Abstract
The mechanisms by which intraperitoneal injection of peripheral blood mono-nuclear cells (PBMCs) from Epstein-Barr virus (EBV)–seropositive donors into severe combined immunodeficient (SCID) mice gives rise to lymphomas (hu/SCID tumors) are far from clear. This study addressed whether chemokine receptors and their ligands could be implicated in this experimental model. CXCR4 was found to be highly expressed in hu/SCID tumors; surface expression of CXCR4 was prevalently limited to a tumor cell subset poorly expressing CD23, whereas the CXCR4 ligand, CXCL12, was predominantly expressed by the tumor subpopulation expressing CD23. In vitro inhibition of this autocrine/paracrine CXCL12/CXCR4 axis significantly inhibited lymphoma proliferation and survival. Furthermore, CXCL12 was expressed in cells recovered from the mouse peritoneal cavity early after PBMC transfer as well as by EBV-transformed B cells but not by resting or activated B lymphocytes; also, lymphoma development was associated with a dramatic increase in the levels of murine CXCL12 present in the peritoneal cavity. Finally, antagonizing the CXCL12/CXCR4 axis in vivo strongly counteracted lymphoma development. These studies demonstrate that CXCL12 expression may be associated with EBV infection and suggest that the CXCR4/CXCL12 axis may participate in the EBV-associated lymphomagenesis process in immunodeficient hosts.
Introduction
Epstein-Barr virus (EBV) is implicated in the pathogenesis of at least 3 malignant disorders of B cells: Burkitt lymphoma, Hodgkin disease, and B-cell lymphoproliferative disease (BLPD) seen in immunosuppressed allograft recipients.1 The severe combined immunodeficient (SCID) mouse, which lacks functional B and T cells,2 is a suitable animal model for the study of EBV-associated lymphoproliferation.3,4 SCID mice injected intraperitoneally with peripheral blood mononuclear cells (PBMCs) from EBV-positive donors develop EBV-positive human B-cell lymphomas (hu/SCID tumors),4 which display a surface phenotype closely resembling that seen in BLPD with low expression of EBV latent proteins and presenting a mixture of lymphoblastoid and plasmacytoid cells.5 The events occurring in the peritoneal cavity of PBMC-injected mice are very complex6 and entail T-cell activation and massive cytokine production, both of which favor the expansion of B lymphocytes, including EBV-positive B-cell precursors. In this regard, we showed that the presence of T lymphocytes within the cellular inoculum is necessary for lymphoma development,7 and treatment of PBMC-injected mice with cyclosporin A paradoxically counteracts tumor generation.7,8 Recently, we also demonstrated that no EBV reactivation occurs during the lymphomagenesis process, and tumor masses originate from the expansion of the very few latently infected B lymphocytes present in the inoculum.9
Hu/SCID tumors consist of multiple masses at the hepatic hilus, lower splanchnic region, and within the mesenteric tissue,7 the privileged sites of primary and/or metastatic localization being peritoneum, liver, and lymph nodes. Interestingly, all these organs have been reported to produce high levels of the CXCR4 ligand (CXCL12, previously called stromal cell–derived factor-1 [SDF-1])10,11 ; indeed, no tumor development occurs when PBMCs from EBV-positive donors are injected by other routes,4 suggesting a critical role of the peritoneal environment for lymphomagenesis. In this regard, it has recently been shown that CXCL12, produced by mesothelial cells lining the peritoneum, is involved in the persistence of peritoneal B lymphocytes in mice11 ; indeed, CXCR4 is expressed on mature B cells.12
In view of these observations, we addressed whether chemokine receptors and their ligands could be implicated in EBV-mediated lymphomagenesis in this experimental model. Aims of our study were (1) to define the chemokine receptor expression profile of lymphoblastoid cell lines (LCLs) and hu/SCID tumors; (2) to assess whether chemokines were differentially expressed following cellular transformation by EBV; and (3) to evaluate whether any of the latter could contribute to the outgrowth of EBV-transformed B cells in the immunocompromised host.
We report here that lymphoma development in PBMC-injected SCID mice is associated with down-regulated expression by EBV-transformed B cells of the chemokine receptors CCR6, CCR7, and CXCR5, while CXCR3 and CXCR4 are expressed at moderately high levels. We show that most hu/SCID tumors express functional CXCR4 receptors and that CXCL12 affects several biologic responses, including migration, adhesion, proliferation, and invasion. In adjunct, we demonstrate that CXCL12 expression may be related to immortalization of B cells by EBV. We also demonstrate that the hu/SCID tumor subset poorly expressing CD23 expresses functional CXCR4; meanwhile, the tumor subset expressing CD23 is the main producer of CXCL12, suggesting a potential chemotactic loop between these 2 cell populations. Finally, antagonizing the CXCR4/CXCL12 axis through the use of a neutralizing anti-CXCL12 antibody (Ab) or a CXCR4 antagonist was able to counteract lymphoma growth. Because CXCR4 is expressed in the human counterpart of hu/SCID tumors,13,14 these observations may be relevant to the human setting and open new avenues to therapeutic approaches in B-cell non-Hodgkin lymphomas.
Materials and methods
Isolation of PBMCs and B-cell purification
PBMCs were isolated by Ficoll-Hypaque (Pharmacia Biotech, Uppsala, Sweden) gradient centrifugation as reported elsewhere,15 washed 4 times with RPMI medium, counted, and used as such for establishment of LCLs and inoculation of SCID mice or separated further. LCLs were generated and maintained as previously reported.9 Purified B cells were obtained from PBMCs by positive selection using magnetic microbeads (Miltenyi Biotec, Bergisch Gladbach, Germany) according to manufacturer's recommendations.9 Recovered B cells were of 95% to 98% purity, as determined by flow cytometric analysis. B-cell cultures were set up as previously described.16
Generation of human B-cell tumors in SCID mice
SCID mice were purchased from Charles River (Wilmington, MA) and maintained in our animal facilities under pathogen-free conditions. Procedures involving animals and their care conformed with institutional guidelines that comply with national and international laws and policies (European Community EEC Council Directive 86/609, OJ L 358, December 12, 1987). Groups of 7- to 9-week-old SCID mice were injected intraperitoneally with 70 × 106 to 100 × 106 unfractionated PBMCs, observed daily for signs of illness, and killed by cervical dislocation when they became sick. Tumors and other tissues of interest were processed as previously described.9
In another set of experiments, hu/SCID tumor cell suspensions were briefly expanded in vitro before being injected intraperitoneally (2 × 106) into SCID mice. Groups of 5 to 8 hu/SCID lymphoma-injected SCID mice were treated with intraperitoneal injections of a polyclonal goat anti-CXCL12 Ab (kindly provided by Prof Robert M. Strieter, UCLA, Los Angeles, CA; 50 μL per mouse per injection); 500 μL of this Ab is sufficient to neutralize 1 μg of either human or murine CXCL12 in leukocyte chemotaxis assays.17 As controls, we used heat-inactivated goat preimmune serum and phosphate-buffered saline (PBS). The animals were treated every day for 3 weeks starting from the day after tumor injection, and the effects of anti-CXCL12 Ab treatment on lymphoma development were assessed by monitoring survival and histologic examination for evidence of tumor. In other groups of animals (6 to 8 mice) the day before transplantation of lymphoma cells, Alzet pumps (duration 14 days; model 1002, ALZA, Mountain View, CA) containing 50 to 80 mg/mL of the CXCR4-specific antagonist 4F-benzoyl-TN1400318 or vehicle were implanted subcutaneously. On day 13, the Alzet pumps were replaced with pumps containing the same amounts of peptide or vehicle.
FACS analysis
The following mouse monoclonal antibodies (mAbs) were used: phycoerythrin (PE)–labeled mAb against CCR1 (IgG2a), CCR2 (IgG2a), CCR4 (IgG1), CCR6 (IgG2b), CXCR4 (IgG2b), CXCR5 (IgG2a) (all from R&D Systems, Minneapolis, MN), and CD23 (IgG3; Immunotech, Marseille, France); and fluorescein isothiocyanate (FITC)–labeled mAb to CCR3 (IgG2a), CCR5 (IgG2a), CCR7 (IgG1), CXCR1 (IgG2a), CXCR2 (IgG2a), CXCR3 (IgG1) (all from R&D Systems), and CD23 (IgG1; Becton Dickinson, Mountain View, CA). Irrelevant conjugated mouse Abs of each isotype (Becton Dickinson) were used to establish specificity of staining. The following Abs also were used: rabbit anti-CXCL12 Ab (PeproTech, London, United Kingdom), irrelevant rabbit immunoglobulin G (IgG) (PeproTech), and Alexa 488–labeled goat antirabbit Ab (Molecular Probes, Leiden, The Netherlands). Results were expressed as percent of the cells expressing the relevant marker.
Intracellular expression of CXCL12 in LCLs and hu/SCID tumor cells was detected by flow cytometry as previously described.19
Isolation of RNA and RT-PCR
Total RNA was isolated using the RNeasy mini kit (QIAGEN, Hilden, Germany), primed (0.5 to 1 μg) with random hexamers (Promega, Madison, WI), and reverse transcribed. Complementary DNA fragments from the reverse transcriptase (RT) reaction mixture were subjected to polymerase chain reaction (PCR) amplification using AmpliTaq Gold polymerase (Applied Biosystems, Foster City, CA) for 35 to 45 cycles (95°C for 45 seconds, 60°C for 45 seconds, 72°C for 1 minute). The following primers were used: human CXCL12: forward, 5′-ATGAACGCCAAGGTCGTGGTCG-3′, reverse (SDF-1α), 5′-AAGTCCTTTTTGGCTGTTGTGC-3′, or reverse (SDF-1β) 5′-TGACCCTCTCACATCTTGAACC-3′ 20 ; human CXCR4: forward, 5′-GGGGATCAGTATATACACTTCAG-3′, reverse, 5′-AGACGCCAACATAGACCAC-3′; mouse CXCL12: forward, 5′-CACCTCGGTGTCCTCTTG-3′, reverse, 5′-GGTCAATGCACACTTGTCTG-3′. For the control of RNA and cDNA preparations, we used RT-PCR for β-actin as previously reported.21 Amplified products were analyzed on a 1.8% agarose electrophoresis gel and visualized under UV rays after ethidium bromide staining.
In vitro adhesion assays
Adhesion of hu/SCID tumor cells to human umbilical vein endothelial cells (HUVECs) and fibronectin was assayed as previously described,22,23 with minor modifications. Briefly, hu/SCID tumor cells (1 × 105) were labeled before the assay with the fluorescent dye calcein acetoxymethyl ester (calcein-am; Molecular Probes) and were subsequently added for 30 to 60 minutes to 96-well culture plates covered by HUVECs, bovine serum albumin (BSA; 40 μg/mL; Sigma, St Louis, MO), or fibronectin (10 μg/mL). After the incubation period, the nonadherent cells were removed by vigorously washing the wells with RPMI 1640 medium (Life Technologies, Grand Island, NY) 3 times. Adherent cells were collected and counted by flow cytometry. A gate was set up using the calcein labeling of the lymphoma cells to exclude HUVECs from the counts. Results were expressed as percentage of adherent cells (ie, number of adherent cells per number of input cells).
For inhibition studies, hu/SCID tumor cells were preincubated with a neutralizing anti-CXCL12 Ab (1:100) or 1 μM TC14012, a CXCR4-specific inhibitor,24 for 30 minutes at 37°C before being plated in HUVEC- or fibronectin-coated wells.
Chemotaxis and chemoinvasion assay
Migration and invasion were assayed in modified Boyden chambers (Neuro Probe, Gaithersburg, MD) using 13-mm polycarbonate filters of 8-μm pore size as previously described.25,26 Membranes were uncoated for chemotaxis or coated with 25 μg Matrigel (Becton Dickinson) for invasion assays. The lower compartments were filled with RPMI supplemented with 0.1% BSA with or without CXCL12 (100 to 200 ng/mL). Hu/SCID tumor cells were preincubated for 30 minutes at 37°C in the presence of 1 μM TC14012, anti-CXCL12 Ab, or in control conditions (RPMI–0.1% BSA) and subsequently loaded (3 × 105) onto the upper compartments of the modified Boyden chambers. After incubation of 2 hours (chemotaxis) or 24 hours (chemoinvasion), the contents of the lower compartments were collected and counted after fixation and staining with crystal violet.
Actin polymerization
To evaluate the effect of CXCL12 on cytoskeleton rearrangement of different tumor subsets, freshly isolated hu/SCID lymphoma cells were labeled with PE-conjugated anti-CD23 mAb at 4°C for 30 minutes. Cells were washed and incubated at 37°C in RPMI medium before assessing cytoskeleton rearrangement. Actin polymerization experiments were performed as previously described.11
Proliferation and survival assays
For proliferation and survival studies evaluating the role of endogenous CXCL12, freshly isolated hu/SCID tumor cell suspensions treated with or without anti-CXCL12 Abs (1:20) or TC14012 (50 to 100 μM) were pulsed for 48 hours with 1 μCi [37 Bq] [methyl-3H]thymidine (Amersham, Arlington Heights, IL) or labeled with FITC-conjugated annexin V (PharMingen, BD Biosciences, San Diego, CA) plus propidium iodide (PI; Molecular Probes).19 Subsequently, thymidine incorporation was evaluated on a scintillation β-plate counter, or the percentage of cells undergoing apoptosis was determined by flow cytometry. Annexin V–positive PI-negative and annexin V–positive PI-positive cells correspond to apoptotic and necrotic cells, respectively.
Confocal microscopy
Confocal microscopic analysis was executed as previously described.27 Cells were stained with the following primary Abs: mouse anti-CXCR4 mAb (R&D Systems) and rabbit anti–human CXCL12 Ab (PeproTech), while the secondary Abs used were Alexa 594–conjugated anti–mouse Ab (Molecular Probes; red signal) and an Alexa 488–conjugated antirabbit Ab (Molecular Probes; green signal).
Murine CXCL12 ELISA
Peritoneal washings were executed as previously described.9 For each time point considered, 3 to 5 mice were killed and the liquid obtained from the peritoneal washings of each time point was pooled together. These pools were concentrated 50 times with Centriplus Centrifugal Filter Devices (YM-3; Millipore, Billerica, MA) to obtain a final volume of 300 to 500 μL. The quantity of murine CXCL12 present in the peritoneal washings was then determined using an antibody sandwich enzyme-linked immunosorbent assay (ELISA) (R&D Systems) with a sensibility of more than 44 pg/mL.
Statistical analysis
Results were expressed as mean value ± SD. Statistical analyses of the in vitro data were performed using the nonparametric Mann-Whitney test; in in vivo experiments, Kaplan-Meier survival curves were analyzed with the Mantel-Haenszel test.
Results
EBV transformation of B cells in vitro (LCLs) and in vivo (hu/SCID tumors) is associated with modulation of chemokine receptor expression
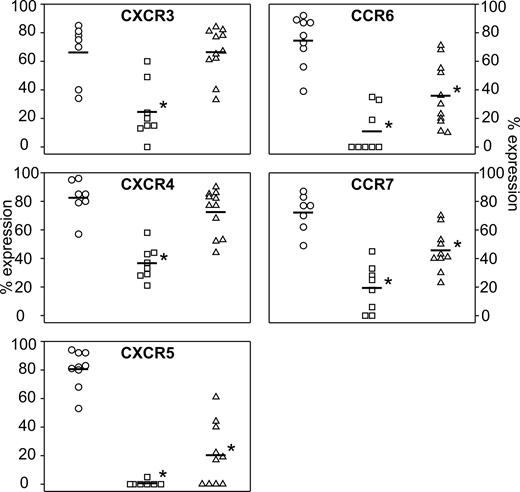
The chemokine receptor expression profile of EBV-transformed B cells has not been as yet extensively addressed. Using a panel of mAbs directed against CCR1, CCR2, CCR3, CCR4, CCR5, CCR6, CCR7, CXCR1, CXCR2, CXCR3, CXCR4, and CXCR5, we compared the chemokine receptor expression profile in freshly isolated B lymphocytes, in vitro EBV-immortalized LCL cells, and in vivo–generated hu/SCID tumors. Data are summarized in Figure 1. Circulating B cells expressed CCR6, CCR7, CXCR3, CXCR4, and CXCR5 (Figure 1, circles); very rarely, CCR1, CCR4, CCR5, and CXCR2 were expressed at low levels (data not shown). In contrast, EBV-immortalized LCL cells expressed CCR7, CXCR3, and CXCR4 at a lower level (P < .05), with CCR6 and CXCR5 being frequently undetectable (Figure 1, squares); rarely, CCR1, CCR3, and CCR4 were positive at low levels (data not shown). Hu/SCID tumors presented a mixed chemokine receptor expression profile (Figure 1, triangles), with CXCR3 and CXCR4 being substantially expressed, while CCR6, CCR7, and CXCR5 were significantly more weakly expressed compared with freshly isolated B lymphocytes (P < .05); rarely, CCR1, CCR2, and CCR4 were positive at low levels (data not shown). In general, a lower percent expression of chemokine receptors was also paralleled by a sizable reduction in mean fluorescence intensity (MFI) of the cells (data not shown). Altogether, these data demonstrate that EBV transformation in vitro is associated with a significant down-regulation of the expression of all chemokine receptors studied (CCR6, CCR7, CXCR3, CXCR4, and CXCR5); on the contrary, EBV-positive hu/SCID tumors generated after PBMC transfer into SCID mice continue to express substantial levels of CXCR3 and CXCR4, comparable to those present in freshly isolated B cells, though down-modulating CCR6, CCR7, and CXCR5.
Modulation of chemokine receptor expression in EBV-transformed B cells in vitro (LCLs) and in vivo (hu/SCID tumors). Flow cytometric analysis for CCR6, CCR7, CXCR3, CXCR4, and CXCR5 expression on B lymphocytes (○), LCL cells (□), and freshly isolated hu/SCID tumor cells (▵). Each point shows the percentage of cells expressing the indicated chemokine receptor. Horizontal bars represent the mean value of each group; the asterisks denote a significant difference compared with B cells (P < .05).
Modulation of chemokine receptor expression in EBV-transformed B cells in vitro (LCLs) and in vivo (hu/SCID tumors). Flow cytometric analysis for CCR6, CCR7, CXCR3, CXCR4, and CXCR5 expression on B lymphocytes (○), LCL cells (□), and freshly isolated hu/SCID tumor cells (▵). Each point shows the percentage of cells expressing the indicated chemokine receptor. Horizontal bars represent the mean value of each group; the asterisks denote a significant difference compared with B cells (P < .05).
CXCR4 is functional and is predominantly expressed by a CD23low hu/SCID tumor cell population
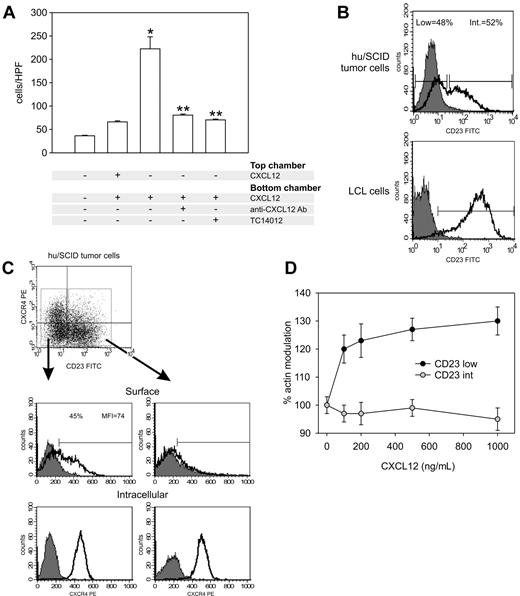
The functionality of the chemokine receptors expressed on hu/SCID tumor cells was evaluated by a chemotaxis assay. In most cases CXCL12 gave the greatest chemotactic response (data not shown), and because tumors in our experimental model consist of multiple peritoneal masses with frequent involvement of liver, spleen, and abdominal lymph nodes, sites known to produce high levels of CXCL12,10 subsequent experiments focused on the CXCL12/CXCR4 axis. As shown in Figure 2A, chemotaxis to CXCL12 could be significantly blocked by pretreatment of cells with neutralizing Abs to CXCL12 and the CXCR4 antagonist TC14012. Further, no significant increase of migration was observed with a uniform distribution of CXCL12, indicating that this is a gradient-dependent chemotactic response. It has been reported that hu/SCID tumors are composed of 2 phenotypically distinct B-cell subsets: CD23low (and CD38high [CD38hi]) and CD23intermediate ([CD23int] and CD38int), demonstrated to harbor a different EBV gene expression profile5 ; on the other hand, LCL cells have been reported to be homogenously CD23hiCD38int/low and only show a reduced CD23 expression when transferred into SCID mice.5,28 We thus investigated whether CXCR4 expression on hu/SCID tumor cells was segregated with the B-cell phenotype. To analyze the coexpression of membrane CXCR4 and CD23 on hu/SCID tumors, the tumor cells were examined cytometrically after staining with FITC anti-CD23 and PE anti-CXCR4 mAbs. As shown in Figure 2B (upper panel), in agreement with previously published data,5 within hu/SCID tumor cells 2 cell subpopulations were discernable on the basis of CD23 expression, one CD23low and one CD23int; this was not the case for LCLs, where virtually all cells showed high CD23 expression (Figure 2B, lower panel). Further, a clear segregation in CXCR4 expression was observed in the 2 hu/SCID tumor cell subpopulations (Figure 2C), with CXCR4 surface expression being predominantly confined to the CD23low subset (Figure 2C, top left panel). On the other hand, both tumor cell subsets expressed consistent levels of internal CXCR4 (Figure 2C). To determine the functional relevance of the generation of the 2 cell subsets, we investigated F-actin modulation following incubation with increasing concentrations of CXCL12. As shown in Figure 2D, a significant increase in F-actin polymerization could be observed only in the CD23low tumor subset, suggesting that this subset is the main responder to CXCL12.
CXCR4 is functional, and its surface expression segregates with B-cell phenotype in hu/SCID tumor cells. (A) Effect of CXCL12 on migration of hu/SCID lymphoma cells. Lymphoma cells were added to the chemotaxis chamber in the presence (+) or absence (–) of the following reagents: 100 ng/mL CXCL12 (SDF-1α), neutralizing anti-CXCL12 Abs (1:100), and TC14012 (1 and 5 μM). Migrated cells were recovered from the lower chamber after 2 hours at 37°C and counted. Results are the mean of 2 separate experiments executed in triplicate (± SD). The single asterisk denotes a significant difference compared with untreated cells (P < .05); 2 asterisks denote a significant inhibition by TC14012 or anti-CXCL12 Abs of the chemotactic properties of CXCL12-stimulated cells (P < .05). (B) Tumor cells were stained with the FITC-conjugated anti-CD23 mAb and the PE-conjugated anti-CXCR4 mAb before being analyzed by cytofluorimetry. The fluorograms represent the expression pattern of CD23 in a representative hu/SCID tumor sample (top) and LCL cells (bottom). (C) The expression of surface and intracellular CXCR4 (upper and lower panels, respectively) in the CD23low (left diagrams) and CD23int (right diagrams) tumor cell subsets is shown. A representative experiment of 3 consecutive experiments is shown. (D) Effect of CXCL12 on actin polymerization in the 2 hu/SCID lymphoma cell subsets. Lymphoma cells were labeled with PE-conjugated anti-CD23 Ab and tested by flow cytometry for CXCL12-induced cytoskeleton rearrangement. Results (mean ± SD from 2 experiments) show the kinetics of actin polymerization following addition of different concentrations of CXCL12; 100% corresponds to the baseline level.
CXCR4 is functional, and its surface expression segregates with B-cell phenotype in hu/SCID tumor cells. (A) Effect of CXCL12 on migration of hu/SCID lymphoma cells. Lymphoma cells were added to the chemotaxis chamber in the presence (+) or absence (–) of the following reagents: 100 ng/mL CXCL12 (SDF-1α), neutralizing anti-CXCL12 Abs (1:100), and TC14012 (1 and 5 μM). Migrated cells were recovered from the lower chamber after 2 hours at 37°C and counted. Results are the mean of 2 separate experiments executed in triplicate (± SD). The single asterisk denotes a significant difference compared with untreated cells (P < .05); 2 asterisks denote a significant inhibition by TC14012 or anti-CXCL12 Abs of the chemotactic properties of CXCL12-stimulated cells (P < .05). (B) Tumor cells were stained with the FITC-conjugated anti-CD23 mAb and the PE-conjugated anti-CXCR4 mAb before being analyzed by cytofluorimetry. The fluorograms represent the expression pattern of CD23 in a representative hu/SCID tumor sample (top) and LCL cells (bottom). (C) The expression of surface and intracellular CXCR4 (upper and lower panels, respectively) in the CD23low (left diagrams) and CD23int (right diagrams) tumor cell subsets is shown. A representative experiment of 3 consecutive experiments is shown. (D) Effect of CXCL12 on actin polymerization in the 2 hu/SCID lymphoma cell subsets. Lymphoma cells were labeled with PE-conjugated anti-CD23 Ab and tested by flow cytometry for CXCL12-induced cytoskeleton rearrangement. Results (mean ± SD from 2 experiments) show the kinetics of actin polymerization following addition of different concentrations of CXCL12; 100% corresponds to the baseline level.
The CXCR4 ligand, CXCL12, is expressed by LCLs and a CD23-expressing hu/SCID tumor cell population but not resting or activated B cells
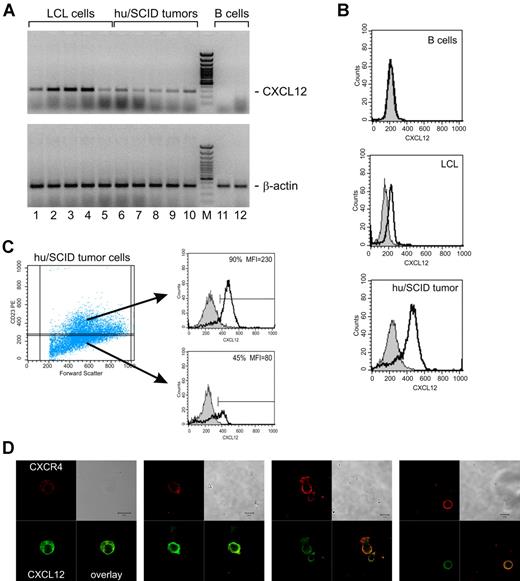
EBV-transformed B lymphocytes have a propensity to produce autocrine factors for their own growth and survival.29,30 We evaluated by both RT-PCR and fluorescence-activated cell sorter (FACS) analysis whether LCLs and hu/SCID tumors expressed the CXCR4 ligand, CXCL12, thus possibly activating an autocrine loop; indeed, this has not been investigated so far. As shown in Figure 3A, all LCLs (lanes 1 to 5) and hu/SCID tumor cells (lanes 6 to 10) tested expressed mRNA for SDF-1α/CXCL12 and most also expressed SDF-1β/CXCL12 (data not shown), with the α isoform being the predominant transcript in most cases. To investigate whether the expression of CXCL12 by LCLs and hu/SCID tumor cells was simply associated to the activation state of these cells or more strictly linked to EBV transformation, B cells were purified and stimulated in vitro with anti-CD40 mAb and interleukin-4 (IL-4) for 3 to 5 days. RT-PCR analysis of these samples disclosed that no specific transcripts for SDF-1α/CXCL12 or SDF-1β/CXCL12 could be evidenced in both freshly isolated (Figure 3A, lane 11 of each panel) and in vitro–activated B cells (Figure 3A, lane 12 of each panel), thus showing that CXCL12 expression was in some way associated with EBV immortalization. The expression of CXCL12 in EBV-transformed B cells was also confirmed at the protein level by cytofluorimetric analysis of both LCLs and hu/SCID tumors (Figure 3B); interestingly, CXCL12 was shown to be preferentially produced by the CD23-expressing (LCL-like) subset of hu/SCID tumors (Figure 3C). To better document the segregation of CXCR4 and CXCL12 expression in hu/SCID tumors, we executed confocal microscopy analysis at the single cell level after staining the cells for surface CXCR4 and intracellular CXCL12. As shown in Figure 3D, on the whole, cells expressing high levels of surface CXCR4 (red signal) expressed much more weakly CXCL12 (green signal) compared with the cell population expressing CXCR4 weakly or not at all.
CXCL12 expression in EBV-transformed B cells and its segregation with B-cell phenotype. (A) The expression of CXCL12 and β-actin in LCL cells and hu/SCID tumor cells was evaluated by RT-PCR. Representative results from 5 LCLs and 5 hu/SCID tumors are shown. Lane M corresponds to 50-bp molecular weight marker. Lanes 12 and 13 correspond to a representative case of purified resting B cells and to the same B cells after 48 hours of in vitro stimulation with anti-CD40/IL-4, respectively. (B-C) The intracellular expression of CXCL12 in LCL cells and hu/SCID tumor cells was evaluated by cytofluorimetric analysis. (B) The fluorograms (from top to bottom) show normal B cells not expressing CXCL12, LCL cells, and hu/SCID tumor cells. (C) The expression of CXCL12 in the CD23low (lower right) and CD23int (upper right) tumor cell subsets is shown. A representative experiment of 3 consecutive experiments is shown. (D) Confocal microscopic analysis evaluating the coexpression of surface CXCR4 and intracellular CXCL12 in hu/SCID tumors. Cells were fixed, stained with anti-CXCR4 Ab, and subsequently permeabilized before incubation with anti-CXCL12 Ab. The signal for CXCR4 is shown in red, while the CXCL12 signal is in green. Areas of colocalization are shown in yellow.
CXCL12 expression in EBV-transformed B cells and its segregation with B-cell phenotype. (A) The expression of CXCL12 and β-actin in LCL cells and hu/SCID tumor cells was evaluated by RT-PCR. Representative results from 5 LCLs and 5 hu/SCID tumors are shown. Lane M corresponds to 50-bp molecular weight marker. Lanes 12 and 13 correspond to a representative case of purified resting B cells and to the same B cells after 48 hours of in vitro stimulation with anti-CD40/IL-4, respectively. (B-C) The intracellular expression of CXCL12 in LCL cells and hu/SCID tumor cells was evaluated by cytofluorimetric analysis. (B) The fluorograms (from top to bottom) show normal B cells not expressing CXCL12, LCL cells, and hu/SCID tumor cells. (C) The expression of CXCL12 in the CD23low (lower right) and CD23int (upper right) tumor cell subsets is shown. A representative experiment of 3 consecutive experiments is shown. (D) Confocal microscopic analysis evaluating the coexpression of surface CXCR4 and intracellular CXCL12 in hu/SCID tumors. Cells were fixed, stained with anti-CXCR4 Ab, and subsequently permeabilized before incubation with anti-CXCL12 Ab. The signal for CXCR4 is shown in red, while the CXCL12 signal is in green. Areas of colocalization are shown in yellow.
In vitro CXCL12 neutralization inhibits hu/SCID lymphoma cell proliferation and survival
We showed thus far that hu/SCID tumors express CXCL12 and that they can efficiently respond to exogenous CXCL12. We thus set out to investigate the possible functional role of endogenous CXCL12 on hu/SCID cell biology. We first investigated whether CXCL12 neutralization by the use of anti-CXCL12 neutralizing Abs or the use of a recently described specific blocking agent for CXCR4,24 such as TC14012, affected lymphoma growth in vitro. Indeed, as shown in Figure 4A, treatment of ex vivo–obtained lymphoma cells for 2 days with neutralizing anti-CXCL12 Abs (1:20 dilution) or TC14012 (50 to 100 μM) significantly reduced lymphoma cell proliferation (P < .05). On the other hand, the goat preimmune serum used as control at the same dilution did not significantly alter lymphoma proliferation (Figure 4A). We next tested the effect of blocking the endogenous CXCL12 on apoptosis of hu/SCID lymphoma cells. To this end, ex vivo–obtained lymphoma cells were treated for 2 days with neutralizing anti-CXCL12 Abs (1:20 dilution) or TC14012 (50 to 100 μM) and then analyzed by flow cytometry after staining with FITC-conjugated annexin V and PI. Treatment of cells with either anti-CXCL12 Abs or TC14012 determined a significant increase in annexin V–positive cells (data not shown). However, a difference seemed to emerge in their mode of action, because the neutralizing anti-CXCL12 Abs determined mainly an increase in early apoptosis (annexin V–positive PI-negative), while the CXCR4 antagonist acted mainly on late apoptosis (annexin V–positive PI-positive) (Figure 4C). Notably, the concentrations of the neutralizing anti-CXCL12 Abs (1:100 dilution) or TC14012 (1 to 5 μM) used in functional assays such as chemotaxis, adhesion, or chemoinvasion did not affect either the proliferation or survival of lymphoma cells (data not shown). On the whole, these data suggest that survival and proliferation of hu/SCID tumors are in part mediated by CXCL12/CXCR4 interactions and autocrine secretion of CXCL12.
Effect of in vitro neutralization of CXCL12 and CXCR4 on hu/SCID lymphoma cell proliferation and survival. (A) Ex vivo–obtained hu/SCID lymphoma cells were cultured for 48 hours with or without TC14012 (50 to 100 μM), neutralizing anti-CXCL12 Abs (1:20), or appropriate controls. Each result is representative of the mean counts per minute (cpm) ± SD of triplicate wells after a 48-hour pulse with [methyl-3H]thymidine. This analysis is representative of 3 separate experiments with different tumors with similar results; the single asterisk denotes a significant difference compared with untreated cells (P < .05). (B) Ex vivo–obtained hu/SCID lymphoma cells were cultured for 48 hours with or without TC14012 (50 to 100 μM), neutralizing anti-CXCL12 Abs (1:20), or appropriate controls. Cells were then harvested and labeled with PI and FITC-conjugated annexin V before being analyzed by flow cytometry. Data are shown as the mean percent change relative to control. One representative experiment of 3 performed is shown.
Effect of in vitro neutralization of CXCL12 and CXCR4 on hu/SCID lymphoma cell proliferation and survival. (A) Ex vivo–obtained hu/SCID lymphoma cells were cultured for 48 hours with or without TC14012 (50 to 100 μM), neutralizing anti-CXCL12 Abs (1:20), or appropriate controls. Each result is representative of the mean counts per minute (cpm) ± SD of triplicate wells after a 48-hour pulse with [methyl-3H]thymidine. This analysis is representative of 3 separate experiments with different tumors with similar results; the single asterisk denotes a significant difference compared with untreated cells (P < .05). (B) Ex vivo–obtained hu/SCID lymphoma cells were cultured for 48 hours with or without TC14012 (50 to 100 μM), neutralizing anti-CXCL12 Abs (1:20), or appropriate controls. Cells were then harvested and labeled with PI and FITC-conjugated annexin V before being analyzed by flow cytometry. Data are shown as the mean percent change relative to control. One representative experiment of 3 performed is shown.
CXCL12 stimulates the adhesive and invasive properties of hu/SCID tumor cells
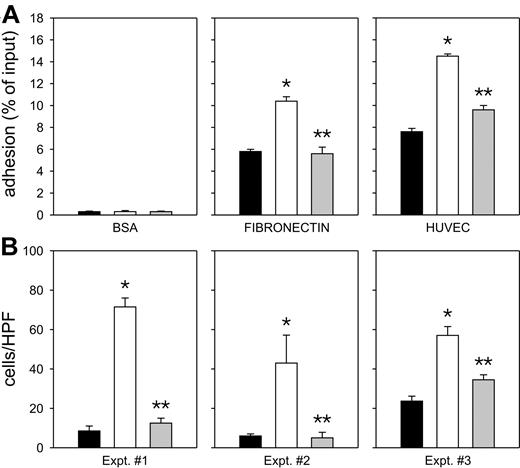
CXCL12 has been reported to regulate the function of several integrins on normal human hematopoietic cells31 ; we thus investigated whether CXCL12 also regulated integrin activity in hu/SCID tumor cells. As shown in Figure 5A, CXCL12 consistently increased the adhesion of hu/SCID tumor cells to both fibronectin (Figure 5A, middle panel) and HUVECs (Figure 5A, right panel), whereas it did not affect their adhesion to BSA (Figure 5A, left panel).
Effect of CXCL12 on the adhesive and invasive properties of hu/SCID tumor cells. (A) Effect of CXCL12 on adhesion of hu/SCID tumor cells to fibronectin and endothelial cells. The panels from left to right represent adhesion of hu/SCID tumor cells to bovine serum albumin (BSA), fibronectin, and HUVECs. Unstimulated cells are shown as black bars; open and gray bars represent hu/SCID tumor cells stimulated with CXCL12/SDF-1α (200 ng/mL) in the absence and in the presence, respectively, of the CXCR4 antagonist TC14012. Data are shown as mean ± SEM of 2 consecutive experiments; the single asterisk denotes a significant difference compared with untreated cells (P < .05); 2 asterisks denote a significant inhibition by TC14012 of the adhesive properties of CXCL12-stimulated cells (P < .05). (B) Cells invading the Matrigel were collected from the lower compartments and counted as detailed in “Materials and methods”; results are expressed as number of cells per high power field (HPF) and represent mean values ± SEM of 3 replicate determinations for each tumor. Hu/SCID lymphoma cells showed significant invasion through Matrigel toward a CXCL12 gradient (open columns); this phenomenon was inhibited by treating the cells with TC14012 (gray columns). The single asterisk denotes a significant difference (P < .05) compared with unstimulated cells; 2 asterisks denote a significant inhibition by TC14012 of the invasive properties of CXCL12-stimulated cells (P < .05). Results obtained in 3 different hu/SCID tumor masses representative of 4 consecutive experiments are shown.
Effect of CXCL12 on the adhesive and invasive properties of hu/SCID tumor cells. (A) Effect of CXCL12 on adhesion of hu/SCID tumor cells to fibronectin and endothelial cells. The panels from left to right represent adhesion of hu/SCID tumor cells to bovine serum albumin (BSA), fibronectin, and HUVECs. Unstimulated cells are shown as black bars; open and gray bars represent hu/SCID tumor cells stimulated with CXCL12/SDF-1α (200 ng/mL) in the absence and in the presence, respectively, of the CXCR4 antagonist TC14012. Data are shown as mean ± SEM of 2 consecutive experiments; the single asterisk denotes a significant difference compared with untreated cells (P < .05); 2 asterisks denote a significant inhibition by TC14012 of the adhesive properties of CXCL12-stimulated cells (P < .05). (B) Cells invading the Matrigel were collected from the lower compartments and counted as detailed in “Materials and methods”; results are expressed as number of cells per high power field (HPF) and represent mean values ± SEM of 3 replicate determinations for each tumor. Hu/SCID lymphoma cells showed significant invasion through Matrigel toward a CXCL12 gradient (open columns); this phenomenon was inhibited by treating the cells with TC14012 (gray columns). The single asterisk denotes a significant difference (P < .05) compared with unstimulated cells; 2 asterisks denote a significant inhibition by TC14012 of the invasive properties of CXCL12-stimulated cells (P < .05). Results obtained in 3 different hu/SCID tumor masses representative of 4 consecutive experiments are shown.
Because tumor cell invasion is one of the major characteristics of malignant cells, we evaluated this feature using a chemoinvasion Matrigel assay. As shown in Figure 5B, the invasive capability of hu/SCID tumors was increased in the presence of a CXCL12 gradient (Figure 5B, open columns). In addition, TC14012 significantly inhibited both the adhesive and invasive properties of these cells (Figure 5, gray columns), thus indicating that the invasion-promoting effect of CXCL12 was a specific phenomenon; similar results were obtained when neutralizing anti-CXCL12 Abs were used (data not shown).
CXCL12 is expressed in vivo during the early phases of EBV-mediated lymphomagenesis, and its neutralization inhibits tumor growth in vivo
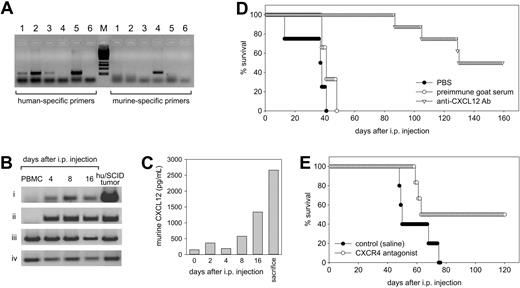
We finally directly addressed the possible relevance of the CXCL12/CXCR4 axis in the lymphomagenesis process of this experimental model. To this end, we first wondered whether CXCL12 and CXCR4 were indeed expressed in the mouse peritoneal microenvironment. Thus, cells were recovered from the peritoneal cavity of SCID mice at 4, 8, and 16 days following intraperitoneal transfer of PBMCs from EBV-positive donors, and the expression of human and murine CXCL12 and human CXCR4 was determined by RT-PCR. In addition, the peritoneal washings were evaluated for the presence of murine CXCL12 by ELISA. We used primers specific for murine and human CXCL12 (Figure 6A), because under our experimental conditions a band corresponding to human CXCL12 could only be evidenced on human cells but not on murine tissues (Figure 6A). At the same time, primers specific for murine CXCL12 gave a band only when mouse tissues were used (Figure 6A). As shown in Figure 6B, both human and mouse CXCL12 transcripts were undetectable in PBMCs immediately prior to injection; meanwhile, they were both readily evidenced in the cell populations recovered at all the time points considered. On the other hand, the CXCR4 transcript was always consistently detected in all cell populations studied (Figure 6B).
Expression of the CXCL12/CXCR4 axis in vivo following PBMC transfer to SCID mice and effects of CXCL12 and CXCR4 neutralization in vivo. (A) The specificity of our primer pairs for human and murine CXCL12 was evaluated on human and murine samples. A band corresponding to human CXCL12 was detected only on human samples: HUVECs (lane 1), MRC-5 (lane 2), human microvascular endothelial cells (lane 3), and LCLs (lane 5) but not on murine tissues such as the peritoneal membrane (lane 4). Conversely, primers specific for murine CXCL12 gave a band only on murine tissues (lane 4). Lane 6 corresponds to the water control. (B) The expression of CXCL12 and CXCR4 in cells recovered from the peritoneal cavity of SCID mice injected with PBMCs from EBV-positive donors was evaluated by RT-PCR. The cells were analyzed before and after various time intervals following intraperitoneal injection for the expression of human CXCL12 (i), murine CXCL12 (ii), human CXCR4 (iii), and β-actin (iv). The first left lane corresponds to the profile obtained with freshly isolated PBMCs; the last lane corresponds to a hu/SCID tumor sample. (C) Expression of murine CXCL12 as evaluated by ELISA in the peritoneal washings obtained at various time intervals following PBMC transfer in SCID mice. (D-E) Effect of CXCL12 and CXCR4 neutralization on tumor growth. Freshly isolated hu/SCID tumor cell suspensions were injected intraperitoneally into naive SCID mice. (D) Mice were treated with intraperitoneal injections of goat anti-CXCL12 Ab, PBS, or heat-inactivated goat preimmune serum every day for 3 weeks starting from the day after cell transfer. The effect of anti-CXCL12 Ab and treatment on lymphoma development was assessed by effects on survival. Five to 8 mice were included in each experimental group, and the experiment was repeated twice. (E) In another group of animals following PBMC transfer, Alzet pumps releasing the CXCR4 antagonist 4F-benzoyl-TN14003 were implanted subcutaneously and changed every 2 weeks for a total of 2 implants. The effect of treatment with 4F-benzoyl-TN14003 on lymphoma development was assessed by effects on survival and tumor dissemination. Six to 8 mice were included in each experimental group.
Expression of the CXCL12/CXCR4 axis in vivo following PBMC transfer to SCID mice and effects of CXCL12 and CXCR4 neutralization in vivo. (A) The specificity of our primer pairs for human and murine CXCL12 was evaluated on human and murine samples. A band corresponding to human CXCL12 was detected only on human samples: HUVECs (lane 1), MRC-5 (lane 2), human microvascular endothelial cells (lane 3), and LCLs (lane 5) but not on murine tissues such as the peritoneal membrane (lane 4). Conversely, primers specific for murine CXCL12 gave a band only on murine tissues (lane 4). Lane 6 corresponds to the water control. (B) The expression of CXCL12 and CXCR4 in cells recovered from the peritoneal cavity of SCID mice injected with PBMCs from EBV-positive donors was evaluated by RT-PCR. The cells were analyzed before and after various time intervals following intraperitoneal injection for the expression of human CXCL12 (i), murine CXCL12 (ii), human CXCR4 (iii), and β-actin (iv). The first left lane corresponds to the profile obtained with freshly isolated PBMCs; the last lane corresponds to a hu/SCID tumor sample. (C) Expression of murine CXCL12 as evaluated by ELISA in the peritoneal washings obtained at various time intervals following PBMC transfer in SCID mice. (D-E) Effect of CXCL12 and CXCR4 neutralization on tumor growth. Freshly isolated hu/SCID tumor cell suspensions were injected intraperitoneally into naive SCID mice. (D) Mice were treated with intraperitoneal injections of goat anti-CXCL12 Ab, PBS, or heat-inactivated goat preimmune serum every day for 3 weeks starting from the day after cell transfer. The effect of anti-CXCL12 Ab and treatment on lymphoma development was assessed by effects on survival. Five to 8 mice were included in each experimental group, and the experiment was repeated twice. (E) In another group of animals following PBMC transfer, Alzet pumps releasing the CXCR4 antagonist 4F-benzoyl-TN14003 were implanted subcutaneously and changed every 2 weeks for a total of 2 implants. The effect of treatment with 4F-benzoyl-TN14003 on lymphoma development was assessed by effects on survival and tumor dissemination. Six to 8 mice were included in each experimental group.
RT-PCR results seemed to indicate that murine CXCL12 is the main isoform present in the early stages of lymphomagenesis. We thus determined whether murine CXCL12 protein was effectively present in the peritoneal cavity and how it varied during the lymphomagenesis process. As shown in Figure 6C, low levels of murine CXCL12 were present in the peritoneal washings prior to PBMC injection; however, during the course of the lymphomagenesis process there was a considerable increase in the protein levels of CXCL12, reaching a peak at the moment of killing.
In view of these data, which suggested the possibility that a CXCL12/CXCR4 autocrine/paracrine loop could be at work in lymphoma development and progression, we wondered whether interfering with the CXCL12/CXCR4 axis could affect lymphoma development in SCID mice. To this end, we chose to use freshly obtained hu/SCID tumor cell suspensions to generate tumors in naive SCID mice. Hu/SCID lymphoma cells were used instead of PBMCs from EBV-positive donors because of the well-known problem of the very high heterogeneity in the generation of lymphomas in SCID mice among different donors,32,33 which would have complicated the interpretation of the effects of neutralization of the CXCR4/CXCL12 axis on lymphoma development. Hu/SCID tumor-injected mice were then treated with intraperitoneal injections of a neutralizing anti-CXCL12 Ab or adequate controls. For CXCR4 neutralization, 4F-benzoyl-TN14003, a recently reported T140 derivative shown to have an increased biostability with respect to TC14012,18 was administered by subcutaneous injection using Alzet pumps beginning from the day preceding transplantation of hu/SCID lymphoma cells for 4 consecutive weeks. As shown in Figure 6D, in experiments evaluating the effect of CXCL12 neutralization, all control animals succumbed within 50 days after tumor cell transfer; meanwhile, mouse treatment with the neutralizing anti-CXCL12 Ab significantly delayed lymphoma development (Figure 6D; P < .0002), with 50% of anti-CXCL12–treated animals being tumor free after 180 days. A similar effect on animal survival was observed when 4F-benzoyl-TN14003 was used (Figure 6E; P < .05).
Discussion
In this article we addressed the contribution of the chemokine system to the outgrowth of EBV-positive B-cell lymphomas in SCID mice injected intraperitoneally with PBMCs from EBV-positive donors. We showed that most in vitro–transformed LCLs strongly down-regulated the expression of most chemokine receptors, compared with normal B lymphocytes; meanwhile, most hu/SCID tumors analyzed expressed CXCR3 and CXCR4 at moderately high levels and CCR6, CCR7, and CXCR5 at intermediate/low levels. That in vivo and in vitro immortalization of circulating B cells by EBV is associated with down-regulation of CCR6, CCR7, and CXCR5 expression may be partially linked to the activation state of these cells.34 Our data concerning LCLs partially confirm those recently reported by other workers,35 in that we also found a down-regulation of CXCR4 and CXCR5; however, we were not able to document any significant up-regulation of CCR6 surface expression in LCL cells. Notably, only a few LCL samples were considered by these authors35 ; in addition, the modulation of chemokine receptor expression (in comparison with a single peripheral blood B-cell sample examined) was solely based on a semiquantitative RT-PCR analysis,35 while our conclusions are based on protein expression by cytofluorimetry on a much larger number of samples. On the other hand, this apparent discrepancy might depend on the very high heterogeneity between LCLs, as also encountered in the present study (Figure 1), and the modulation of some chemokine receptors associated with in vitro culture (data not shown). The differential profile of chemokine receptor expression between LCLs and hu/SCID tumor cells, with these latter expressing moderately high CXCR3 and CXCR4 levels, may also partially reflect a different stage of maturation of these 2 cell populations.28 It is well known that plasma cells express CXCR4 while down-modulating CCR7 and CXCR5,36 whereas activated B cells down-modulate CCR6 (Brandes et al34 and data not shown). Thus, the down-regulation of CCR6, CCR7, and CXCR5 seen in hu/SCID tumors may partially reflect their stage of differentiation and their activation state.
Recently, it has been described that EBV latent genes can also modify chemokine receptor expression.35 Hu/SCID tumors in general exibit low levels of latent EBV transcripts such as Epstein-Barr nuclear antigen-1 (EBNA-1), EBNA-2, and latent membrane protein-1 (LMP-1) with respect to LCLs.5 Thus, differential expression of EBV latent genes between LCLs and hu/SCID tumors may also help explain the differences in the chemokine receptor profile between LCLs and hu/SCID tumors. In this regard, as outlined previously and reported by others,5,28 hu/SCID tumors are composed of 2 phenotypically distinct B-cell subsets: CD23lowCD38hi and CD23intCD38int; meanwhile, LCLs have been reported to be homogenously CD23hiCD38int/low.5,28 The CD23lowCD38hi cell population present within hu/SCID tumors expresses lytic cycle EBV transcripts, while the CD23intCD38int population only expresses the latent cycle transcripts EBNA-1, EBNA-2, and LMP-1.5 We demonstrated that surface CXCR4 is predominantly expressed by the CD23low tumor cell subset, previously shown to have a low proliferative index and high-level Ig secretion, typical of plasma cells.5 Thus, it is conceivable that differential expression of EBV genes could translate into modulation of chemokine receptor expression, with CXCR4 up-regulation by lytic gene products.
Given these premises, we concentrated our attention on the CXCR4 ligand, CXCL12, to evaluate whether a putative autocrine loop could be at work in the lymphomagenesis process of this experimental model. We showed for the first time that EBV-transformed B cells, both LCLs and hu/SCID tumors, are able to express CXCL12, in contrast to resting and in vitro–activated B lymphocytes. Interestingly, CXCL12 was shown to be produced predominantly by the CD23int tumor cell subset, which expresses low levels of surface CXCR4 (sCXCR4) but high levels of internal CXCR4. It has been previously shown that the transfer of a tumor cell line (generated after in vitro growth of hu/SCID lymphomas) expressing CD23 at intermediate levels to SCID mice entails a substantial shift to low CD23 expression. Because the former expresses low levels of sCXCR4, while the latter expresses good levels of sCXCR4, it could be inferred that the CD23intCD38intsCXCR4low (lymphoblastoid-like) subset shifts phenotype to CD23lowCD38hisCXCR4int (plasmacytoid-like) after transfer and growth in SCID mice with gain of CXCL12 responsiveness and that the plasmacytoid cells originate from the lymphoblastoid cells.28 We thus expand the current knowledge regarding this experimental model, proposing that the differentiation of plasmacytoid cells from lymphoblastoid cells involves gain of CXCR4 surface expression (and responsiveness) and reduced production of CXCL12 and that the CXCL12/CXCR4 axis may be important in the homeostasis of the 2 tumor subsets that are found within lymphomas. Furthermore, in vitro treatment of hu/SCID lymphoma cells with neutralizing ant-CXCL12 Abs or TC14012 decreased cell survival and proliferation, implying autocrine regulation of hu/SCID lymphoma cell biology by endogenous CXCL12. However, the fact that blocking CXCR4 or CXCL12 reduced but did not completely inhibit the proliferation and survival of hu/SCID lymphoma cells suggests that factors and pathways other than CXCR4/CXCL12 interactions are involved in the regulation of these processes. Further work is warranted to clarify this issue.
Along with the demonstration that CXCL12 expression by B lymphocytes could be related to EBV transformation, one major finding of this work is the demonstration that interfering with the CXCL12/CXCR4 axis may affect EBV-transformed cell behavior, both in vitro and in vivo. Indeed, we found that the lymphomagenesis process was associated with a dramatic increase in the levels of murine CXCL12 present within the peritoneal cavity, suggesting a role for this chemokine in lymphoma growth and progression. Notably, with the ELISA kit that was used, human CXCL12 (SDF-1α) exhibits 26% to 42% cross-reactivity at all concentrations, so the contribution of human CXCL12 to tumor growth cannot be completely excluded. In vivo neutralization of the CXCL12/CXCR4 axis by a neutralizing anti-CXCL12 Ab or CXCR4 antagonist had a dramatic effect on lymphoma development, even though the anti-CXCL12 antiserum seemed more effective. The CXCR4 antagonist used in vivo was 4F-benzoyl-TN14003 instead of TC14012, because the latter has been shown to be relatively unstable in rat liver homogenates while 4F-benzoyl-TN14003 is relatively stable in vivo.18 The minor efficacy of 4F-benzoyl-TN14003 compared with the neutralizing anti-CXCL12 Abs may reflect the greater difficulties of the former with respect to administration modality, schedule, and in vivo stability or half-life. Indeed, only high doses of this antagonist inhibited proliferation and cell survival in vitro.
The neutralization of CXCR4 through the use of an anti-CXCR4 Ab has been shown to be able to prevent tumor growth in nonobese diabetic (NOD)/SCID mice injected intraperitoneally with the Namalwa cell line13 ; however, compared with PBMC-injected SCID mice, this experimental model may be more distant from the human lymphomagenesis setting. In addition, the use of an anti-CXCR4 Ab as a tumor-preventing tool may give a less mechanistic insight into the relevance of the CXCL12/CXCR4 axis in the lymphomagenesis process; in fact, it could be argued that the effect on lymphoma growth could be due to tumor cell elimination by the mouse reticuloendothelial system. We chose to prevent this possible criticism through the use of an Ab directed against CXCL12 and a CXCR4-specific antagonist. In any case, these findings strongly suggest that the CXCR4/CXCL12 axis could play a significant role in favoring the outgrowth and dissemination of EBV-transformed cells in PBMC-injected SCID mice. Because CXCR4 expression has been proven in the human tumor counter-part of this experimental model,13,14 the CXCL12/CXCR4 axis could indeed become an attractive target for therapy in lymphoma-bearing patients, possibly through the use of small molecular antagonists of CXCR4.
Prepublished online as Blood First Edition Paper, September 28, 2004; DOI 10.1182/blood-2004-03-0799.
Supported in part by grants from Ministero dell'Instruzione, dell' Universitá e della Ricerca (MIUR; FIRB and PRIW and MUIR 60%); Istituto Superiore di Sanità (AIDS Project); Italian Association for Research on Cancer (AIRC) and Italian Foundation for Research on Cancer (FIRC); and Padua University grants. V.T. is a recipient of an AIRC fellowship.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We are grateful to Dr J. Gordon (Birmingham, United Kingdom) for providing us with the CD-32L cells. We are particularly indebted to Prof R. M. Strieter (UCLA) for providing the goat anti-CXCL12 serum. We also thank Dr A. Janowska-Wieczorek (Edmonton, AB, Canada) for kind advice on the chemoinvasion assay and Dr K. Balabanian (INSERM U131, Institut Paris-Sud sur les Cytokines, Clamart, France) for help with actin polymerization experiments. Also, the great expertise of Dr A. Rosato in animal procedures is acknowledged. The invaluable help of P. Gallo in artwork preparation is gratefully acknowledged.

![Figure 4. Effect of in vitro neutralization of CXCL12 and CXCR4 on hu/SCID lymphoma cell proliferation and survival. (A) Ex vivo–obtained hu/SCID lymphoma cells were cultured for 48 hours with or without TC14012 (50 to 100 μM), neutralizing anti-CXCL12 Abs (1:20), or appropriate controls. Each result is representative of the mean counts per minute (cpm) ± SD of triplicate wells after a 48-hour pulse with [methyl-3H]thymidine. This analysis is representative of 3 separate experiments with different tumors with similar results; the single asterisk denotes a significant difference compared with untreated cells (P < .05). (B) Ex vivo–obtained hu/SCID lymphoma cells were cultured for 48 hours with or without TC14012 (50 to 100 μM), neutralizing anti-CXCL12 Abs (1:20), or appropriate controls. Cells were then harvested and labeled with PI and FITC-conjugated annexin V before being analyzed by flow cytometry. Data are shown as the mean percent change relative to control. One representative experiment of 3 performed is shown.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/105/3/10.1182_blood-2004-03-0799/6/m_zh80030573180004.jpeg?Expires=1765931988&Signature=Ov2YQCYh68t6jVEt~2xSDJ1RYpamkZ5SEkjb84xtyuEqnEoMpdbEI6gOUBP~E2AcHKJ-CdvSqTAKXwJ1cJeLBB5UfePmey4-KIg1n7rK-8xSWkYx6CRQmXXAxN2MY5EOdV0y8niUfPusc9zzU3D~cmbqO-mh7IaeQ8Wcd6XiRq9ScUPJvPO2gDIv5UsL~cv5gWMZ8-Fu0oNq2XaCjQYOztr5HOrtBwBuRV7akA5qRFz1Zfi5Uyv1CDl9HJ4hOPySd67lzNoXMcv8ttKlTI4qzF6dGzbyywZgx-68TfesP~Kax2ps397KjU7IHXVLXMjjBhgfMqqyiow1-cE2SHAz-A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal