Abstract
Angiopoietin-1 (Ang1) and -2 (Ang2) are endothelial growth factors that bind to the tyrosine kinase receptor Tie2 and contribute to orchestrate blood vessel formation during angiogenesis. Ang1 mediates vessel maturation and integrity by the recruitment of pericytes. In contrast, Ang2 is classically considered as a Tie2 antagonist, counteracting the stabilizing action of Ang1. Inflammation exists in a mutually dependent association with angiogenesis and we have therefore studied the capacity of angiopoietins to modulate proinflammatory activities, namely P-selectin translocation and neutrophil adhesion onto endothelial cells. We observed that both Ang1 and Ang2 increased these biologic activities. Furthermore, combination of Ang1/Ang2 induced an additive effect on neutrophil adhesion but not on P-selectin translocation. In an attempt to clarify this phenomenon, we found that angiopoietins can directly activate neutrophils through Tie2 signaling as well as modulate platelet-activating factor (PAF) synthesis and β2 integrin functional up-regulation. Together, our data demonstrate that angiopoietins could promote acute recruitment of leukocytes, which might contribute to facilitate vascular remodeling and angiogenesis.
Introduction
Angiogenesis is characterized by the formation of new microvessels from preexisting vasculature. This process is tightly regulated by the action of different families of growth factors. Along with the well-characterized vascular endothelial growth factor (VEGF) family, the angiopoietins have been shown to be critical in orchestrating blood vessel formation (Carmeliet1 for review).
Angiopoietin-1 (Ang1) and -2 (Ang2) are structurally related endothelial growth factors. They bind with similar specificity and affinity to the tyrosine kinase receptor with immunoglobulin and epidermal growth factor homology domain 2 (Tie2) expressed on endothelial cells (ECs) and hematopoietic cells.2-4 Studies with knockout mice showed that the Ang1/Tie2 system plays an essential role in embryonic vascular remodeling.2,5 The current model suggests that under physiologic conditions, Ang1, through Tie2 signaling, mediates vessel maturation and maintains vessel integrity by the recruitment of periendothelial cells. In contrast, Ang2 is classically considered as a Tie2 antagonist and it is accepted that at sites of vascular remodeling, Ang2 counteracts the stabilizing action of Ang1 by exposing the endothelium to proangiogenic factors such as VEGF. This antagonistic role of Ang2 was first suggested when its overexpression resulted in the impairment of blood vessel formation in transgenic mice, a phenotype similar to the one obtained in Ang1 and Tie2 knockout mice.4 However, studies with Ang2 knockout mice suggest that its role would not be restricted to counteracting Ang1 activities. Ang2 may also act as a Tie2 agonist, being involved in postnatal remodeling events.6 Recently, this hypothesis was supported by the abilities of Ang2 to activate Tie2, stimulate EC migration, and stimulate EC capillarylike tube formation in vitro.7,8 Hence, the role of Ang2 is still unclear since it appears to possess both agonist and antagonist functions.
Postnatal angiogenesis is associated with numerous inflammatory conditions such as atherosclerosis, arthritis, retinopathy, and tumor growth.9 Recent investigations provided evidence that inflammation exists in a mutually dependent association with angiogenesis.10,11 During inflammatory processes, newly formed vessels supply the inflamed tissues with nutrients and oxygen allowing the transport of inflammatory cells. Among these, neutrophils are the first cells recruited in the angiogenic bed and provide cytokines, growth factors, and proteolytic enzymes, which together contribute to regulate angiogenesis.12,13 The recruitment of neutrophils implies a distinct but overlapping succession of adhesive events implying neutrophil tethering, rolling, and firm adhesion to ECs. These processes require the interaction of different adhesion molecules between ECs and neutrophils. Stimulation of ECs with inflammatory mediators including thrombin, histamine, and VEGF can promote a rapid and transient P-selectin translocation at their surface. P-selectin is then able to interact with its high-affinity counterreceptor, P-selectin glycoprotein ligand-1 (PSGL-1), expressed on neutrophils and promote their rolling and transient adhesion.14-16 Inflammatory mediators may also lead to an equivalent rapid and transient synthesis of platelet-activating factor (PAF) by ECs and/or neutrophils. Newly synthesized PAF can then bind to its receptor expressed on neutrophils and induce a rapid functional up-regulation of the β2-integrin complex (CD11/CD18) and favor the binding to its endothelial counterreceptors, intracellular adhesion molecules-1 and -2 (ICAM-1 and ICAM-2). This latter interaction increases the adhesion of neutrophils onto activated ECs, which are critical in the initiation of the inflammatory process at injury sites14,17,18 Carlos and Harlan,19 for review).
Many proangiogenic factors have been proposed to play a role in the setting of inflammatory angiogenesis. Our laboratory demonstrated that VEGF-A165 inflammatory effects are mediated through the synthesis of PAF by ECs.20 We showed that the acute induction of PAF synthesis by VEGF-A165 is driven by the activation of VEGF receptor-2/neuropilin-1 complex21,22 and that PAF contributes to the induction of endothelial P-selectin translocation and subsequent neutrophil adhesion onto activated ECs.16
Since angiopoietins act in concert with VEGF to modulate vascular plasticity during postnatal neovascularization,23 we sought to assess the proinflammatory potential of Ang1 and Ang2 by investigating their capacity to modulate P-selectin translocation and neutrophil recruitment onto endothelial cells.
Materials and methods
Cell culture
Human umbilical vein ECs (HUVECs) were isolated by collagenase treatment from fresh umbilical cords, seeded on gelatin-coated (0.25%) plates, and cultured in endothelial basal medium 2 (EBM-2; Clonetics, San Diego, CA) containing 10% fetal bovine serum (Hyclone, Logan, UT), endothelial growth medium 2 (EGM-2) singlequot (Clonetics), and 2% antibiotics (penicillin and streptomycin; Sigma, St Louis, MO). HUVECs were used at passage 1 or 2.
Cell-surface ELISA
Endothelial P-selectin translocation was measured by cell surface enzyme-linked immunosorbent assay (ELISA) as described previously,16 following stimulation of HUVECs with Ang1 and Ang2 (R&D Systems, Minneapolis, MN) alone or combined.
Neutrophil purification
Venous blood was obtained from healthy donors free from medication for at least 10 days prior to the experiments. Neutrophils were isolated using Ficoll-Hypaque gradient, as described previously.16,24 Ninety-five percent of the isolated cells were neutrophils as determined by a Coulter counter and Wright-Giemsa staining, and viability was found to be greater than 98% as assessed by trypan blue dye exclusion assay.
Neutrophil adhesion assay
Neutrophil adhesion onto HUVECs or human extracellular matrix (hECM; BD Bioscience, Mississauga, ON, Canada) was measured under static conditions as described previously.16 HUVECs were pretreated or not with (1) recombinant soluble P-selectin glycoprotein ligand immunoglobulin molecule (rPSGL-Ig; kindly provided by Dr A. Kumar, Genetics Institute, Andover, MA) or (2) blocking goat polyclonal anti–human Tie2 (anti-hTie2) IgG (R&D Systems). In some experiments, neutrophils were pretreated with either (1) anti-hTie2 IgG, (2) CV-3988 (Biomol Research Laboratories, Plymouth Meeting, PA), or (3) blocking mouse monoclonal antihuman CD18 IgG (BD Biosciences). Due to slight variations of basal adhesion between experiments, we then reported our data as relative neutrophil adhesion (%).
Reverse transcriptase–polymerase chain reaction (RT-PCR) analyses
Total RNAs were obtained from freshly isolated human neutrophils (2 × 107) and cultured HUVECs (5 × 106 at passage 2) by using the RNeasy extraction kit (Qiagen, Mississauga, ON, Canada). One microgram of total RNAs was reverse transcribed using random hexamers and the Moloney murine leukemia virus (MMLV) reverse transcriptase (Invitrogen, Burlington, ON, Canada) as described by the manufacturer. The PCR reactions were performed as follows: cDNAs were denatured (94°C for 5 minutes), submitted to 30 cycles of amplification for Tie2 and 33 cycles for Tie1 (94°C for 1 minute, 62°C for 1 minute, and 72°C for 1 minute), and submitted to a final elongation (72°C for 10 minutes). Primers were used to amplify a 943–base pair (bp) fragment of Tie2 cDNA (GenBank no. BC035514; forward: 5′-GGAACTGTGGAAGGTGCC-3′, and reverse: 5′-GCGATCACACATCTCCCC-3′) or a 588-bp of Tie1 cDNA (GenBank no. BC038239; forward: 5′-CCTGCGTGTCTGGTGAGGCC-3′, and reverse: 5′-GCCATAGGGATCCGGGAGGC-3′). Upon purification on a QIAquick PCR Purification column (Qiagen), the Tie2 PCR products were subcloned into the pCRII vector using the TOPO TA cloning kit (Invitrogen) and sequenced using m13 reverse and forward primers.
Immunocytochemistry
Freshly isolated human neutrophils (1 × 108) were centrifuged in a 15-mL tube for 10 minutes at 500g. The pellet was fixed in 10% formalin for 1 hour, dehydrated in a graded series of ethanol solutions and xylene, and embedded in paraffin. Six-micrometer–thick sections were submitted to immunohistochemistry by using rabbit polyclonal anti–human Tie2 IgG (1:200 dilution; Santa Cruz Biotechnology, Santa Cruz, CA) as described previously.25
Confocal microscopy
Neutrophils were isolated as described for neutrophil purification and led to adhere on glass coverslips precoated with 1% poly-l-lysine for 1 hour, fixed with a 2% paraformaldehyde solution. Nonspecific binding of primary antibodies was prevented by preincubating fixed neutrophils with 10% serum from the species used to raise the secondary antibodies. Neutrophils were submitted to confocal microscopy using rabbit polyclonal anti–human Tie2 IgG and Cy3 dye as a fluorochrome. Glass coverslips were mounted using DABCO (1,4-diazabicyclo-2-2-2-octane)/glycerol (1:1) solution. Neutrophils were observed on a Zeiss Axiovert 100 M microscope equipped with a 63×/1.4 Plan-Apochromat oil objective lens (Zeiss, Oberkochen, Germany) adapted with an LSM 510 confocal system. Images were recorded with LSM 510 software (Zeiss) and exported in tagged-image file format (TIFF). Observation of Mayer hematoxylin-and-eosin–counterstained sections mounted in Permount was performed using an Olympus BX60 microscope equipped with a UPlanFI 100×/1.30 oil objective lens (Olympus, Melville, NY). Images were acquired with a Sony DKC-5000 3CCD digital camera (Sony, Toronto, ON, Canada) and Adobe Photoshop 5.0.2 software (Adobe, San Jose, CA). Original magnification, × 1000. Image processing was done with Adobe Photoshop 7.0 software (Adobe).
Western blot analysis of Tie2
HUVECs stimulated with angiopoietins were lysed with Laemmli buffer. Immunoprecipitation of Tie2 proteins was performed with rabbit polyclonal anti–human Tie2 IgG prior to Western blot analysis. Membranes were probed with mouse monoclonal antiphosphotyrosine IgG (Clone 4G10; 1:1000; Upstate Biotechnology, Lake Placid, NY) to detect Tie2 phosphorylation. Polyvinylidene difluoride (PVDF) membranes were subsequently stripped in 0.2 M NaOH and Tie2 protein expression was determined with rabbit polyclonal anti-hTie2 IgG antibodies (1:1000). Neutrophils (2.5 × 107/mL) were stimulated with or without angiopoietins. Cells were lysed in 2 × Laemmli buffer. Whole-cell extract (125 μg) was used for Western blot analysis. Membranes were probed with rabbit polyclonal anti–phospho-specific Tie2 IgG (1:1000; Oncogene Research, Cambridge, MA). Membranes were subsequently stripped in NaOH and Tie2 protein expression was determined with rabbit polyclonal anti-hTie2 IgG (1:1000). Immunoreactive bands were visualized by enhanced chemiluminescence, digitized using a 2-dimensional gel scanner, and quantified using Quantity One software (Bio-Rad, Hercules, CA).
Measurement of PAF synthesis
PAF synthesis was measured by determining the incorporation of [3H]-acetate into lyso-PAF as described previously.20,26 Briefly, HUVECs or neutrophils (5 × 106/mL) were stimulated with angiopoietins or VEGF-A165 (PeproTech, Rocky Hill, NJ). Reactions were stopped and synthesized [3H]-PAF was extracted, purified by high-performance liquid chromatography (HPLC), and quantified by β counting.26
Statistical analysis
Data are presented as mean ± SEM. Statistical comparisons were made by a one-way analysis of variance (ANOVA), followed by a Bonferroni test for multiple comparisons. Differences were considered significant at P values less than .05.
Results
Ang1 and Ang2 induce endothelial P-selectin translocation and neutrophil adhesion onto endothelial cells
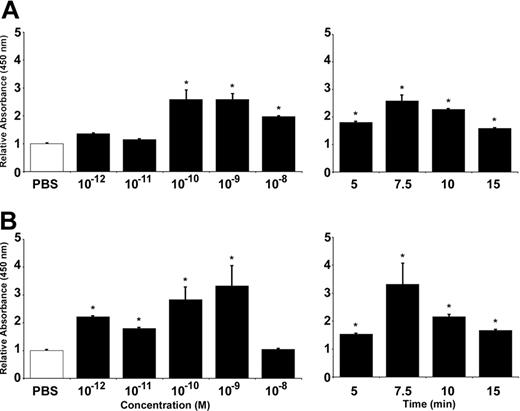
We first assessed the capacity of angiopoietins to modulate the translocation of endothelial P-selectin by ELISA on HUVECs. Treatment of HUVECs with Ang1 induced P-selectin translocation in a concentration-dependent (10–12 to 10–8 M) and time-dependent (5 to 15 minutes) manner. Maximal increase was achieved at 10–9 M (159% increase compared with phosphate-buffered saline [PBS]–treated cells) and within 7.5 minutes of stimulation (Figure 1A). The same conditions were used to characterize P-selectin translocation induced by Ang2. Again, maximal effect was obtained at 10–9 M (232% increase) and within 7.5 minutes (Figure 1B).
Ang1 and Ang2 induce endothelial P-selectin translocation in HUVECs. P-selectin translocation in HUVECs was quantified by cell surface ELISA. PBS was used as a control and the basal levels of cell surface P-selectin were normalized to 1. Translocation of P-selectin induced by Ang1 (A) and Ang2 (B), both at 7.5 minutes, was determined at different concentrations (10–12 to 10–8 M; left panels) and in function of time at 10–9 M (5 to 15 minutes; right panels). Data are means ± SEM of at least 7 experiments; *P = .01 compared with PBS.
Ang1 and Ang2 induce endothelial P-selectin translocation in HUVECs. P-selectin translocation in HUVECs was quantified by cell surface ELISA. PBS was used as a control and the basal levels of cell surface P-selectin were normalized to 1. Translocation of P-selectin induced by Ang1 (A) and Ang2 (B), both at 7.5 minutes, was determined at different concentrations (10–12 to 10–8 M; left panels) and in function of time at 10–9 M (5 to 15 minutes; right panels). Data are means ± SEM of at least 7 experiments; *P = .01 compared with PBS.
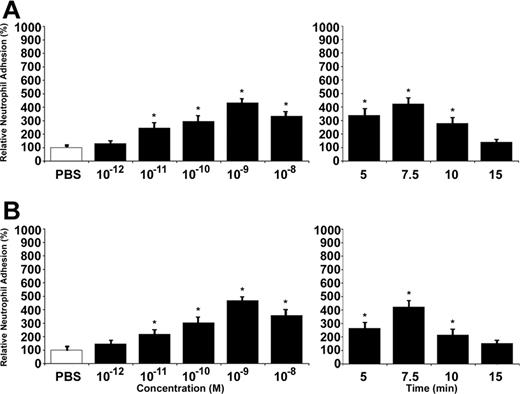
Since both angiopoietins were able to promote P-selectin translocation, we then assessed their capacity to induce neutrophil adhesion onto activated ECs. Cell adhesion assays were performed under static conditions and stimulations were also performed in a concentration- and time-dependent manner. Treatment with Ang1 increased neutrophil adhesion up to 343% at 10–9 M and within 7.5 minutes (Figure 2A). Treatment with Ang2 had similar effect, within 7.5 minutes, and at 10–9 M it increased neutrophil adhesion by 368% compared with PBS-treated cells (Figure 2B). As positive control, HUVECs were treated with VEGF-A165 (10–9 M) for 7.5 minutes, which increased endothelial P-selectin by 220% and neutrophil adhesion onto activated HUVECs by 367% (data not shown).
Ang1 and Ang2 induce neutrophil adhesion to HUVECs. Neutrophil adhesion to HUVECs was determined by cell surface adhesion assay under static conditions. PBS was used as a control. Due to slight variations in basal neutrophil adhesion between different experiments, we reported our data as relative neutrophil adhesion (%). Neutrophil recruitment induced by Ang1 (A) and Ang2 (B), both at 7.5 minutes, was determined at different concentrations (10–12 to 10–8 M; left panels) and in function of time at 10–9 M (5 to 15 minutes; right panels). Data are means ± SEM of at least 12 experiments; *P = .01 compared with PBS.
Ang1 and Ang2 induce neutrophil adhesion to HUVECs. Neutrophil adhesion to HUVECs was determined by cell surface adhesion assay under static conditions. PBS was used as a control. Due to slight variations in basal neutrophil adhesion between different experiments, we reported our data as relative neutrophil adhesion (%). Neutrophil recruitment induced by Ang1 (A) and Ang2 (B), both at 7.5 minutes, was determined at different concentrations (10–12 to 10–8 M; left panels) and in function of time at 10–9 M (5 to 15 minutes; right panels). Data are means ± SEM of at least 12 experiments; *P = .01 compared with PBS.
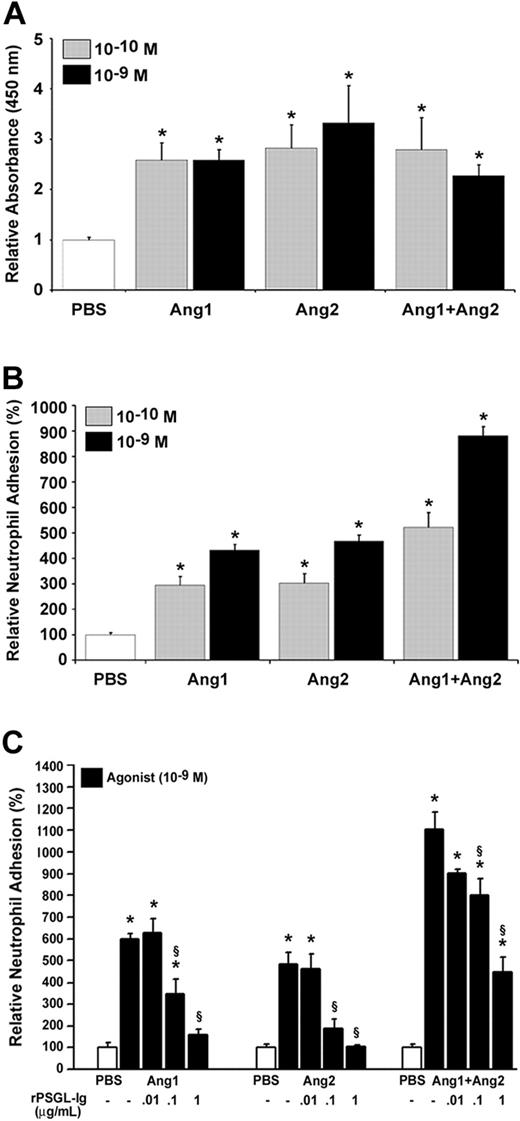
We then assessed whether the combination of Ang1 and Ang2 would affect those biologic activities. We selected the most effective concentrations (10–10 and 10–9 M) and the optimal time (7.5 minutes) to determine the effect of Ang1 plus Ang2 (Ang1/Ang2) on P-selectin translocation and neutrophil adhesion. The combination of Ang1/Ang2 either at 10–10 or 10–9 M did not further increase P-selectin translocation compared with Ang1 or Ang2 alone (Figure 3A). However, the combination of both angiopoietins elicited an additive effect on neutrophil adhesion at both 10–10 and 10–9 M (421% and 781% increase, respectively) compared with Ang1 (10–10 M; 194% and 10–9 M; 332% increase) or Ang2 alone (10–10 M; 203% and 10–9 M; 368% increase; Figure 3B).
Stimulation with Ang1 and Ang2 induces an additive effect on neutrophil adhesion but not on P-selectin translocation. Endothelial P-selectin translocation (A) and neutrophil adhesion onto HUVECs (B) were quantified upon stimulation with either Ang1, Ang2, or both Ang1 and Ang2 (10–10 M or 10–9 M; 7.5 minutes). The requirement of endothelial P-selectin translocation for neutrophil adhesion (C) was demonstrated after pretreating HUVECs with rPSGL-Ig (0.01 to 1 μg/mL; 15 minutes) prior to stimulation with Ang1, Ang2, or both Ang1 and Ang2 (10–9 M; 7.5 minutes). Data are means ± SEM of at least 7 experiments; *P = .01 compared with PBS, §P = .01 compared with corresponding agonist.
Stimulation with Ang1 and Ang2 induces an additive effect on neutrophil adhesion but not on P-selectin translocation. Endothelial P-selectin translocation (A) and neutrophil adhesion onto HUVECs (B) were quantified upon stimulation with either Ang1, Ang2, or both Ang1 and Ang2 (10–10 M or 10–9 M; 7.5 minutes). The requirement of endothelial P-selectin translocation for neutrophil adhesion (C) was demonstrated after pretreating HUVECs with rPSGL-Ig (0.01 to 1 μg/mL; 15 minutes) prior to stimulation with Ang1, Ang2, or both Ang1 and Ang2 (10–9 M; 7.5 minutes). Data are means ± SEM of at least 7 experiments; *P = .01 compared with PBS, §P = .01 compared with corresponding agonist.
We then assessed the contribution of endothelial P-selectin to neutrophil adhesion induced by angiopoietins. HUVECs were pretreated with a P-selectin antagonist, rPSGL-Ig, which prevents the interaction of the neutrophil receptor PSGL-1 with its endothelial P-selectin ligand.16,24 Pretreatment of HUVECs with increasing concentrations of rPSGL-Ig (0.01 to 1 μg/mL) 15 minutes prior to the addition of neutrophils and stimulation of HUVECs with either Ang1 or Ang2 alone provided a concentration-dependent and complete inhibition of neutrophil adhesion. Pretreatment with rPSGL-Ig up to 1 μg/mL reduced by 66% the adhesion of neutrophils mediated by the Ang1/Ang2 combination (Figure 3C).
Expression and regulation of Tie2 in HUVECs and neutrophils
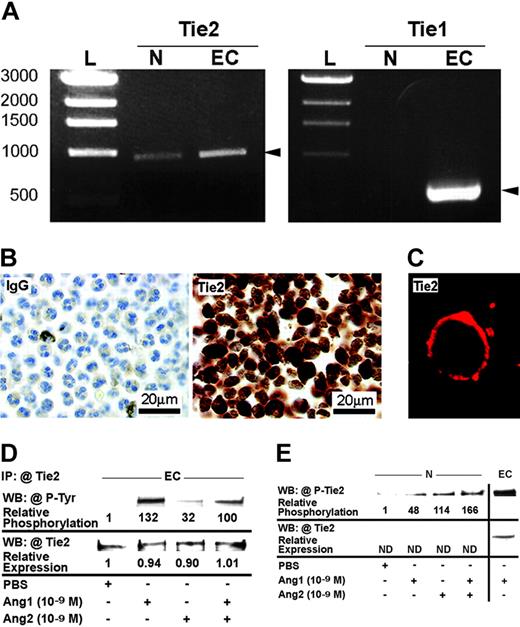
Since the combination of Ang1/Ang2 induced an additive effect on neutrophil adhesion without increasing P-selectin translocation, we postulated that neutrophils might be activated by angiopoietins. As the presence of Tie1 and/or Tie2 on neutrophils or any other leukocytes has never been described, we performed a series of experiments to determine their expression on neutrophils. By RT-PCR, we detected the expression of Tie2 but not Tie1 in neutrophil mRNAs, with HUVEC mRNA used as positive control (Figure 4A). The specificity of the PCR reaction was validated by sequencing the Tie2 product amplified in neutrophils. By immunocytochemistry and confocal microscopy, we then confirmed the presence of Tie2 receptor and its localization on neutrophil cytoplasmic membrane (Figure 4B-C). In both experiments, normal rabbit IgG was used as negative control. We also investigated by Western blot analyses the expression and the activation of Tie2 in HUVECs and neutrophils. HUVECs were treated with PBS solution, and Ang1 and Ang2 alone or combined (10–9 M; 7.5 minutes) and Tie2 proteins were immunoprecipitated with anti-hTie2 IgG. Phosphorylation of Tie2 was confirmed by using antiphosphotyrosine IgG. Treatment of HUVECs with PBS provided marginal phosphorylation of Tie2 compared with Ang1 and Ang2 alone or combined (10–9 M; 7.5 minutes), which induced a marked increase (131-, 31-, and 99-fold, respectively) of Tie2 phosphorylation. The equivalent loading was confirmed by reblotting the membranes with anti-Tie2 IgG (Figure 4D).
Expression and activation of Tie2 receptors in HUVECs and neutrophils. Tie2 mRNA expression in neutrophil (N) was revealed by RT-PCR, HUVEC (EC) mRNA was used as a positive control, and base pairs DNA ladder (L) was used as the molecular-weight standard. In contrast, Tie1 was present in HUVECs but not in neutrophils (A). Tie2 expression in neutrophils was confirmed at the protein level by immunocytochemistry, using anti-hTie2 IgG, and normal IgG was used as negative control (B). Tie2 protein was located at the cytoplasmic membrane of neutrophils as demonstrated by confocal microscopy (C). HUVECs were treated with either Ang1, Ang2, or Ang1 and Ang2 (10–9 M; 7.5 minutes). Tie2 proteins were first immunoprecipitated (IP) with anti-hTie2 IgG and Tie2 phosphorylation was analyzed by immunoblotting using antiphosphotyrosine IgG (4G10 clone). WB indicates Western blot. Membranes were subsequently stripped and the detection of Tie2 protein was performed to confirm equal protein loading (D). Neutrophils were treated with Ang1, Ang2, or Ang1 and Ang2 (10–9 M; 7.5 minutes). Cell lysates equivalent to 125 μg total proteins were loaded in each lane. Membranes were subsequently probed with antiphospho–hTie2 IgG (E).
Expression and activation of Tie2 receptors in HUVECs and neutrophils. Tie2 mRNA expression in neutrophil (N) was revealed by RT-PCR, HUVEC (EC) mRNA was used as a positive control, and base pairs DNA ladder (L) was used as the molecular-weight standard. In contrast, Tie1 was present in HUVECs but not in neutrophils (A). Tie2 expression in neutrophils was confirmed at the protein level by immunocytochemistry, using anti-hTie2 IgG, and normal IgG was used as negative control (B). Tie2 protein was located at the cytoplasmic membrane of neutrophils as demonstrated by confocal microscopy (C). HUVECs were treated with either Ang1, Ang2, or Ang1 and Ang2 (10–9 M; 7.5 minutes). Tie2 proteins were first immunoprecipitated (IP) with anti-hTie2 IgG and Tie2 phosphorylation was analyzed by immunoblotting using antiphosphotyrosine IgG (4G10 clone). WB indicates Western blot. Membranes were subsequently stripped and the detection of Tie2 protein was performed to confirm equal protein loading (D). Neutrophils were treated with Ang1, Ang2, or Ang1 and Ang2 (10–9 M; 7.5 minutes). Cell lysates equivalent to 125 μg total proteins were loaded in each lane. Membranes were subsequently probed with antiphospho–hTie2 IgG (E).
Upon treatment of neutrophils with angiopoietins alone or combined (10–9 M; 7.5 minutes), cells were lysed and Tie2 proteins were immunoprecipitated. However, Tie2 protein levels in neutrophils appear insufficient to be detected by Western blot analysis (Figure 4E). Nevertheless, by using antiphospho–hTie2 IgG on total proteins, we detected a marked increase of Tie2 phosphorylation in neutrophils mediated by Ang1, Ang2, and the Ang1/Ang2 combination (47-, 113-, and 166-fold, respectively) compared with PBS-treated neutrophils (Figure 4E).
Direct activation of Tie2 in neutrophils by angiopoietins is necessary to further increase neutrophil adhesion
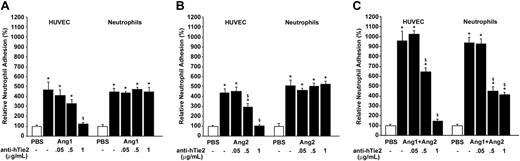
Since our data suggest that angiopoietins are able to activate Tie2 in neutrophils, we investigated how its activation might contribute to neutrophil recruitment. First, we used a blocking anti-hTie2 IgG selected for its property to prevent angiopoietins/Tie2 interaction.27 In each adhesion assay, either HUVECs or neutrophils were pretreated (15 minutes) with different concentrations of anti-hTie2 (0.05 to 1 μg/mL) prior to stimulation with angiopoietins. Pretreatment of HUVECs with 1 μg/mL of anti-hTie2 abrogated the adhesion of neutrophils induced by Ang1, Ang2, and the Ang1/Ang2 combination (10–9 M; 7.5 minutes; Figure 5A-C left histograms). Pretreatment of neutrophils with increasing concentrations of anti-hTie2 up to 1 μg/mL prior to their addition to HUVECs had no inhibitory effect on neutrophil recruitment induced by Ang1 or Ang2 alone (10–9 M; 7.5 minutes; Figure 5A-B right histograms) but reduced by 63% their adhesion induced by Ang1/Ang2 (10–9 M; 7.5 minutes), which corresponds to the additive effect produced by the angiopoietin combination (Figure 5C right histogram). Pretreatments of HUVECs or neutrophils with anti-hTie2 IgG prior to PBS treatment (1 μg/mL) or with normal IgG (1 μg/mL) prior to stimulation with angiopoietins had no effect on neutrophil adhesion (data not shown).
Neutrophil adhesion induced by angiopoietins: implication of Tie2 receptors on HUVECs and neutrophils. HUVECs (left) or neutrophils (right) were pretreated for 15 minutes with blocking anti-hTie2 IgG (0.05 to 1 μg/mL) prior to stimulation with Ang1 (A), Ang2 (B), or the Ang1/Ang2 combination (10–9 M; 7.5 minutes; C). Data are means ± SEM of at least 8 experiments; *P = .01 compared with PBS, §P = .01 compared with corresponding agonist.
Neutrophil adhesion induced by angiopoietins: implication of Tie2 receptors on HUVECs and neutrophils. HUVECs (left) or neutrophils (right) were pretreated for 15 minutes with blocking anti-hTie2 IgG (0.05 to 1 μg/mL) prior to stimulation with Ang1 (A), Ang2 (B), or the Ang1/Ang2 combination (10–9 M; 7.5 minutes; C). Data are means ± SEM of at least 8 experiments; *P = .01 compared with PBS, §P = .01 compared with corresponding agonist.
Mechanism of neutrophil activation by angiopoietins
VEGF secretion. Since the combination of Ang1 and Ang2 provides maximal adhesion of neutrophils onto ECs, we wished to establish the contribution of neutrophil activation to this additive effect. Neutrophils, upon their stimulation with various mediators, including phorbol-12-myristate (PMA), fMET-Leu-Phe (fMLP), and tumor necrosis factor-α (TNF-α), can rapidly release their intracellular pool of VEGF.28 Recently, our laboratory studied the mechanisms underlying VEGF-A165 induction of neutrophil adhesion onto HUVECs.16 In order to verify the possibility that VEGF secretion following neutrophil activation by the Ang1/Ang2 combination might contribute to the increase of neutrophil adhesion, we pretreated HUVECs with a selective inhibitor of VEGF receptors (VEGFR-1 and VEGFR-2; VEGFR tyrosine kinase inhibitor [VTK], 10–5 M; inhibitory concentration of 50% [IC50] = 2 × 10–6 M and 1 × 10–7 M for VEGFR-1 and VEGFR-2, respectively)29 and with a selective VEGFR-2 inhibitor (SU1498; 5 × 10–6 M; IC50 = 7 × 10–7 M),30 these concentrations being selected for their capacity to abrogate VEGF-A165–mediated biologic activities.16 Treatment with these inhibitors did not significantly reduce the adhesion of neutrophils induced by the combination of angiopoietins (10–9 M; 7.5 minutes; data not shown).
PAF synthesis. We recently showed that endothelial PAF synthesis was necessary for VEGF-A165–mediated P-selectin translocation and neutrophil adhesion onto HUVECs.16 Consequently, we raised the hypothesis that angiopoietins might also induce PAF synthesis. Treatment of HUVECs with angiopoietins alone or combined (10–9 M; 7.5 minutes) did not provide a significant increase of endothelial PAF synthesis (Table 1). Treatment of HUVECs with VEGF-A165 (10–9 M; 7.5 minutes) was performed as positive control, resulting in a 167% increase in endothelial PAF synthesis. Treatment of neutrophils with Ang1 or Ang2 alone (10–9 M; 7.5 minutes) enhanced PAF synthesis by 456% and 675%, respectively, compared with PBS-treated cells. The combination of Ang1/Ang2 was even more potent and increased the level of PAF synthesis in neutrophils by 1894% (Table 2). As negative control, neutrophils, which do not bear VEGF receptors, were stimulated with VEGF-A165 (10–9 M; 7.5 minutes), which failed to mediate PAF synthesis (Table 2).
PAF synthesis induced by angiopoietins in HUVECs
Treatment . | [3H]-PAF synthesis, dpm . |
|---|---|
| PBS | 311 ± 101 |
| Ang1, 10-9 M | 215 ± 62 |
| Ang2, 10-9 M | 388 ± 41 |
| Ang1+Ang2, 10-9 M | 337 ± 56 |
| VEGF, 10-9 M | 829 ± 64* |
Treatment . | [3H]-PAF synthesis, dpm . |
|---|---|
| PBS | 311 ± 101 |
| Ang1, 10-9 M | 215 ± 62 |
| Ang2, 10-9 M | 388 ± 41 |
| Ang1+Ang2, 10-9 M | 337 ± 56 |
| VEGF, 10-9 M | 829 ± 64* |
Data are means ± SEM of at least 12 experiments.
dpm indicates disintegration per minute.
P ≤ .01 compared with PBS.
PAF synthesis induced by angiopoietins in neutrophils
Treatment . | [3H]-PAF synthesis, dpm . |
|---|---|
| PBS | 16 ± 11 |
| Ang1, 10-9 M | 89 ± 19* |
| Ang2, 10-9 M | 124 ± 17* |
| Ang1+Ang2, 10-9 M | 319 ± 50* |
| VEGF, 10-9 M | 23 ± 14 |
Treatment . | [3H]-PAF synthesis, dpm . |
|---|---|
| PBS | 16 ± 11 |
| Ang1, 10-9 M | 89 ± 19* |
| Ang2, 10-9 M | 124 ± 17* |
| Ang1+Ang2, 10-9 M | 319 ± 50* |
| VEGF, 10-9 M | 23 ± 14 |
Data are means ± SEM of at least 12 experiments.
P ≤ .01 compared with PBS.
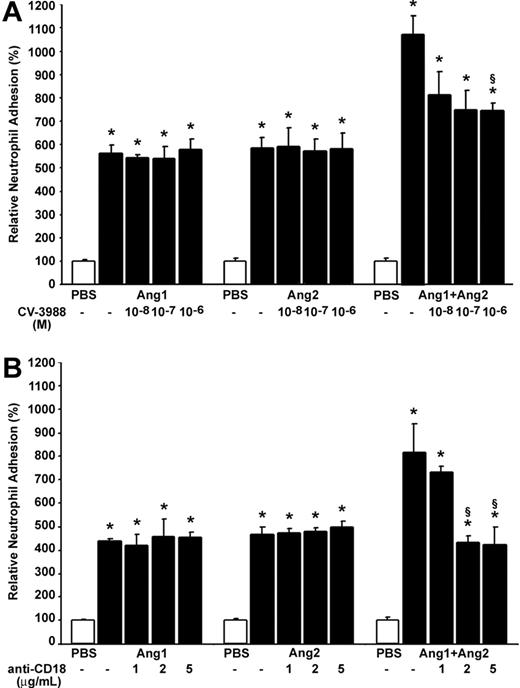
In light of these results, we postulated that newly synthesized PAF in neutrophils might contribute to the additive effect observed in neutrophil adhesion following activation by Ang1/Ang2. Consequently, we pretreated neutrophils with increasing concentrations of a selective PAF receptor antagonist CV-3988 (10–8 to 10–6 M; IC50 = 2.5 × 10–7 M)31,32 15 minutes prior to their addition to ECs. Such pretreatment did not reduce the adhesion of neutrophils induced by Ang1 or Ang2 alone (10–9 M; 7.5 minutes). However, pretreatment of neutrophils with CV-3988 at 10–7 and 10–6 M prior to stimulation with the combination of Ang1/Ang2 (10–9 M; 7.5 minutes) reduced by 33.1% and 33.4%, respectively, the adhesion of neutrophils (Figure 6A). It is worth noting that this 33% reduction represents a 67% reduction of the additive effect on neutrophil adhesion onto HUVECs induced by the combination of angiopoietins.
Contribution of PAF and β2 integrin on angiopoietin-induced neutrophil adhesion onto HUVECs. Neutrophils were pretreated with increasing concentrations of CV-3988 (10–8 to 10–6 M; A) and blocking anti-CD18 IgG (1 to 5 μg/mL; B) for 15 minutes prior to the adhesion assay. Neutrophils were added to HUVECs concomitantly to their stimulation with Ang1, Ang2 alone, or the Ang1/Ang2 combination (10–9 M; 7.5 minutes). Data are means ± SEM of at least 8 experiments; *P = .01 compared with PBS, §P = .01 compared with corresponding agonist.
Contribution of PAF and β2 integrin on angiopoietin-induced neutrophil adhesion onto HUVECs. Neutrophils were pretreated with increasing concentrations of CV-3988 (10–8 to 10–6 M; A) and blocking anti-CD18 IgG (1 to 5 μg/mL; B) for 15 minutes prior to the adhesion assay. Neutrophils were added to HUVECs concomitantly to their stimulation with Ang1, Ang2 alone, or the Ang1/Ang2 combination (10–9 M; 7.5 minutes). Data are means ± SEM of at least 8 experiments; *P = .01 compared with PBS, §P = .01 compared with corresponding agonist.
β2 Integrin implication. PAF induces a rapid functional up-regulation of the CD11/CD18 β2-integrin complex in neutrophils and favors the binding of this complex to their endothelial counterreceptors, ICAM-1 and ICAM-2, promoting adhesion of neutrophils onto activated ECs.18 Since the combination of angiopoietins induced a marked increase of PAF synthesis in neutrophils, and since pretreatment with a selective PAF receptor antagonist provided a significant reduction of neutrophil adhesion onto HUVECs mediated by Ang1/Ang2 combination, we thus hypothesized that the additive effect might involve the functional up-regulation of the CD11/CD18 β2-integrin complex. To assess this hypothesis, neutrophils were pretreated with increasing concentrations of blocking antihuman CD18 IgG (anti-CD18; 1 to 5 μg/mL)33 15 minutes prior to their addition to HUVECs. Pretreatment of neutrophils with blocking anti-CD18 IgG had no effect on neutrophil adhesion induced by Ang1 or Ang2 alone (10–9 M; 7.5 minutes). However, such pretreatment prevented the additive effect produced by the combination of Ang1/Ang2 (10–9 M; 7.5 minutes) on neutrophil adhesion (Figure 6B). In each case, pretreatment of neutrophils with CV-3988 (10–6 M) or blocking anti-CD18 IgG (5 μg/mL) did not significantly modulate the basal level of neutrophil adhesion onto ECs. In addition, pretreatment of neutrophils with normal IgG (5 μg/mL) did not decrease the adhesion induced by Ang1, Ang2, or the Ang1/Ang2 combination (data not shown).
The Ang1/Ang2 combination induces a proadhesive state of neutrophils independently from HUVEC stimulation
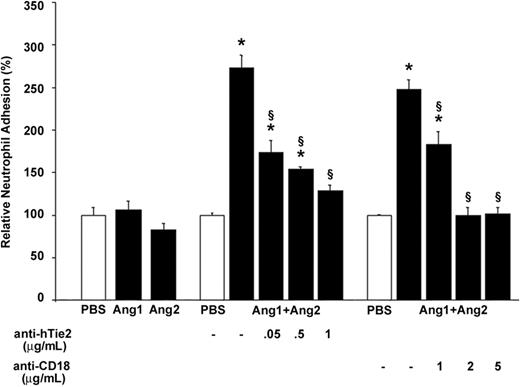
To ascertain that the stimulation of neutrophils by the Ang1/Ang2 combination was sufficient to induce an adhesive state, we performed an adhesion assay of neutrophils on hECM.34 Stimulation of neutrophils with Ang1 or Ang2 alone (10–9 M; 7.5 minutes) did not increase their adhesion onto hECM compared with PBS-treated cells (Figure 7 left histograms). In contrast, treatment of neutrophils with the Ang1/Ang2 combination (10–9 M) for 7.5 minutes increased by 173% their adhesion onto hECM (Figure 7 middle histograms). Such adhesion was dependent on Tie2 activation, since pretreatment of neutrophils with increasing concentrations of blocking anti-hTie2 IgG (up to 1 μg/mL) reduced their adherence by 83% (Figure 7 middle histograms). The implication of β2 integrin in this adhesion assay was also verified by pretreating neutrophils with increasing concentrations of blocking anti-CD18 IgG (1 to 5 μg/mL). Pretreatment with 2 μg/mL of blocking anti-CD18 IgG was sufficient to abrogate the adhesion of neutrophils onto hECM induced by the Ang1/Ang2 combination (10–9 M; 7.5 minutes; Figure 7 right histograms).
Combination of Ang1 and Ang2 promotes neutrophil adhesion on hECM: implication of Tie2 and β2 integrin. Neutrophils were pretreated with increasing concentrations of blocking anti-hTie2 IgG (0.05 to 1 μg/mL) or blocking anti-CD18 IgG (1 to 5 μg/mL) antibodies 15 minutes prior to the adhesion assay on hECM. Neutrophils were stimulated with either Ang1, Ang2, or the Ang1/Ang2 combination (10–9 M; 7.5 minutes). Data are means ± SEM of at least 8 experiments; *P = .01 compared with PBS, §P = .01 compared with corresponding agonist.
Combination of Ang1 and Ang2 promotes neutrophil adhesion on hECM: implication of Tie2 and β2 integrin. Neutrophils were pretreated with increasing concentrations of blocking anti-hTie2 IgG (0.05 to 1 μg/mL) or blocking anti-CD18 IgG (1 to 5 μg/mL) antibodies 15 minutes prior to the adhesion assay on hECM. Neutrophils were stimulated with either Ang1, Ang2, or the Ang1/Ang2 combination (10–9 M; 7.5 minutes). Data are means ± SEM of at least 8 experiments; *P = .01 compared with PBS, §P = .01 compared with corresponding agonist.
Viability of HUVECs and neutrophils
To ensure that neutrophil adhesion onto ECs or hECM induced by angiopoietins or that the reduction of neutrophil recruitment by inhibitors or antagonists used in these studies were not due to cytotoxicity, the effect of angiopoietins and all inhibitors or antagonists on cell viability was assessed by trypan blue exclusion assay at the highest concentrations used. In all cases, the reagents and conditions used did not increase cell mortality (data not shown).
Discussion
In the present study, we demonstrate for the first time the proinflammatory activities of both angiopoietins to mediate endothelial P-selectin translocation and neutrophil adhesion onto activated ECs. The combination of Ang1 and Ang2 provides an additive effect on neutrophil adhesion but not on P-selectin translocation. This phenomenon is related to the unexpected expression of Tie2 receptor on the cell surface of neutrophils. Angiopoietins appear to directly activate neutrophils and modulate PAF synthesis. The combination of Ang1 and Ang2, cooperatively with PAF signaling, may promote a rapid functional up-regulation of the β2-integrin complex (CD11/CD18), contributing to increase the adhesion of neutrophils onto activated ECs.
Angiopoietin-mediated neutrophil adhesion to HUVECs is P-selectin–dependent
Previous studies reported that Ang1 has anti-inflammatory properties, thus contributing to the maturation of neovessels (Thurston35 for review). In vivo, it prevents VEGF-mediated vascular permeability36,37 and in vitro it reduces the basal activation of vascular endothelial cadherin (VE-cadherin) and β-catenin, concomitantly with a reduction of VEGF-mediated EC permeability.38,39 Sustained treatment of ECs with Ang1 reduces endothelial expression of ICAM-1, vascular cell adhesion molecule-1 (VCAM-1), and E-selectin,40 leading to a marked reduction of VEGF-mediated neutrophil adhesion.39,40 On the other hand, Ang2 might display proinflammatory activities, since it negatively controls vascular maturation, stability, and quiescence by antagonizing the effects of Ang1. It remains that there are no studies defining the capacity of Ang2 to regulate selective proinflammatory activities.
In this study, we observed that both angiopoietins possess a similar agonistic capacity to mediate an early and transient endothelial P-selectin translocation as well as neutrophil adhesion onto activated HUVECs. The agonistic function of Ang2 herein described strengthens the current view, which tends to reconsider the purely antagonist role of this growth factor and suggests discrepant actions on its receptor Tie2, depending on the cell types or pathophysiologic conditions.
The kinetic of neutrophil adhesion was in line with the level of endothelial P-selectin translocation: in both cases, the peaks were achieved within 7.5 minutes and nearly returned to the baseline levels after 15 minutes. Translocated P-selectin on EC surface is indeed rapidly reinternalized and/or cleaved and can therefore no longer interact with its neutrophil counterreceptor to maintain their acute adhesion onto ECs.14,16,41,42 This might explain why other authors could not observe the capacity of angiopoietins to induce neutrophil adhesion, as their studies were performed for extended periods of time.39,40 Using a selective P-selectin antagonist, we showed that endothelial P-selectin translocation is essential for the adhesion of neutrophils. Although it did not further increase P-selectin translocation compared with Ang1 or Ang2 alone, the combination of both angiopoietins provided an additive effect on neutrophil adhesion. This led us to hypothesize that the Ang1/Ang2 combination may induce the synthesis and/or the activation of other molecule(s) either on HUVECs and/or on neutrophils, thus contributing to increase their adhesion on activated ECs.
Expression and activation of Tie2 receptor in neutrophils
Our study is the first to demonstrate the expression of Tie2 receptor in neutrophils. Moreover, we demonstrated the capacity of angiopoietins to activate Tie2 phosphorylation in neutrophils. Although Tie2 is generally considered as an endothelial-specific receptor, it is also expressed in EC precursors and hematopoietic stem cells.43 Its presence on neutrophils implicitly suggests that the expression of Tie2 is maintained during the commitment of hematopoietic progenitor cells to the neutrophil lineage. This surprising observation opens up intriguing new perspectives in the understanding of the physiopathologic role of the angiopoietin receptor Tie2.
Activation of Tie2 receptor in neutrophils increases their adhesion onto HUVECs
The pretreatment of neutrophils with a blocking anti-hTie2 IgG demonstrated that activation of this receptor in neutrophils was necessary for the additive effect mediated by the Ang1/Ang2 combination. Moreover, we performed an adhesion assay on hECM that confirmed that stimulation with the Ang1/Ang2 combination was able to promote a direct, Tie2-dependent, adhesive state in neutrophils, independently from the potential contribution of activated ECs. These effects were not observed with stimulation with Ang1 or Ang2 alone. In a recent study, Autiero et al44 have shown that the stimulation of VEGFR-1 with different VEGF analogs resulted in a different profile of effector activation. Stimulation with VEGF-A165 induced a strong phosphorylation of VEGFR-1 tyrosine (Tyr) residue Tyr1213 and to a lesser extent Tyr1242 and Tyr1333, whereas the stimulation with placental growth factor (PlGF) induced the phosphorylation of Tyr1309 but not Tyr1213.44 Such differences in the activation of VEGFR-1 by various agonists, termed “agonist trafficking,” may explain the distinct biologic activities of VEGF-A165 and its analogs. Consequently, it is conceivable that in neutrophils the combination of Ang1 and Ang2 also provides a different effector activation profile upon their interaction with Tie2, which could be responsible for their additive effect on neutrophil adhesion. Hence, we have searched for a particular signaling pathway in neutrophils, which could explain the additive effect induced by the combination of angiopoietins. Our results showed that the VEGF system is not involved in this process. However, stimulation of neutrophils with Ang1/Ang2 induced a synergistic effect on PAF synthesis compared with a treatment with Ang1 or Ang2 alone. The subsequent PAF signaling was critical in the additive effect observed with the Ang1/Ang2 combination on neutrophil adhesion. The binding of PAF to its receptor on neutrophils induces a functional up-regulation of the β2-integrin complex (CD11/CD18), which contributes to increase neutrophil adhesion.14,17 Using a blocking antihuman CD18 IgG prior to stimulation, we confirmed that the additive effect induced by the Ang1/Ang2 combination was dependent on the β2-integrin complex. It is worth noting that interleukin-8 (IL-8) can also contribute to β2-integrin activation.45 In neutrophils, IL-8 transcripts are constitutively expressed but the release of this inflammatory mediator requires de novo synthesis of the protein. Consequently, the acute response in neutrophil adhesion described in this study (within 7.5 minutes) cannot be dependent on IL-8, produced by neutrophils in response to the Ang1/Ang2 combination.46 However, under prolonged treatment, it would be interesting to assess if angiopoietins like other inflammatory mediators can induce protein expression of IL-8 both in HUVECs and neutrophils.47
In conclusion, this study demonstrates that Ang1 and/or Ang2 induce a rapid and transient endothelial P-selectin translocation, which is essential for the adhesion of neutrophils onto activated ECs. In addition, we showed, also for the first time, that angiopoietins combined can activate Tie2 receptor expressed on neutrophils, thus leading to PAF synthesis, functional up-regulation of β2-integrin complex, and subsequent increase in neutrophil adhesion. Our data bring us to suggest that angiopoietins should be considered as acute proinflammatory mediators, which may contribute to initial steps of pathologic angiogenesis.
Prepublished online as Blood First Edition Paper, October 21, 2004; DOI 10.1182/blood-2004-09-3531.
Supported by grants from the Canadian Institutes of Health Research (CIHR; MOP-43919) and from the Heart and Stroke Foundation of Québec (M.G.S.). M.G.S. is recipient of a scholarship from the CIHR.
R.M. and J.F. contributed equally to this study.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We are grateful to Dr Danielle Libersan, Louis R. Villeneuve, and Dominique Lauzier for their technical assistance.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal