.4 × 106 cells).
Proliferation
Purified CTCL cells (2 × 106/mL, 100 μL/well) were added to autologous or allogeneic DCs (1 × 105/well) that either were not treated or were loaded with CTCL cells rendered apoptotic by CD3 antibody. Cells were pulsed with 1 μCi/well (37 × 103 Bq/well) 3[H]-thymidine and were cocultured overnight. Wells were harvested (PhD Harvester; Cambridge Technology, Cambridge, MA), and incorporation of the radioisotope was quantified with a liquid scintillation counter.
Cytokine secretion
Supernatants were harvested from CTCL cells that had been cultured overnight alone or after stimulation with autologous DCs, which either were not treated or were loaded with apoptotic tumor cells. IL-10 or TGF-β secretion was detected using ELISA kits, as described by the manufacturer (R&D Systems).
To measure the suppression of cytokine release, normal control leukocytes were isolated from a known responder and were stimulated with tetanus toxoid (10 μg/mL, RPMI 1640/15% autologous serum) or were cultured with media and serum to obtain background counts. Control cells were pulsed with 3[H]-thymidine and demonstrated a proliferative response at day 5 (19 175 ± 3068 delta cpm). CTCL cells were induced to adopt a Treg phenotype using DCs fed CD3-treated apoptotic tumor cells; as a control, the DCs were coincubated with viable CD4-treated CTCL cells. Treg CTCL cells were added to the antigen-stimulated control cells at ratios of 1:1, 1:10, and 1:100 CTCL cells/normal control cells and were incubated for 5 days. CTCL cells that did not assume a Treg phenotype, after incubation with DCs pulsed with viable CTCL cells, were added at the same ratios to the antigen-stimulated control leukocytes. Supernatants were harvested at day 5 and were tested for IL-2 or interferon-γ (IFN-γ) production by enzyme-linked immunosorbent assay (ELISA) (R&D Systems).
Neutralizing antibodies to IL-10 or TGF-β (1-10 μg/mL; R&D Systems) were tested to determine whether the inhibition of IFN-γ secretion, noted after Treg CTCL cells were added to normal T cells (1:1 ratio) responding to tetanus toxoid was caused by immunosuppressive cytokines (Supplemental Figure 4A-B). Antibody doses were confirmed to be sufficient to neutralize Treg CTCL cell cytokine secretion using ELISA. In addition, Treg and CTCL cells were separated by a transwell membrane (0.40 μM membrane; Corning, NY) from normal lymphocytes stimulated by tetanus toxoid, and the level of IFN-γ secretion was measured using ELISA (Supplemental Figure 4C).
Calcium flux
Production of a calcium flux was monitored in CTCL cells as they encountered tumor-loaded DCs. Responding purified CTCL cells (107) were resuspended in loading buffer (RPMI 1640/5% AB serum, 1 mM CaCL2, 1 mM MgCL2, and 4 mM probenecid) and were loaded with 2 μg/mL indo-1 am (pentaacetoxymethyl ester; Molecular Probes, Eugene, OR) for 30 minutes at 37°C. Cells were resuspended in fresh loading media and were analyzed with a flow cytometer equipped with a UV light source and a 37°C sample chamber (FACS; Becton Dickinson, San Diego, CA). Optimal indo-1 loading was confirmed by the addition of 2 μg/mL ionomycin and the detection of immediate calcium flux in 100% of the cells. Autologous tumor-loaded DCs (3 × 105 DCs loaded with 1 × 106 apoptotic tumor, nonloaded DCs, or DCs loaded with γ-irradiated normal control cells (2 × 106) were injected into the flow system, and the presence of a calcium flux was monitored over time.
Inhibition of MHC class 2 pathway
To block MHC class 2, an antibody to the DR molecule (anti-HLA-DR purified; Becton Dickinson) on the DC was used. Because binding of MHC class 2 antibodies to mature DCs has been shown to induce apoptosis,21 an anti-DR antibody was chosen that did not cause DC apoptotic cell death.22 Anti-DR antibody was added (12.5 μg/0.5 mL) at the time of DC loading with apoptotic CTCL cells and was readded when freshly purified CTCL cells were cocultivated with the tumor-loaded DCs. The anti-DR antibody blocked 83% of the DC membrane MHC class 2 expression detected by staining with anti-class 2 antibody. In addition, the transport of newly synthesized MHC class 2 molecules was inhibited by the addition of Brefeldin A23 (10 μg/mL; Sigma-Aldrich, St Louis, MO). Induction of a CTCL-cell Treg phenotype was evaluated after anti-HLA-DR blocking or Brefeldin A treatment by immunophenotyping. Controls included nonloaded DCs, cocultures of DCs loaded with CD4-treated viable CTCL cells incubated with anti-HLA-DR or Brefeldin A, and DCs loaded with apoptotic tumor and incubated with an isotype-matched control.
Statistical analysis
Statistical evaluation was performed with an unpaired, 2-tailed Student t test. Three or 5 replicate cultures were averaged, and the mean and standard deviation were reported and used for statistical comparisons.
Results
Induction of a Treg phenotype
To induce a Treg phenotype, isolated DCs were pulsed with purified CTCL cells that had been rendered apoptotic by incubation with CD3 antibody. CD3 treatment of CTCL cells was chosen because of the rapid time course (Supplemental Figure 2A-B) and the efficiency in generating apoptotic material for DC loading in comparison with other mediators of apoptosis, such as γ-irradiation,24 staurosporine,25 anti-Fas antibody,26 or 8-methoxypsoralen27 (Supplemental Figure 3A-B).
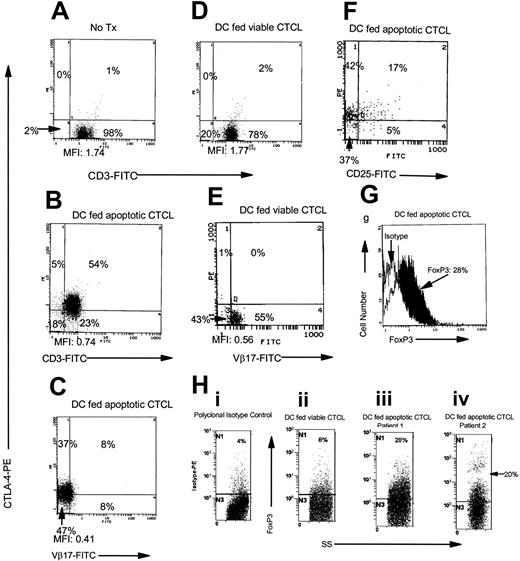
When freshly purified CTCL cells were incubated overnight with DCs that had ingested apoptotic tumor cells, CTLA-4 expression was up-regulated in the cytoplasm of the CTCL cells (Figure 1B-C). Coincubation of CTCL cells with DCs that had not been pulsed (Figure 1A) or that had been pulsed with viable CTCL cells did not result in CTLA-4 up-regulation (Figure 1D-E). Membrane expression of CTCL cell TCR decreased after incubation with DCs that had ingested apoptotic CTCL cells, as shown by reduced membrane CD3 (Figure 1B) and by the clonally expressed Vβ region of the TCR (Figure 1C) compared with exposure to DCs pulsed with viable CTCL cells (Figure 1D-E). Taken together, the demonstration of up-regulated CTLA-4 (which occurs after formation of the TCR-MHC-peptide complex28 ) and the loss of the TCR from the CTCL cell membrane (which supports binding to the TCR29 ) indicated that CTCL cell TCR engagement occurred after encounter with apoptotic tumor-loaded DCs.
Induction of CTLA-4 expression in CTCL cells. (A) Nonloaded DC-stimulated fresh CTCL cells and membrane CD3/cytoplasmic CTLA-4 expression was measured. Treg-cell induction was monitored by measuring the coexpression of (B) membrane CD3/cytoplasmic CTLA-4 and (C) membrane Vβ17 (antibody to the variable region of the beta chain of the TCR that is clonotypically expressed on the tumor cells)/cytoplasmic CTLA-4. (D) DCs pulsed with viable CTCL cells (incubated with CD4 antibody) provided a control for tumor-loaded DCs by measuring membrane CD3/cytoplasmic CTLA-4 and (E) membrane Vβ17/cytoplasmic CTLA-4. (F) Induced Treg cells coexpressed membrane CD25/cytoplasmic CTLA-4. Results are reported as percentage of positive cells and mean fluorescence intensity (MFI). Results are representative of 10 separate experiments performed on cells isolated from cultures of 8 different CTCL patients. (G) CTCL cells were induced to express cytoplasmic FoxP3, compared with isotype-matched control (1-color histogram), by overnight incubation with DCs loaded with apoptotic tumor cells in. (H) Isotype control (donkey antigoat PE) staining in CTCL cells is compared (i) with CTCL cocultivated overnight with DCs exposed to viable CTCL cells (ii) and CTCL cells isolated from 2 patients and cultured with DCs that had ingested apoptotic tumor cells (iii and iv). Histograms demonstrate side scatter gating on the lymphocyte population compared with FoxP3 PE staining.
Induction of CTLA-4 expression in CTCL cells. (A) Nonloaded DC-stimulated fresh CTCL cells and membrane CD3/cytoplasmic CTLA-4 expression was measured. Treg-cell induction was monitored by measuring the coexpression of (B) membrane CD3/cytoplasmic CTLA-4 and (C) membrane Vβ17 (antibody to the variable region of the beta chain of the TCR that is clonotypically expressed on the tumor cells)/cytoplasmic CTLA-4. (D) DCs pulsed with viable CTCL cells (incubated with CD4 antibody) provided a control for tumor-loaded DCs by measuring membrane CD3/cytoplasmic CTLA-4 and (E) membrane Vβ17/cytoplasmic CTLA-4. (F) Induced Treg cells coexpressed membrane CD25/cytoplasmic CTLA-4. Results are reported as percentage of positive cells and mean fluorescence intensity (MFI). Results are representative of 10 separate experiments performed on cells isolated from cultures of 8 different CTCL patients. (G) CTCL cells were induced to express cytoplasmic FoxP3, compared with isotype-matched control (1-color histogram), by overnight incubation with DCs loaded with apoptotic tumor cells in. (H) Isotype control (donkey antigoat PE) staining in CTCL cells is compared (i) with CTCL cocultivated overnight with DCs exposed to viable CTCL cells (ii) and CTCL cells isolated from 2 patients and cultured with DCs that had ingested apoptotic tumor cells (iii and iv). Histograms demonstrate side scatter gating on the lymphocyte population compared with FoxP3 PE staining.
CTCL cells coexpressed membrane CD25/CTLA-4 (Figure 1F) after encountering DCs loaded with autologous apoptotic tumor cells, whereas coincubation with viable CTCL cells did not up-regulate CD25/CTLA-4 (not shown). FoxP3 expression was found in the cytoplasm of CTCL cells that had been exposed to tumor-loaded DCs (Figure 1G-H). Thus, CTCL cells adopt phenotypic features of Treg cells on incubation with DCs that have been pulsed with apoptotic tumor cells.
CTLA-4 expression is dependent on the dose of tumor-loaded DCs and the number of apoptotic tumor cells loaded into DCs
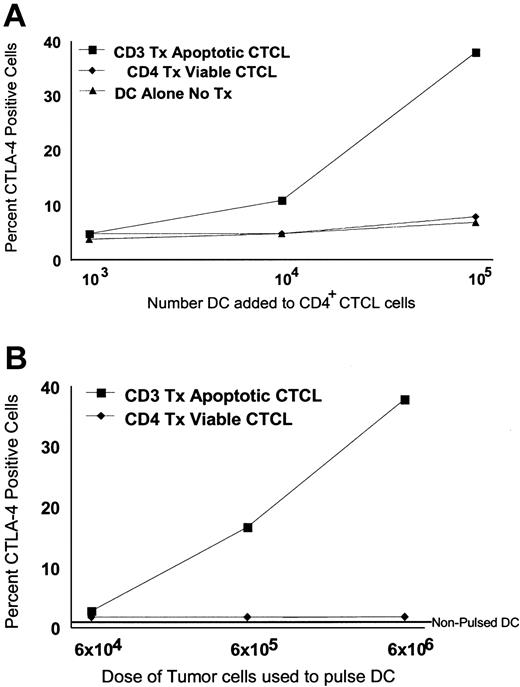
CTCL cell expression of cytoplasmic CTLA-4 increased in a dose-dependent fashion after exposure to increasing numbers of DCs pulsed with a constant number of apoptotic tumor cells (Figure 2A). Only baseline stimulation of cytoplasmic CTLA-4 was found when CTCL cells were cocultured overnight with 103 DCs that had been pulsed with apoptotic CTCL cells. When 104 tumor-loaded DCs were used to stimulate CTCL cells, the level of CTLA-4 expression increased in 11% of the cells. A further increase in cytoplasmic CTLA-4 was detected (38% CTLA-4+) when the dose of tumor-loaded DCs was increased to 105 cells. The addition of increasing numbers of DCs that had been pulsed with viable CTCL cells or DCs that had not been pulsed did not increase CTLA-4 expression.
Dose-response curve of CTLA-4 induction in CTCL cells. (A) Increasing numbers of DCs (103, 104, and 105 DCs/well) loaded with a constant number of apoptotic CTCL cells (▪) (2 × 105 cells/well) were used to stimulate a constant number of cocultured freshly purified CTCL cells (4 × 105 cells/well), isolated from the same original cultures. As controls, increasing numbers of nonloaded DCs (▴) or DCs pulsed with viable CTCL cells (treated with CD4 antibody, ♦) were added to the same number of freshly purified CTCL cells and were cocultured overnight. The percentage of cytoplasmic CTLA-4+ tumor cells was measured by flow cytometry. (B) CTCL cells and DCs were purified from cultures, and a constant number of DCs (1 × 106/well) were loaded with increasing numbers of apoptotic CTCL cells (6 × 104-6 × 106 CD3-treated apoptotic CTCL cells/well) and were used to stimulate 1.4 × 106 freshly purified responding CTCL cells. Results are presented as the percentage of cytoplasmic CTLA-4+ CTCL cells.
Dose-response curve of CTLA-4 induction in CTCL cells. (A) Increasing numbers of DCs (103, 104, and 105 DCs/well) loaded with a constant number of apoptotic CTCL cells (▪) (2 × 105 cells/well) were used to stimulate a constant number of cocultured freshly purified CTCL cells (4 × 105 cells/well), isolated from the same original cultures. As controls, increasing numbers of nonloaded DCs (▴) or DCs pulsed with viable CTCL cells (treated with CD4 antibody, ♦) were added to the same number of freshly purified CTCL cells and were cocultured overnight. The percentage of cytoplasmic CTLA-4+ tumor cells was measured by flow cytometry. (B) CTCL cells and DCs were purified from cultures, and a constant number of DCs (1 × 106/well) were loaded with increasing numbers of apoptotic CTCL cells (6 × 104-6 × 106 CD3-treated apoptotic CTCL cells/well) and were used to stimulate 1.4 × 106 freshly purified responding CTCL cells. Results are presented as the percentage of cytoplasmic CTLA-4+ CTCL cells.
As the dose of apoptotic CTCL cells used to pulse a constant number of DCs was increased (Figure 2B), the percentage of CTCL cells that expressed cytoplasmic CTLA-4 also increased. A dose of 6 × 104 apoptotic CTCL cells induced only 3% of the freshly added CTCL cells to express cytoplasmic CTLA-4. As the dose of apoptotic CTCL cells rose 10-fold, to 6 × 105, the level of cytoplasmic CTLA-4 expression also rose to 17%. The highest level of CTLA-4 expression was found when the dose of apoptotic CTCL cells added to the DCs was increased to 6 × 106, causing 38% of the added CTCL cells to express CTLA-4 in the cytoplasm. In contrast, increasing the number of viable CTCL cells cocultivated with a constant number of DCs did not enhance the expression of CTLA-4 in freshly purified, cocultivated CTCL cells. Adding nonpulsed DCs did not increase CTLA-4 expression. The dose dependency of CTLA-4 expression in CTCLs indicated that a threshold level of T-cell apoptosis and DC uptake of apoptotic tumor is required to induce CTLA-4 expression. Therefore, the generation of CTLA-4+ Treg cells may be controlled by the level of apoptotic cell death and the number of immature phagocytic DCs available for ingestion of apoptotic cells.
Proliferative response to tumor-loaded DCs
When CTCL cells were purified and cultured alone with supportive cytokines, they did not proliferate (Figure 3A). Purified CTCL cells cocultured overnight with autologous nonloaded DCs demonstrated only a small proliferative response. CTCL cell stimulation with allogeneic non-tumor-loaded DCs did not induce CTCL cells to proliferate. However, significantly increased proliferation (P ≤ .001) was obtained when the CTCL cells were cocultivated overnight with autologous tumor-loaded DCs.
Proliferative response to DCs loaded with apoptotic CTCL cells. (A) CTCL cells (2 × 106/well) cocultivated overnight alone without DCs did not proliferate substantially (0). Adding purified CTCL cells (2 × 106/well) to autologous DCs (1 × 105/well) overnight induced a small proliferative response (auto DC), whereas adding allogeneic DCs (allo DC, 1 × 105/well) did not stimulate proliferation. When autologous DCs were pulsed overnight with CD3 treated apoptotic CTCL cells (1 × 106/well) and then cocultivated with freshly purified CTCL cells (2 × 106/well), a significant (P ≤ .001) proliferative response was obtained. The proliferative response was determined by measuring the counts per minute (CPM) incorporated after an 18-hour pulse of 3[H]-thymidine. Results presented are the mean ± SD of 5 replicate cultures. (B) CTCL cells mobilize calcium stores after stimulation with tumor-loaded DCs. Purified CTCL cells (107) were loaded with indo-1 am and flowed through a cytometer equipped with a UV laser. DCs (3 × 105) loaded overnight with apoptotic CTCL cells (1 × 106) were injected into the flow stream, and induction of a calcium flux was measured over time. Controls included DCs loaded with viable CTCL cells or γ-irradiated normal control CD4 T cells.
Proliferative response to DCs loaded with apoptotic CTCL cells. (A) CTCL cells (2 × 106/well) cocultivated overnight alone without DCs did not proliferate substantially (0). Adding purified CTCL cells (2 × 106/well) to autologous DCs (1 × 105/well) overnight induced a small proliferative response (auto DC), whereas adding allogeneic DCs (allo DC, 1 × 105/well) did not stimulate proliferation. When autologous DCs were pulsed overnight with CD3 treated apoptotic CTCL cells (1 × 106/well) and then cocultivated with freshly purified CTCL cells (2 × 106/well), a significant (P ≤ .001) proliferative response was obtained. The proliferative response was determined by measuring the counts per minute (CPM) incorporated after an 18-hour pulse of 3[H]-thymidine. Results presented are the mean ± SD of 5 replicate cultures. (B) CTCL cells mobilize calcium stores after stimulation with tumor-loaded DCs. Purified CTCL cells (107) were loaded with indo-1 am and flowed through a cytometer equipped with a UV laser. DCs (3 × 105) loaded overnight with apoptotic CTCL cells (1 × 106) were injected into the flow stream, and induction of a calcium flux was measured over time. Controls included DCs loaded with viable CTCL cells or γ-irradiated normal control CD4 T cells.
CTCL cells stimulated by tumor-loaded DCs mobilize calcium
When CTCL cells encounter tumor-loaded DCs, their TCR is triggered, as shown by the detection of a calcium flux (Figure 3B). CTCL cells that remained viable after CD4 binding and normal CD4 T cells that were γ-irradiated did not stimulate calcium flux in CTCL cells. Thus, CTCL cells encountering autologous tumorloaded DCs manifested calcium flux, thereby confirming that TCR engagement occurred.
CTCL cells stimulated with tumor-loaded DCs secrete IL-10 and TGF-β
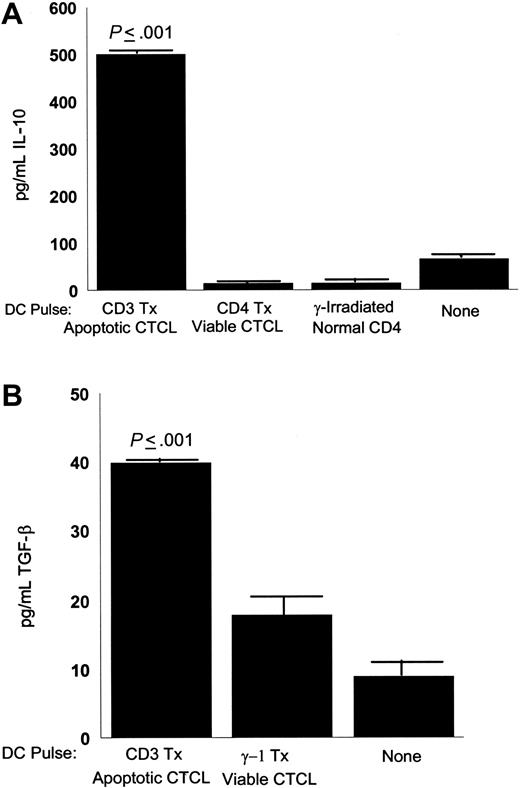
CTCL cells stimulated by apoptotic tumor-loaded DCs secreted increased amounts of immunosuppressive cytokines. In Figure 4A, CTCL cells produced significantly increased amounts of IL-10 (P ≤ .001) after stimulation with DCs loaded with autologous apoptotic tumor cells, in comparison with DCs pulsed with viable CTCL cells or CD4 T cells isolated from a healthy donor and γ-irradiated. As a baseline control, the level of IL-10 was determined in the supernatant obtained from the cultures in which the CTCL cells and the DCs had been grown.
Secretion of inhibitory cytokines by CTCL cells stimulated with apoptotic tumor cell-loaded DCs. (A) Supernatants were harvested from overnight cultures of CTCL cells (1 × 106/well) stimulated with DCs (1 × 105/well) that had been loaded with apoptotic tumor cells (1 × 106/well, CD3 treated), viable tumor cells (1 × 106/well, CD4 treated), γ-irradiated normal control CD4 T cells (1 × 106/well), or culture supernatant (none). Secreted IL-10 was detected by ELISA. Results are expressed as the mean ± SD of 3 cultures and are representative of 3 experiments. (B) Supernatants harvested from overnight cultures of CTCL cells (1 × 106/well) stimulated with DCs (1 × 105/well) that had been loaded with apoptotic tumor cells (1 × 106/well, CD3-treated), viable tumor cells (1 × 106/well, γ-1 isotype control treated), or culture supernatant (none). Secreted TGF-β was measured by ELISA. Results are expressed as the mean ± SD of 3 cultures and are representative of 3 experiments.
Secretion of inhibitory cytokines by CTCL cells stimulated with apoptotic tumor cell-loaded DCs. (A) Supernatants were harvested from overnight cultures of CTCL cells (1 × 106/well) stimulated with DCs (1 × 105/well) that had been loaded with apoptotic tumor cells (1 × 106/well, CD3 treated), viable tumor cells (1 × 106/well, CD4 treated), γ-irradiated normal control CD4 T cells (1 × 106/well), or culture supernatant (none). Secreted IL-10 was detected by ELISA. Results are expressed as the mean ± SD of 3 cultures and are representative of 3 experiments. (B) Supernatants harvested from overnight cultures of CTCL cells (1 × 106/well) stimulated with DCs (1 × 105/well) that had been loaded with apoptotic tumor cells (1 × 106/well, CD3-treated), viable tumor cells (1 × 106/well, γ-1 isotype control treated), or culture supernatant (none). Secreted TGF-β was measured by ELISA. Results are expressed as the mean ± SD of 3 cultures and are representative of 3 experiments.
CTCL cells also produced significantly increased amounts of TGF-β1 (P ≤ .001) after stimulation with DCs loaded with apoptotic CTCL cells (Figure 4B), in comparison with viable CTCL cells pulsed onto DCs. In addition, the baseline level of TGF-β1 was determined in the supernatant fluid of cultured CTCL cells and DCs.
Treg CTCL cells suppress antigen-stimulated normal T-cell cytokine responses
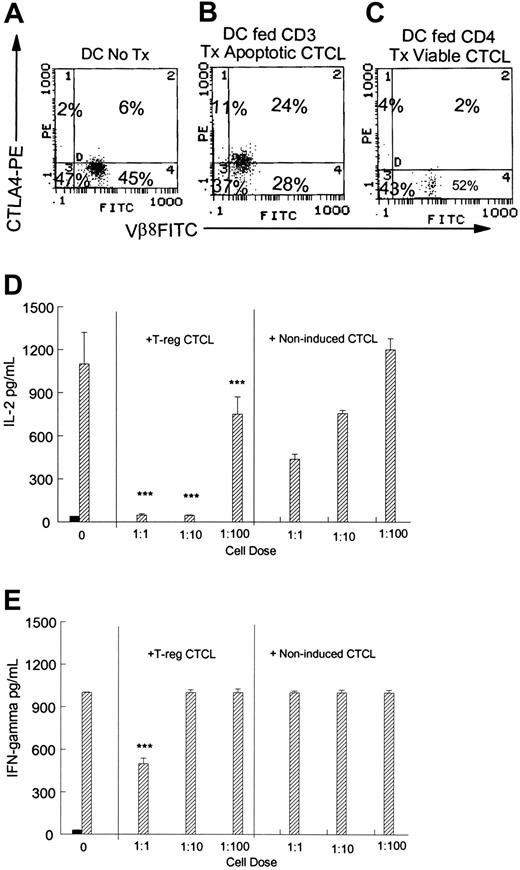
CTCL cells were stimulated to adopt a Treg phenotype by culture with tumor-loaded DCs or, as a control, DCs pulsed with viable CTCL cells (Figure 5A-C). Induced Treg CTCL cells or control CTCL cells were then added in graded doses to normal control cells stimulated by the addition of tetanus toxoid. All ratios of Treg CTCL cells (35% CTLA4+) added to responding normal T cells significantly (P ≤ .001) suppressed IL-2 production (Figure 5D) by antigen-stimulated normal T cells. For comparison, CTCL cells that were not induced to adopt a Treg profile (6% CTLA-4+) were added at the same ratios to the antigen-stimulated control cells. Adding CTCL cells that did not express a Treg phenotype reduced the concentration of IL-2 present in the culture supernatants but did not mediate the almost complete suppression of IL-2 production found when Treg CTCL cells were added at ratios of 1:1 and 1:10.
Treg CTCL cells inhibit normal control antigen-stimulated cytokine production. Normal control lymphocytes (2 × 105/well) were stimulated to proliferate with tetanus toxoid. CTCL cells were induced to assume a Treg phenotype, as previously described, and were added at graded doses to the normal control lymphocytes. Supernatants were harvested at day 5 and were tested for cytokine concentration by ELISA. Results are presented as the mean ± SD of 3 replicate wells. Coexpression of membrane Vβ8/cytoplasmic CTLA-4 was determined in responding CTCL cells by flow cytometry. (A) Nonpulsed DCs cocultivated with Vβ8+ CTCL cells overnight. (B) Responding CTCL cells cocultured with DCs pulsed with CD3-treated apoptotic CTCL. (C) DCs pulsed with CD4-treated viable CTCL cells and cultured with responding CTCL cells. (D) CTCL cells that were induced to assume a Treg phenotype by cocultivation with tumor-loaded DCs (B) or noninduced CTCL cells cultured with viable CTCL cells (C) were added to antigen-stimulated normal control cells (1:1, 1:10, 1:100 CTCL cells/normal cells), and the production of IL-2 was measured by ELISA. Significant suppression was obtained when Treg CTCL cells were added compared with the addition of the same number of noninduced CTCL cells (P ≤ .001). (E) Adding a 1:1 ratio of Treg CTCL cells to antigen-stimulated normal control cells significantly reduced the production of IFN-γ (P ≤ .001) compared with the addition of the same number of noninduced CTCL cells. For panels D and E, ▪ indicates no antigen; ▨, tetanus.
Treg CTCL cells inhibit normal control antigen-stimulated cytokine production. Normal control lymphocytes (2 × 105/well) were stimulated to proliferate with tetanus toxoid. CTCL cells were induced to assume a Treg phenotype, as previously described, and were added at graded doses to the normal control lymphocytes. Supernatants were harvested at day 5 and were tested for cytokine concentration by ELISA. Results are presented as the mean ± SD of 3 replicate wells. Coexpression of membrane Vβ8/cytoplasmic CTLA-4 was determined in responding CTCL cells by flow cytometry. (A) Nonpulsed DCs cocultivated with Vβ8+ CTCL cells overnight. (B) Responding CTCL cells cocultured with DCs pulsed with CD3-treated apoptotic CTCL. (C) DCs pulsed with CD4-treated viable CTCL cells and cultured with responding CTCL cells. (D) CTCL cells that were induced to assume a Treg phenotype by cocultivation with tumor-loaded DCs (B) or noninduced CTCL cells cultured with viable CTCL cells (C) were added to antigen-stimulated normal control cells (1:1, 1:10, 1:100 CTCL cells/normal cells), and the production of IL-2 was measured by ELISA. Significant suppression was obtained when Treg CTCL cells were added compared with the addition of the same number of noninduced CTCL cells (P ≤ .001). (E) Adding a 1:1 ratio of Treg CTCL cells to antigen-stimulated normal control cells significantly reduced the production of IFN-γ (P ≤ .001) compared with the addition of the same number of noninduced CTCL cells. For panels D and E, ▪ indicates no antigen; ▨, tetanus.
IFN-γ production (Figure 5E) was also significantly (P ≤ .001) inhibited by the addition of high numbers of Treg CTCL cells (1:1 ratio; Figure 5B) in comparison with the addition of CTCL cells not induced to adopt a Treg phenotype. Therefore, when CTCL cells are induced to adopt a Treg phenotype, they are functional suppressors of antigen-stimulated normal T-cell cytokine production.
In supplementary studies (Figure S4A&B), neutralization of IL-10 or TGF-β could not reverse the inhibition of IFN-γ secretion by antigen-activated normal lymphocytes mediated by Treg CTCL cells. In addition, separation of the Treg cells from the activated normal lymphocytes by a membrane did not inhibit the suppression of IFN-γ secretion (Supplemental Figure 4C), indicating that a soluble factor that does not require cell contact mediates the suppression of normal T-cell responses demonstrated in the presence of Treg CTCL cells.
Blocking the MHC class 2 pathway inhibits CTLA-4 expression
To confirm that MHC class 2 presentation was required for the induction of CTLA-4 expression in CTCL cells, a monoclonal antibody that binds to the DC MHC class 2 molecule or a drug (Brefeldin A) that interferes with the transport of newly synthesized MHC class 2 molecules23 was added. CTCL cells cocultivated with nonloaded DCs (Figure 6A, D) expressed baseline levels of CTLA-4. An isotype-matched control antibody (Figure 6B) did not block induction of a Treg phenotype when CTCL cells were stimulated with tumor-loaded DCs. In the presence of the anti-MHC class 2 antibody (Figure 6C), the induction of CTLA-4 was reduced by 33%, demonstrating that interference with CTCL cell TCR access to MHC class 2 molecules diminishes the up-regulation of CTLA-4. In a similar fashion (Figure 6F), the addition of Brefeldin A caused a 65% reduction in cytoplasmic CTLA-4 expression in CTCL cells compared with DCs fed apoptotic tumor cells in the absence of Brefeldin A (Figure 6E). Adding anti-MHC class 2 antibody or Brefeldin A to CTCL cells stimulated with DCs pulsed with viable CTCL cells did not affect the baseline level of CTLA-4 expression in responding CTCL cells (not shown). Therefore, access to newly synthesized MHC class 2 molecules is required for CTLA-4 expression and for induction of a Treg phenotype in CTCL.
Interference with the MHC class 2 pathway inhibits CTCL cell expression of a Treg phenotype. Coexpression of membrane CD3 and cytoplasmic CTLA-4 was measured in responding CTCL cells by flow cytometry. (A) CTCL cells cocultured overnight with nonloaded DCs. (B) CTCL cells stimulated with DCs that had been pulsed with CD3-treated apoptotic tumor cells and cultured in the presence of an isotype-matched control antibody (immunoglobulin G2a [IgG2a]). (C) An anti-DR antibody was added to cocultures of tumor-loaded DCs and responding CTCL cells. Coexpression of the clonotypic TCR was measured by monitoring the expression of membrane Vβ8 and cytoplasmic CTLA-4 in responding CTCL cells. (D) CTCL cells were incubated overnight with nonpulsed DCs. (E) CTCL cells were stimulated with DCs that had ingested apoptotic tumor cells. (F) CTCL cells were stimulated with tumor-loaded DCs in the presence of Brefeldin A.
Interference with the MHC class 2 pathway inhibits CTCL cell expression of a Treg phenotype. Coexpression of membrane CD3 and cytoplasmic CTLA-4 was measured in responding CTCL cells by flow cytometry. (A) CTCL cells cocultured overnight with nonloaded DCs. (B) CTCL cells stimulated with DCs that had been pulsed with CD3-treated apoptotic tumor cells and cultured in the presence of an isotype-matched control antibody (immunoglobulin G2a [IgG2a]). (C) An anti-DR antibody was added to cocultures of tumor-loaded DCs and responding CTCL cells. Coexpression of the clonotypic TCR was measured by monitoring the expression of membrane Vβ8 and cytoplasmic CTLA-4 in responding CTCL cells. (D) CTCL cells were incubated overnight with nonpulsed DCs. (E) CTCL cells were stimulated with DCs that had ingested apoptotic tumor cells. (F) CTCL cells were stimulated with tumor-loaded DCs in the presence of Brefeldin A.
Apoptotic material from malignant and normal T cells stimulates a Treg phenotype
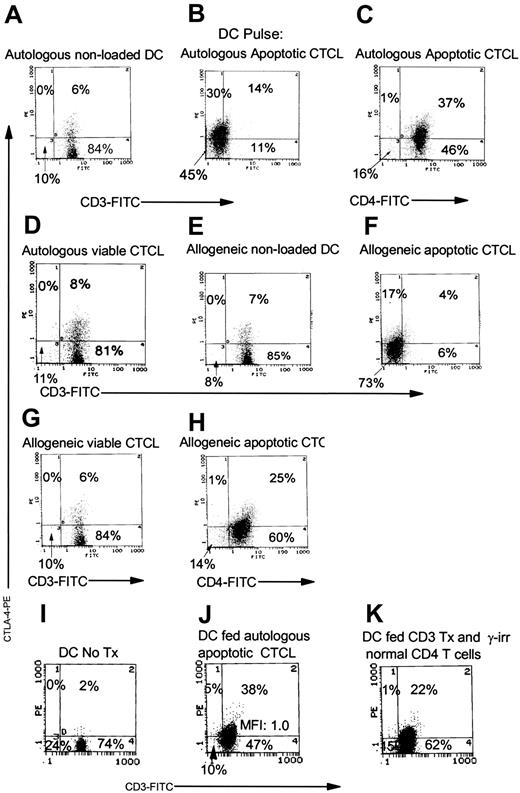
As shown in Figure 7, CTCL cells and DCs were isolated from cultures established from 2 patients with CTCL. Baseline CTLA-4 expression was found in cocultures of freshly purified CTCL cells stimulated by autologous or allogeneic nonloaded DCs (Figure 7A, E). Cocultivation of CTCL cells with DCs that had been loaded with autologous apoptotic tumor cells resulted in the up-regulation of cytoplasmic CTLA-4 (Figure 7B-C) and the loss of expression of cell membrane CD3 (Figure 7B). Pulsing viable autologous CTCL cells onto autologous DCs did not induce CTLA-4 expression in CTCL cells (Figure 7D). When allogeneic CTCL cells were rendered apoptotic and were used to pulse DCs autologous to the responding CTCL cells, CTLA-4 expression was up-regulated (Figure 7F, H) and loss of membrane TCR expression was found (Figure 7H). Viable allogeneic CTCL cells pulsed onto DCs, autologous to the responding CTCL cells, did not trigger CTLA-4 expression (Figure 7G). Therefore, when autologous or allogeneic apoptotic CTCL cells were used as a source of apoptotic material to pulse DCs autologous to the responding CTCL cells, a Treg phenotype could be induced.
The source of apoptotic material that drives the induction of a Treg phenotype in CTCL cells is common to CTCL and normal CD4 T cells. Coexpression of membrane CD3 or CD4 and cytoplasmic CTLA-4 was measured in responding CTCL cells by flow cytometry. (A) CTCL cells cultivated with nonloaded autologous DCs. (B) CTCL cells cultured with autologous CD3-treated apoptotic tumor-loaded DCs. (C) CTCL cells cultured with autologous apoptotic tumor-loaded DCs. (D) CTCL cells cultured with DCs pulsed with viable CD4-treated autologous CTCL cells. (E) CTCL cells cultured with nonloaded allogeneic DCs. (F) CTCL cells stimulated by autologous DCs loaded with allogeneic apoptotic CTCL cells. (G) CTCL cells cultured with autologous DCs pulsed with viable allogeneic CTCL cells. (H) CTCL cells stimulated by autologous DCs pulsed with allogeneic apoptotic CTCL cells. (I) CTCL cells cocultivated with nonloaded DCs. (J) CTCL cells responding to DCs fed autologous apoptotic CTCL cells. (K) DCs fed normal control CD4 T cells, treated with CD3, and γ-irradiated.
The source of apoptotic material that drives the induction of a Treg phenotype in CTCL cells is common to CTCL and normal CD4 T cells. Coexpression of membrane CD3 or CD4 and cytoplasmic CTLA-4 was measured in responding CTCL cells by flow cytometry. (A) CTCL cells cultivated with nonloaded autologous DCs. (B) CTCL cells cultured with autologous CD3-treated apoptotic tumor-loaded DCs. (C) CTCL cells cultured with autologous apoptotic tumor-loaded DCs. (D) CTCL cells cultured with DCs pulsed with viable CD4-treated autologous CTCL cells. (E) CTCL cells cultured with nonloaded allogeneic DCs. (F) CTCL cells stimulated by autologous DCs loaded with allogeneic apoptotic CTCL cells. (G) CTCL cells cultured with autologous DCs pulsed with viable allogeneic CTCL cells. (H) CTCL cells stimulated by autologous DCs pulsed with allogeneic apoptotic CTCL cells. (I) CTCL cells cocultivated with nonloaded DCs. (J) CTCL cells responding to DCs fed autologous apoptotic CTCL cells. (K) DCs fed normal control CD4 T cells, treated with CD3, and γ-irradiated.
In addition, when normal control CD4 T cells were stimulated by CD3 treatment and then rendered apoptotic with γ-irradiation, they also served as a source of apoptotic material that could induce a Treg phenotype in responding CTCL cells. CTCL cells stimulated with nonloaded DCs expressed baseline levels of CTLA-4 (Figure 7I), but, when the CTCL cells responded to DCs fed apoptotic autologous tumor, they up-regulated CTLA-4 expression (43% CTLA-4+; Figure 7J). When normal control CD4 T cells were first activated with CD3 and then rendered apoptotic by γ-irradiation and fed to the DCs isolated from patients with CTCL, they were approximately half as efficient as autologous apoptotic CTCL cells in stimulating the up-regulation of CTLA-4 expression (23% CTLA-4+; Figure 7K). Therefore, the source of apoptotic material that drives a Treg phenotype in CTCL is not restricted to tumor cells and can be derived from normal CD4 T cells.
Discussion
Our results support the hypothesis that CTCL is a malignancy of CD4 T cells driven to assume the properties of Treg cells when stimulated by DC MHC class 2 presentation of antigens generated by apoptotic cell death. CTCL cells adopt a Treg phenotype (CD25+, CTLA-4+, FoxP3+) and function after stimulation by DCs loaded with apoptotic T cells. Treg CTCL cells secrete IL-10, a cytokine that maintains DC immaturity and can thereby ensure the continued phagocytosis20,30 and presentation of antigens derived from apoptotic cells, inducing further CTCL cell proliferation and up-regulation of a Treg profile. In addition, secretion of TGF-β by Treg CTCL cells can provide a continual source of immature DCs through the differentiation of LCs from monocyte and CD34 precursors in the epidermis.31 In this manner, secretion of TGF-β by epidermal Treg CTCL cells may replace any LC emigrants that have matured and transited to lymph nodes.
Increased expression of CTLA-4 in the cytoplasm of CTCL cells is consistent with engagement of the CTCL cell TCR, putatively as a result of DC display in MHC class 2 molecules of self-antigen(s) derived from processed proteins obtained from ingested apoptotic tumor cells. In addition, loss of membrane TCR expression and mobilization of calcium stores supports our contention that TCR engagement occurs after CD4+ CTCL cells encounter tumor-loaded DCs. Direct blocking of the DC MHC class 2 molecules or interference with the MHC class 2 pathway23 diminishes CTCL cell conversion to a CTLA-4+ phenotype.
Other studies demonstrate that CTLA-4 is induced after TCR engagement in the cytoplasm of Treg cells and that CTLA-4 is rapidly translocated to the membrane site of the TCR-MHC-peptide trimolecular complex,28 where it binds with a higher affinity to the B7 costimulatory molecule than CD28 does,29 providing a mechanism for direct interference with immune responses through the blocking of costimulatory signals. CTLA-4 plays a central role in the down-regulation of a large variety of immune responses mediated by CD4 and CD8 T cells.18 The mechanism of Treg CTCL cell immunosuppression remains to be determined but may be mediated through secretion of one or more soluble inhibitory factors.
Features of Treg cells suggest a role in the immunopathology of CTCL and offer an explanation for the immunosuppression that accompanies the evolution of the disease. Patients with CTCL frequently die of opportunistic infections rather than of complications from the tumor burden, indicating that they have compromised immune systems,32 a disease feature consistent with the immunosuppressive nature of Treg cells. In advanced CTCL, the normal T-cell compartment is depleted, suggesting that CTCL cells have an inhibitory influence on normal T-cell poiesis,33 perhaps explained by their adoption of Treg functions. We confirm the immunosuppressive nature of the malignancy by demonstrating that Treg CTCL cells inhibit antigen-stimulated normal T-cell production of IL-2 and IFN-γ. CTCL cells have been shown to produce IL-1034 and TGF-β.35 Our results demonstrate that these cytokines are produced by CTCL cells, stimulated by apoptotic cell-loaded DCs, to become Treg cells.
The immunopathology of CTCL, in which during early disease the malignant tumor cells preferentially migrate to the epidermis and spare the bone marrow, can be understood in light of the growth dependency of Treg CTCL cells on an immature DC, the LC. Epidermal association of proliferating clonal CD4 tumor cells surrounding a central LC6 may represent an interaction with immature DCs driven by DC uptake and presentation of antigen(s) derived from apoptotic cells. In situ studies have demonstrated that LCs in the epidermis of patients with tumor-stage CTCL contain apoptotic cells associated with IL-10-positive cells, suggesting that in vivo uptake of apoptotic cells may parallel the in vitro induction of IL-10-producing CTCL cells.36 In addition, cells expressing CTLA-4 have been described in the epidermal infiltrate of patients with CTCL.37
These studies do not preclude the use of DC immunotherapy in the treatment of CTCL. We demonstrate that the induction of a Treg phenotype is controlled by the dose of apoptotic T cells available and the dose of tumor-loaded DCs, indicating that a threshold level of signaling is required for Treg-cell production.28 Our results show that substantial levels of apoptosis, such as induced by CD3-binding, are required for Treg-cell induction. We have demonstrated that extracorporeal photopheresis (ECP) and its replacement therapy, transimmunization, operate through the production of apoptotic tumor-loaded DCs.38 Substantial clinical improvement has been noted in ECP-treated patients, including the development of a CD8 T-cell response.39 We have noted a Treg phenotype in vivo in occasional patients with CTCL (2 of 50 patients studied), indicating that though Treg cells can occur and may limit the immune response in some patients, most treated patients do not regularly develop Treg cells in response to ECP or transimmunization. The limited induction of a Treg phenotype in treated CTCL patients may relate to the relatively slow apoptotic cell death induced by 8-methoxypsoralen/ultraviolet A (8-MOP/UVA) treatment40 ; it may be sufficient for immune recognition, but it remains beneath the threshold level of cell death required for Treg-cell stimulation.
We demonstrate that apoptotic cell death of activated T cells through binding to the CD3 molecule provides an excellent source of material for Treg-cell induction. Other mediators of apoptosis were less effective, perhaps because of the prolonged time course required to generate high levels of apoptotic cells24 or because of an intrinsic resistance of CTCL cells to apoptosis, as reported for tumor cell lines.26 CD3 binding alone, in the absence of DCs, was unable to induce a Treg phenotype in CTCL cells, indicating a requirement for a second signal. In studies of normal CD4 T cells, CD3 binding alone did not induce Treg cells, and coengagement of the complement receptor was required.41
Other investigators have implicated apoptotic cells in the induction of immunosuppression42 and tolerance.43 It has been proposed that previously cryptic antigens are revealed by apoptotic cell death and that presentation of these neoantigens plays a role in suppression of the immune response.44 Our results indicate that the mechanism that may drive immunosuppression after apoptotic cell death may be mediated through the induction of Treg cells by DC ingestion and the presentation of self-peptides to CD4 T cells.
Although Treg cells may be undesirable for tumor immunotherapy, their generation could be of value in autoimmune disease45 and transplantation tolerance.17 Through improved knowledge of T cell and DC interactions, we may gain the ability to manage a spectrum of maladies ranging from cancer to disorders of T-cell regulation by inhibiting Treg-cell generation for cancer immunotherapy and promoting Treg development to induce tolerance in autoimmune disease and transplantation.
Prepublished online as Blood First Edition Paper October 28, 2004; DOI 10.1182/blood-2004-06-2181.
Supported by the New York Cardiac Foundation and the National Institutes of Health and by Yale Skin Disease Research Core Center grant 5 P30 AR 41942.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

![Figure 3. Proliferative response to DCs loaded with apoptotic CTCL cells. (A) CTCL cells (2 × 106/well) cocultivated overnight alone without DCs did not proliferate substantially (0). Adding purified CTCL cells (2 × 106/well) to autologous DCs (1 × 105/well) overnight induced a small proliferative response (auto DC), whereas adding allogeneic DCs (allo DC, 1 × 105/well) did not stimulate proliferation. When autologous DCs were pulsed overnight with CD3 treated apoptotic CTCL cells (1 × 106/well) and then cocultivated with freshly purified CTCL cells (2 × 106/well), a significant (P ≤ .001) proliferative response was obtained. The proliferative response was determined by measuring the counts per minute (CPM) incorporated after an 18-hour pulse of 3[H]-thymidine. Results presented are the mean ± SD of 5 replicate cultures. (B) CTCL cells mobilize calcium stores after stimulation with tumor-loaded DCs. Purified CTCL cells (107) were loaded with indo-1 am and flowed through a cytometer equipped with a UV laser. DCs (3 × 105) loaded overnight with apoptotic CTCL cells (1 × 106) were injected into the flow stream, and induction of a calcium flux was measured over time. Controls included DCs loaded with viable CTCL cells or γ-irradiated normal control CD4 T cells.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/105/4/10.1182_blood-2004-06-2181/6/m_zh80040574310003.jpeg?Expires=1768591449&Signature=Pk3Qf5nj94jsvtcYuPLVNXzYg3WD6JUk~oXubJjwFX1L~mMWOuZf18tc8Gn6d8EHI5pyi5pCt0Uqxq7JPviARGdfoXu9hhE63dh5FgU54oeOZge~spoqQsTPGQFJOCEg6Ee0shaWU0li0B-apTKHiqfH6Z4rqyGoO-wchJLQYqRmnbGTvnEQNkY~JuYem1RcwMMlscesa3L12fa6e-k31THnDR~YR6gicfq3qJvXFwvjwlPe0bC-JW3evhpFvngk3GT6wvtKsfOHgeqwgusg0ggoRnuLuHwiuQFcEGjKgJtexXU~Op2e4JUKWD1U4-ouwf9-QDGPI6SNjmj4IqgIUQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 6. Interference with the MHC class 2 pathway inhibits CTCL cell expression of a Treg phenotype. Coexpression of membrane CD3 and cytoplasmic CTLA-4 was measured in responding CTCL cells by flow cytometry. (A) CTCL cells cocultured overnight with nonloaded DCs. (B) CTCL cells stimulated with DCs that had been pulsed with CD3-treated apoptotic tumor cells and cultured in the presence of an isotype-matched control antibody (immunoglobulin G2a [IgG2a]). (C) An anti-DR antibody was added to cocultures of tumor-loaded DCs and responding CTCL cells. Coexpression of the clonotypic TCR was measured by monitoring the expression of membrane Vβ8 and cytoplasmic CTLA-4 in responding CTCL cells. (D) CTCL cells were incubated overnight with nonpulsed DCs. (E) CTCL cells were stimulated with DCs that had ingested apoptotic tumor cells. (F) CTCL cells were stimulated with tumor-loaded DCs in the presence of Brefeldin A.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/105/4/10.1182_blood-2004-06-2181/6/m_zh80040574310006.jpeg?Expires=1768591449&Signature=ondzHx56xy-kk4ZprT9J6idjFyHw4TDUB9ECqt1Jnwjubt2hlmzm1PxPmcaD9~gAYesy7nffQ9U-jCfXIMwfAMLoufNMSy9EShk8hGAUzpXwdhMgXtt~~r5zaXuTeZsXkQliYoHlGwuex9O~ZbZhjBHSuOCDkm0y3Wz8zRTaI-Y7W6m2AQGOghEoNUKFdUrEIx7NaeuaZgGoxgyrcAfEpFwZ-ZhQdGVR-47q17wY5xcUl7dvSdLLa80rTTZzaRhTg1t0EdEaXnF2vh2krBxetZIq0FPpgDC9MGNvFLj2AhSkFmXn~Kj1PnKrsqFRu93AYnPRgXHaxuh5TWoeNfZq9Q__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal