Abstract
Endostatin is a proteolytic fragment of collagen XVIII that inhibits endothelial cell migration in vitro and experimental tumor growth in vivo. To determine how endostatin affects the in vivo behavior of endothelial cells, we took advantage of a surrogate model of human angiogenesis, in which human endothelial cells are transferred to immunodeficient mice and develop into complex vessels in the course of 30 days. Systemic delivery of human yeast-derived endostatin (serum levels of 30-35 ng/mL) inhibited the number of human vessels dramatically (95% at day 20), as most endothelial cells remained suspended as single cells. The fraction of cells with a migratory phenotype (F-actin–positive, extending pseudopods) was strongly reduced (from 50% to 13% at day 10), while the number of apoptotic and mitotic cells remained unchanged. Endostatin also hampered the recruitment of α-smooth muscle actin–expressing perivascular cells and thus reduced the number of mature vessels (from 64.3% to 28.6% at day 30). Moreover, transcripts of pericyte-recruiting platelet-derived growth factor-B (PDGFB) were strongly reduced in endothelial cells of endostatin-treated mice. Our results are strong evidence that endostatin inhibits angiogenesis at several levels in vivo, including perivascular cell recruitment.
Introduction
The formation of new capillaries from pre-existing vessels (angiogenesis) appears to be essential for tumor growth and survival.1-6 Whereas hypoxic conditions in expanding tumors often initiate a cascade of endothelial cell sprouting and formation of new vessels, tumor growth can be arrested by inadequate blood supply and a lack of metabolic exchange, often leading to tumor necrosis and regression.7
Several tumor-derived, circulating angiogenesis inhibitors generated in vivo by proteolytic degradation have been recently identified (reviewed in Cao8 and Marneros and Olsen9 ). In particular, a 20-kDa C-terminal proteolytic fragment of collagen XVIII, termed endostatin, inhibits tumor growth in several animal models.10-15 Although the mechanisms of action are incompletely understood, endostatin reportedly interacts with several endothelial cell-surface receptors that are critically involved in angiogenesis. Endostatin binds directly to the fibronectin receptor α5β116,17 and also interacts with αvβ3 and αvβ5 integrins.17 In keeping with these interactions, endostatin inhibits endothelial cell binding to the αvβ3-ligand gelatin17 and also their binding to collagen type I in a dose-dependent manner.18 Moreover, endostatin binds to heparan sulfate,19-22 tropomyosin,23 caveolin-1,16 VEGFR-1 (vascular endothelial growth factor receptor-1; Flt-1), and VEGFR-2 (Flk-1)24 and was recently shown to depend on E-selectin expression to be effective in vivo.25 Interaction of endostatin with endothelial cells leads to a variety of downstream effects,26-28 including inhibition of the Wnt/β-catenin pathway,29 and actin reorganization in endothelial cells.16,30-33 In fact, this wide variety of effects elicited by 1 endogenous inhibitor of angiogenesis was recently supported by 2 studies of global gene expression in endostatin-treated endothelial cells.27,34
There is general agreement that the in vitro action of endostatin involves inhibition of endothelial cell migration, but less clear whether endostatin also affects endothelial cell apoptosis and proliferation.6,12,26,35-39 Furthermore, even less is known about how endostatin affects endothelial cell function in vivo, as readouts have been restricted to analysis of vascular density or blood flow in tumors,12-14,35,40 or neovascularization of the chick allantoic membrane.10,12,39 Moreover, studies that focused in more detail on endothelial cell behavior in vivo either failed to detect substantial effects of endostatin41 or found subtle signs of impaired blood vessel maturation.42 Finally, several studies failed to observe an antitumor effect of endostatin (reviewed in Marshall43 ). Thus, there is an obvious need to more carefully analyze the effect of endostatin at the level of single endothelial cells during angiogenesis.
Here, we describe the effect of endostatin in an in vivo model of human endothelial cell behavior in which the final stages of angiogenesis, ie, assembly of single endothelial cells to complex microvessels, can be observed directly. In the absence of endostatin, human endothelial cells suspended in Matrigel form complex human vessels that make xenogeneic contact with the murine vascular system, carry murine blood, and recruit perivascular α-smooth muscle actin–positive cells.44 However, systemic delivery of yeast-derived, recombinant human endostatin leading to serum levels of 30 to 35 ng/mL during 30 days almost completely abolished endothelial cell migration and capillary morphogenesis and also inhibited the recruitment of perivascular cells.
Materials and methods
Chemicals, growth factors, and other reagents
Collagenase (C-7926) and Dulbecco phosphate-buffered saline (PBS) were purchased from Sigma Chemical (St Louis, MO). Trypsin/EDTA (ethylenediaminetetraacetic acid) solution was from BIOWhittaker (Walkersville, MD), Dispase from Roche Diagnostics (Mannheim, Germany), bovine dermal type I collagen (Vitrogen 100) from Celtrix Laboratories (Palo Alto, CA), and DMSO (dimethyl sulfoxide, dried) from Merck (Darmstadt, Germany). Optimal cutting temperature (OCT) compound was from Tissue Tec, Miles Laboratories (Elkart, IN). Matrigel basement membrane matrix was from Becton Dickinson Labware (Bedford, MA), soluble recombinant human endostatin expressed in Pichia pastoris was a gift from EntreMed (Rockville, MD) and purified according to Sim et al.45 The enzyme immunoassay (EIA) kit for human endostatin was from CytImmune Sciences (College Park, MD) (also a gift from EntreMed), the ApopTag Peroxidase In Situ Detection Kit from Intergen (Oxford, United Kingdom), Dynabeads from Dynal (Oslo, Norway), and Alzet osmotic pumps from Durect (Cupertino, CA). Fluorescein isothiocyanate (FITC)–conjugated Ulex europaeus Lectin I was from Vector Laboratories (Burlingame, CA). Rhodamine-conjugated phalloidine (R415) was from Molecular Probes (Eugene, OR).
Primary antibodies and secondary reagents
The primary antibodies (Abs) used in this study were mouse anti–human CD31 (hec7, 0.4 μg/mL; courtesy of W. A. Muller [Weill Medical College and Graduate School of Medical Sciences of Cornell University, New York, NY]), mouse anti–human Ki67 (MIB-1, 10 μg/mL; Dako, Glostrup, Denmark), mouse anti–α-smooth muscle actin (M0851, 10 μg/mL; Dako) and rabbit anti-TGFβ1 (transforming growth factor β1; 10 μg/mL; Promega, Phoenix, AZ). Irrelevant control Abs raised to tomato or potato viruses, respectively, were matched for concentration and isotype (courtesy of R. Burns, Scottish Agricultural Science Agency, Edinburgh, United Kingdom). Texas red (TR)–conjugated donkey anti–mouse immunoglobulin G (IgG; H+L) and Streptavidin–carbocyanine 3 (Cy3) were from Jackson ImmunoResearch Laboratories (West Grove, PA), and biotinylated donkey anti–rabbit IgG (H+L) was from Vector Laboratories.
In vivo experiments
Animal experiments were performed in accordance with institutional guidelines and national legislation. Specific pathogen-free (SPF) BALB/c Rag2–/– mice (age, 6-8 weeks; weight, 20-22 g) were purchased from Taconic (Ry, Denmark), and fed SDS Rat and Mouse diet No. 3 (SDS, Essex, United Kingdom) and tap water ad libitum. Anesthesia was induced by intraperitoneal injection of a combination of fentanyl + fluanisone (Hypnorm) + midazolam (Dormicum) solutions.
Alzet osmotic pumps (model 2004; 0.25 μL/h, 28 days) were filled with 200 μL endostatin (7.9 mg/mL) and implanted subcutaneously on the back of anesthetized mice for systemic delivery at approximately 2 μg/h. Human umbilical vein–derived endothelial cells (HUVECs) and nasal polyp–derived microvascular endothelial cells (PMECs) were isolated and cultured as detailed elsewhere.44,46 HUVECs or PMECs suspended in liquid Matrigel were subsequently injected subcutaneously, as detailed in previous work.44 Briefly, endothelial cells (3 × 105) suspended in 200 μL Matrigel were injected in the midline of the abdomen, carefully positioning the needle between the epidermis and the muscle layer. The liquid Matrigel stiffens at body temperature to form a solid plug.
Upon termination of the experiment, the Matrigel plugs were removed by a wide excision of the abdominal wall, including the skin and all muscle layers, and transferred to PBS on ice. Each specimen was then divided into 2 pieces, 1 being immediately snap-frozen (liquid N2) in OCT compound for storage at –70°C until use, the other being fixed for 24 hours in methanol (–20°C) or formalin (4°C) and further processed as follows: 96% ethanol (24 hours, 4°C), absolute ethanol (2 hours, 4°C) twice, xylene twice (20 hours, 4°C and 30 minutes, 22°C) prior to paraffin embedding.
RT-PCR analysis
Matrigel plugs were harvested on ice and separated from surrounding mouse tissue with a scalpel. The plugs were cut into small pieces and digested in collagenase 1 mg/mL and Dispase 2 mg/mL dissolved in endothelial basal medium (EBM; 40 minutes, 37°C) on a magnetic stirrer. The cell suspension was next washed twice with PBS (4°C), sequentially incubated with mouse anti–hCD31 antibody (15 minutes on ice) and para-magnetic Dynabeads coated with rat anti–mouse IgG (20 minutes, 4°C on a roller) before extraction of human endothelial cells. Total RNA isolation and on-column DNase treatment were performed using the RNeasy Mini Kit according to instructions of the manufacturer (Qiagen GmbH, Hilden, Germany). Total RNA was reverse transcribed using Oligo(dT) and Superscript III Reverse Transcriptase (Invitrogen, Carlsbad, CA). Human platelet–derived growth factor-B (PDGFB) and transforming growth factor-β (TGFβ) mRNA transcripts were quantified by real-time polymerase chain reaction (PCR) using the Light Cycler (Roche Diagnostics). TGFβ copy numbers were quantified by using the TGF-β1 kit from Search-LC (GmbH, Heidelberg, Germany) according to instructions of the manufacturer. Human PDGFB-specific transcripts were amplified using 5′-CAT AGA CCG CAC CAA CGC CAA CTT C-3′ as forward primer and 5′-ATC TTT CTC ACC TGG ACA GGT CGC AG-3′ as reverse primer at 3.0 mM MgCl2 and 68°C annealing temperature. Copy numbers were determined using the standard from the PDGF-β LightCycler kit from Search-LC. As internal control for the amount of human RNA input we quantified the human housekeeping gene hydroxymethylbilane synthase using the QuantiTect SYBR Green PCR kit (Qiagen GmbH) at 60°C annealing temperature, 3.5 mM MgCl2 concentration with forward primer 5′-GTA ACG GCA ATG CGG CTG-3′ and reverse primer 5′-GGT ACG AGG CTT TCA ATG-3′.
Immunostaining protocols
All of the following steps were performed at 22°C unless otherwise noted. Frozen tissue sections (8 μm) were air-dried overnight and subsequently acetone-fixed (10 minutes). Paraffin sections (4 μm) were dried for 24 hours at 4°C, dewaxed with xylene (10 minutes), and rehydrated through graded alcohols before staining. Cryosections were incubated with the primary and secondary reagents for 1 hour each, whereas paraffin sections were incubated with primary Abs for 20 hours, secondary reagents for 3 hours, and tertiary reagents for 1 hour, respectively.
Tissue sections were immunostained with a mixture of primary mouse Abs and FITC-conjugated Ulex lectin (10 μg/mL), and then with TR-conjugated donkey anti–mouse IgG. In other protocols we either combined FITC-conjugated Ulex lectin with rhodamine-conjugated phalloidin (1/200). Detection of TGFβ1 was performed by microwave treatment of the tissue sections before incubation with an antibody to TGFβ1 in combination with FITC-conjugated Ulex lectin followed by biotinylated donkey anti–rabbit IgG (7.5 μg/mL) and finally Streptavidin-Cy3 (0.5 μg/mL).
The ApopTag kit was used to detect apoptotic cells in the Matrigel plugs, based on the transferase-mediated deoxyuridine triphosphate (dUTP) nick-end labeling (TUNEL) assay by detection of nuclear DNA fragmentation.47 Peroxidase staining of pretreated cryosections was done as described by the manufacturer's instructions. Positive cells were counted in gels in both treated and untreated groups.
Negative controls were tissue sections incubated with primary irrelevant isotype- and concentration-matched Abs, whereas tissue sections from human tonsils or lingual cancer served as positive controls. For the apoptosis detection kit we used sections of Matrigel plugs from mice receiving serial injections of TNF-α as positive controls. Microscopy was performed with a Nikon E-800 fluorescence microscope (Nikon, Tokyo, Japan) equipped with 10 ×/0.30, 20 ×/0.50, 40 ×/0.75, and 60 ×/0.85 Nikon Plan-Fluor objective lenses and an F-view digital camera controlled by AnalySIS 3.2 software (Soft Imaging System GmbH, Munster, Germany).
In situ determination of PDGFB in human endothelium
One kilobase (kb) digoxigenin (DIG)–labeled sense and antisense riboprobes were generated from the coding region of human PDGFB with a DIG RNA labeling kit according to the manufacturer's directions (Boehringer Mannheim, Mannheim, Germany), and applied to air-dried (60°C, overnight), formalin-fixed paraffin sections (4 μm) after dilution in RiboHybe (Ventana Medical Systems, Illkirch, France). Deparaffinization, pretreatment, and hybridization were performed according to manufacturer's instructions on a Ventana Discovery automated histology robot (Ventana). Pretreatment consisted of mild alkaline protease (0.02 U/mL, 4 minutes, 37°C) and Ribo CC (mild protocol). Hybridization was performed at 60°C (6 hours) and stringency washes with 3 rounds of 2 × saline sodium citrate (SSC; 6 minutes, 60°C). Riboprobes were detected with biotinylated anti-Digoxin (1:500, 32 minutes, 37°C; Jackson Immunoresearch Laboratories) and the BlueMap Kit (Ventana) that develops the nitroblue tetrazolium salt 5-bromo-4-chloro-3-indolyl phosphate (NBC/BCIP) alkaline phosphatase substrate.
ELISA determination of osmotic pump–delivered endostatin
Blood samples were collected after 20, 30, and 40 days from the tail artery. Serum levels of human endostatin were quantified by an ELISA that according to the manufacturer's instructions shows less than 5% cross-reactivity with murine endostatin.
Quantitative analyses of vascular morphogenesis
Vascular structures in the Matrigel plugs were classified as either immature or mature, complex vessels. Immature vessels were defined as vessel structures composed of more than 1 endothelial cell, whereas complex, mature vessels were defined as vessel structures either containing erythrocytes or being associated with α-smooth muscle actin–positive cells at less than 10 μm distance. Vascular morphogenesis was quantified by enumerating the ratio of Σvessel structures to Σsingle cells per cross section and expressing the mean ± SEM obtained for each group of animals. The number of proliferating and apoptotic cells was related to the area of the corresponding Matrigel plug. Enumerations were performed with AnalySIS 3.2 software. Independence between the endostatin-treated groups and the nontreated groups was determined by means of the Mann-Whitney U test. Two-sided tests were performed, and P values less than .05 were considered to be statistically significant. Statistical analyses were implemented by means of SPSS 9.0 (SPSS, Chicago, IL).
Results
Serum levels of endostatin
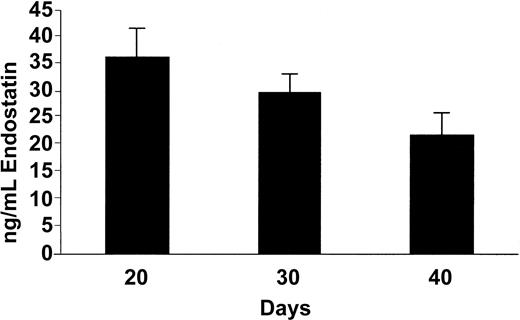
To test the capability of osmotic pumps to deliver endostatin (systemic release of 2 μg/h, resulting in 2 mg/kg/d) throughout the experiment, we measured serum levels of human endostatin by means of the EIA kit, finding that the endostatin levels decreased from 36.4 ± 5.3 ng/mL (mean ± SEM) at day 20 to 29.7 ± 3.6 ng/mL at day 30 (Figure 1), perhaps reflecting the reduction of serum levels upon exhaustion of the pump capacity as they were designed for 28-day delivery. However, endostatin remained detectable at lower levels at day 40 (22.0 ± 3.8 ng/mL). Serum levels from control littermates (pumps releasing saline) were at all time points below the detection limit of the assay (data not shown), which in our hands was estimated at 7 ng/mL.
Serum concentrations of human endostatin. Enzyme-linked immunoassay. Data are expressed as mean value + SEM.
Serum concentrations of human endostatin. Enzyme-linked immunoassay. Data are expressed as mean value + SEM.
Endostatin inhibits vascular morphogenesis of human endothelial cells in vivo
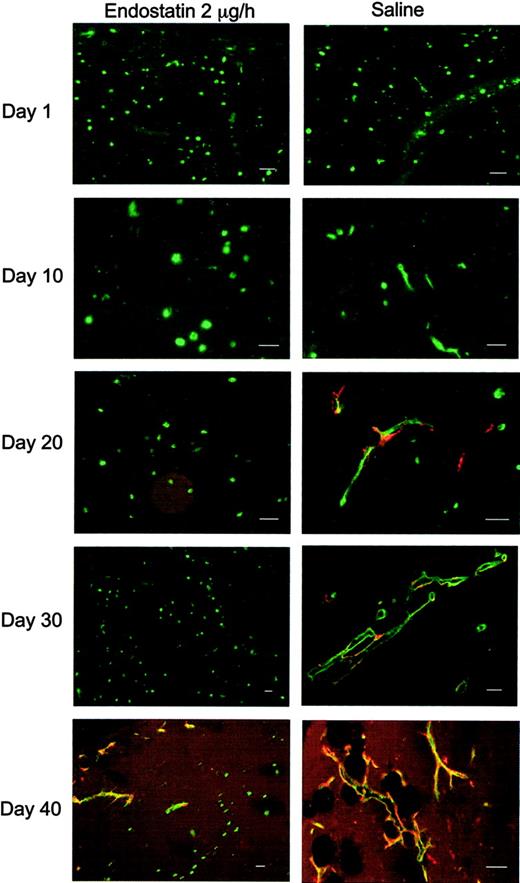
We have previously shown that adoptively transferred HUVECs suspended in Matrigel form functional human vessels that make xenogeneic contact with the murine vascular system, carry mouse blood and recruit vascular smooth muscle cells.44 Human endothelial cells were unambiguously discriminated from murine endothelial cells by Ulex binding to the former. Thus, in the absence of endostatin, single, Ulex-positive HUVECs developed in the predicted manner to initially extend pseudopods and form vessel-like structures within 10 days, predominantly in the periphery of cross-cut matrigel sections. By day 20, 50% of the vessels were considered mature as they were covered by α-smooth muscle actin–positive cells or contained erythrocytes (Figure 2). By day 30 more human vessels contained erythrocytes, and 64.3% were covered by α-smooth muscle actin–positive cells. On day 40 nearly all human vessels were fully mature, containing erythrocytes and surrounded by α-smooth muscle actin–expressing cells (94.5%). By contrast, in the presence of endostatin single HUVECs almost completely failed to migrate and accumulate into vessels (Figure 2), and, at days 20 and 30, only a small percentage of vessel-like structures were found. However, given the limited capacity of the pumps and the sinking endostatin levels, we were not surprised to note assembly of some vessels at day 40, however not by far as many as in the untreated day-40 mice.
Endostatin inhibits capillary morphogenesis. Immunofluorescence staining for α-smooth muscle actin (red) and Ulex lectin-staining (green) of Matrigels from endostatin-treated and control animals. Note the few pericyte-covered vessels in endostatin-treated animals after 40 days. Scalebars, 50 μm.
Endostatin inhibits capillary morphogenesis. Immunofluorescence staining for α-smooth muscle actin (red) and Ulex lectin-staining (green) of Matrigels from endostatin-treated and control animals. Note the few pericyte-covered vessels in endostatin-treated animals after 40 days. Scalebars, 50 μm.
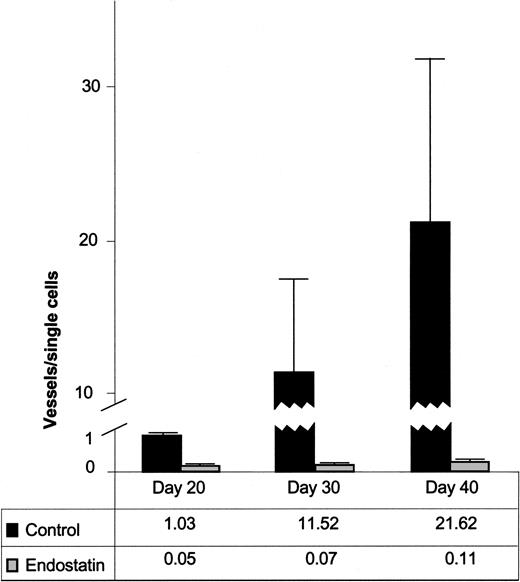
To quantitatively assess the effect of endostatin on vascular morphogenesis, we counted single cells, immature (α-actin–) and mature (α-actin+) vessels of human origin (Ulex+) and related them to the total Matrigel area of the tissue sections. By calculating the ratios of the total number of vessels divided by the number of single cells, we found that the ratio of Σvessels to Σcells in the endostatin-treated group was dramatically lower than in the control group at day 20, 30, and 40 after injection (Figure 3). Thus, the mean ratio at day 20 was 1.03 (range, 1.02-1.04; n = 2) for the control group and 0.05 (range, 0.03-0.07; n = 2) for the endostatin group. By day 30, the mean ratio for the control group was 11.52 (range, 0.40-51.75; n = 6), whereas the ratio in the endostatin-treated mice remained almost unaltered (mean, 0.07; range, 0.03-0.14; n = 6). For mock-treated mice in the day-40 group, the mean was 21.62 (range, 0.56-70.25; n = 6), and for the endostatin-treated mice 0.11 (range, 0.03-0.18; n = 6) (Figure 3). The differences between the endostatin and control groups obtained at day 30 and 40 were significant (P = .004 for both groups) using the Mann-Whitney U test.
Quantitative assessment of capillary morphogenesis in endostatin-treated and control mice. Ratios of total number of vessels (immature and mature) divided by total number of single cells. Data are expressed as mean value + SEM.
Quantitative assessment of capillary morphogenesis in endostatin-treated and control mice. Ratios of total number of vessels (immature and mature) divided by total number of single cells. Data are expressed as mean value + SEM.
To further investigate the effects of endostatin on human endothelial cell function in vivo, we also used microvascular endothelial cells derived from nasal polyps (PMECs) in some experiments. These endothelial cells behaved similarly to HUVECs in that they extended pseudopods at early time points, recruited α-smooth muscle actin–positive cells, and developed into vessels connected to the circulation within 20 days. As shown in this section for HUVECs, vascular morphogenesis was also strongly inhibited for microvascular cells, as only a few vessels were observed in endostatin-treated mice (of which none were connected to the circulation). The ratios of Σvessels to Σcells obtained for PMECs were similar to those calculated for HUVECs in endostatin and control littermates, being reduced by 97.1% and 99.2% in endostatin-treated mice at days 20 and 30, respectively.
Endostatin inhibits the development of a migratory endothelial cell phenotype
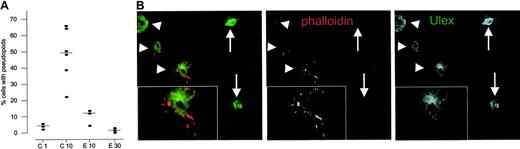
When analyzing the Matrigel plugs at early time points, we found 2 distinct phenotypes of Ulex-positive, single endothelial cells.44 Cells from Matrigel plugs harvested at day 1 were mainly of a round phenotype, and only a small portion of the cells had extended pseudopod-like cytoplasmic extensions (median, 4%; range, 2.4%-5.1%; n = 3; Figure 4A). In control mice, this fraction of pseudopod-extending cells was increased to include 50.2% (range, 22.9%-65.3%; n = 6) of all cells at day 10 but was less prominent or no longer present after 20 days, because at this time point most cells were organized into vascular structures (Figures 2 and 4A).
Endostatin inhibits the extension of F-actin–positive pseudopods in single endothelial cells. (A) Percentage of single cells extending pseudopods in endostatin-treated and control mice. (B) Single endothelial cells are either of an F-actin–expressing, pseudopod-extending phenotype (arrowheads) or a round phenotype with a low or absent expression of F-actin (arrows). Inserts show higher magnification. Scalebars, 50 μm.
Endostatin inhibits the extension of F-actin–positive pseudopods in single endothelial cells. (A) Percentage of single cells extending pseudopods in endostatin-treated and control mice. (B) Single endothelial cells are either of an F-actin–expressing, pseudopod-extending phenotype (arrowheads) or a round phenotype with a low or absent expression of F-actin (arrows). Inserts show higher magnification. Scalebars, 50 μm.
To associate the apparently migrating endothelial cell phenotype (ie, pseudopod extension) with a molecular marker of cell migration, we stained tissue sections with phalloidin to detect polymerized F-actin, as assembly of actin polymers appears to be an absolute requirement for pseudopod extension and cell migration.48-50 F-actin was strongly expressed in single cells that extended pseudopods at days 1 and 10, whereas cells of the round phenotype at all time points had a weak or almost absent expression of F-actin (Figure 4B). Moreover, mature vessel structures strongly expressed F-actin at all time points (data not shown). We therefore conclude that single endothelial cells in Matrigel plugs are of 2 distinct phenotypes: the first being a nonmigratory phenotype that extends no pseudopods and is F-actin–low, and the second a migratory phenotype that strongly expresses F-actin and avidly extends pseudopods. This interpretation is well in line with the recent description of an F-actin–positive, migrating endothelial tip cell phenotype that is seen at the tips of vascular sprouts during retinal angiogenesis51 as well as in sprouting angiogenesis of the zebra fish embryo.52
These 2 distinct endothelial phenotypes were also found in Matrigel plugs in mice treated with endostatin, but in these animals, single cells at day 10 were mainly of the round, F-actin–negative phenotype, as only 13% of the cells extended pseudopods (median range, 4.9%-13.5%; n = 3). This was even more pronounced at day 20 and beyond when gels of endostatin-treated animals remained populated by single cells of which only 1% to 3% were of the pseudopod-extending phenotype (Figure 4A).
Endostatin inhibits maturation of human vessels
In addition to acting as an antiangiogenic agent by dramatically reducing the number of human vascular constructs, endostatin also altered their subsequent maturation into functional vessels. Thus, endostatin also affected the occurrence of α-smooth muscle actin–expressing cells, as these were not observed at day 20, and occurred only on a small number of vessels at day 30 (28.6% in the presence of endostatin versus 64.3% in the untreated group, median values; n = 6; P = .091; Figure 5). Furthermore, although a substantially higher fraction of human vessels was covered by α-smooth muscle actin–positive cells at day 40 than at day 30 (83.1% versus 28.6%), it was nevertheless a lower fraction than in their untreated counterparts (94.5%, n = 6, P = .089) (Figure 5). Moreover, human vessels containing erythrocytes were never observed in endostatin-treated mice.
Endostatin inhibits recruitment of α-smooth muscle actin-positive cells to human vessels in Matrigel. Percentage of vascular constructs covered by α-smooth muscle actin-positive cells (median and counts from individual gels).
Endostatin inhibits recruitment of α-smooth muscle actin-positive cells to human vessels in Matrigel. Percentage of vascular constructs covered by α-smooth muscle actin-positive cells (median and counts from individual gels).
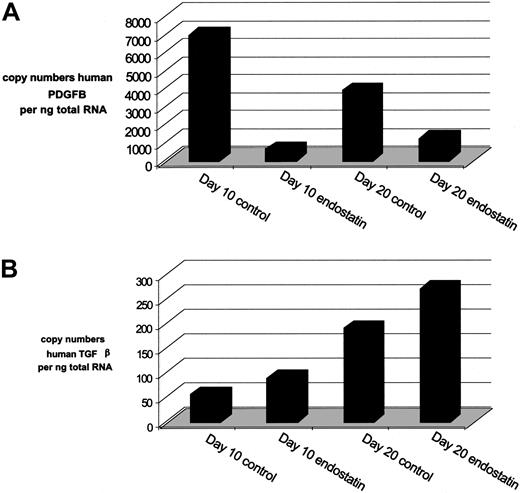
To investigate the possible mechanisms that may have reduced the ratio of α-smooth muscle actin–positive cell-covered vessels due to endostatin treatment we analyzed endothelial cell–expressed PDGFB (currently considered the most important recruiter of pericytes during angiogenesis53,54 ), by means of in situ hybridization. As our probe revealed some hybridization to mouse PDGFB, we costained with FITC-labeled Ulex lectin to relate the signal to human endothelium in the Matrigel. While PDGFB transcripts were found in most vascular constructs in both groups, the single, round cells that dominate the gels of endostatin-treated mice were negative (Figure 6A-B). To more accurately quantify the mRNA levels of hPDGFB at days 10 and 20 we also performed quantitative PCR based on mRNA from immunopurified human endothelial cells of enzyme-digested Matrigel plugs. In line with our in situ observations, we found that endostatin reduced the copy numbers of hPDGFB by 90% and 70% at days 10 and 20, respectively (Figure 7A).
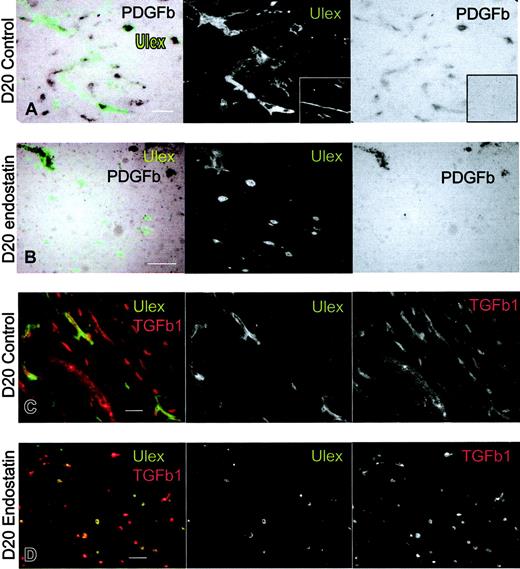
Endostatin inhibits the expression of endothelial PDGFB but not TGFβ1. Combined in situ hybridization of PDGFB (black) and immunofluorescense staining with Ulex lectin (green) in Matrigels from (A) control and (B) endostatin-treated animals at day 20. Note that the probe PDGFB also hybridizes to cells of mouse origin. Insert shows sense probe to PDGFB. (C-D) Immunofluorescence staining of TGFβ1 (red) and Ulex lectin (green) in Matrigels from day-20 control (C) and endostatin-treated (D) mice. Note that the antibody to hTGFβ1 also recognizes mouse TGFβ. Scalebars, 50 μm.
Endostatin inhibits the expression of endothelial PDGFB but not TGFβ1. Combined in situ hybridization of PDGFB (black) and immunofluorescense staining with Ulex lectin (green) in Matrigels from (A) control and (B) endostatin-treated animals at day 20. Note that the probe PDGFB also hybridizes to cells of mouse origin. Insert shows sense probe to PDGFB. (C-D) Immunofluorescence staining of TGFβ1 (red) and Ulex lectin (green) in Matrigels from day-20 control (C) and endostatin-treated (D) mice. Note that the antibody to hTGFβ1 also recognizes mouse TGFβ. Scalebars, 50 μm.
Endostatin reduces mRNA expression of hPDGFB but not hTGFβ. Human PDGFB and TGFβ mRNA expression determined by real-time PCR analysis using the Light Cycler on cDNA synthesized from RNA of human CD31+ cells isolated from control- or endostatin-treated Matrigel plugs. (A) Copy numbers of human PDGFB transcripts in CD31+ cell fraction per nanogram RNA. (B) TGFβ mRNA copy numbers in CD31+ cells per nanogram RNA.
Endostatin reduces mRNA expression of hPDGFB but not hTGFβ. Human PDGFB and TGFβ mRNA expression determined by real-time PCR analysis using the Light Cycler on cDNA synthesized from RNA of human CD31+ cells isolated from control- or endostatin-treated Matrigel plugs. (A) Copy numbers of human PDGFB transcripts in CD31+ cell fraction per nanogram RNA. (B) TGFβ mRNA copy numbers in CD31+ cells per nanogram RNA.
We also analyzed the expression of TGFβ, which is known to be an important mediator of mural cell differentiation.54 We first costained with Ulex and an antibody to TGFβ1, finding that all human endothelial cells were positive at all stages and in both groups (Figure 6C-D). Furthermore, we analyzed the mRNA levels of hTGFβ, finding the levels to be considerably lower than those of hPDGFB in all samples, and slightly increased in endostatin-treated mice compared with the nontreated mice (Figure 7B).
To control for the amount of human mRNA following isolation we measured the human housekeeping gene hydroxymethylbilane synthase and found no differences between the samples (data not shown).
Endostatin has no effect on proliferation and apoptosis of endothelial cells in vivo
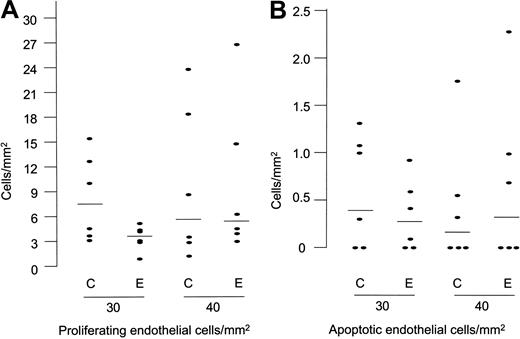
To test whether HUVEC proliferation was affected by endostatin treatment, we stained for Ki67, a nuclear antigen expressed by proliferating cells, but down-regulated in cells re-entering the G0 phase, and therefore suitable for comparing the growth fraction in the 2 groups.55 To this end, we counted the number of Ki67-positive cells and related them to the corresponding gel areas. We subsequently tested independence of the obtained ratios and found no differences between the endostatin- and saline-treated groups at days 10 (n = 1), 20 (n = 2), 30 (n = 6; P = .15), and 40 (n = 6; P = .63) (Figure 8A).
Endostatin has no obvious in vivo effect on endothelial cell proliferation and apoptosis. (A) Number of proliferating endothelial cells (Ki67 positive) per square millimeter cross-cut Matrigel plug area (median and counts from individual gels). (B) Number of apoptotic endothelial cells divided on the Matrigel plug area (median and counts from individual gels).
Endostatin has no obvious in vivo effect on endothelial cell proliferation and apoptosis. (A) Number of proliferating endothelial cells (Ki67 positive) per square millimeter cross-cut Matrigel plug area (median and counts from individual gels). (B) Number of apoptotic endothelial cells divided on the Matrigel plug area (median and counts from individual gels).
To determine whether endostatin induced apoptosis of HUVECs in the Matrigel plug we used the ApopTag kit which detects DNA fragmentation in cells that are apoptotic or preapoptotic using the TUNEL method.47 When counting positive cells in the Matrigel plug and relating them to the cross-cut area, we found the number of apoptotic cells in the plugs to be low (0-5 cells per cross-section at all time points), and a difference between the endostatin- and the saline-treated group was not observed at days 10 (n = 1), 20 (n = 2), 30 (n = 6; P = .41), and 40 (n = 6; P = .80) (Figure 8B).
Discussion
This study shows that endostatin efficiently inhibits the assembly of human endothelial cells into complex vessels. By introducing sustained release of endostatin to endothelial cells in Matrigel, we evaluated the effect of the protein at a given dose by measuring and comparing the serum levels with immunohistochemical findings in the course of 40 days. During the first 30 days of endostatin treatment (at serum levels in the range of 30-35 ng/mL), the normal assembly of endothelial cells in Matrigel was dramatically disturbed as only few vascular structures were detectable, of which none were developed into mature vessels (ie, surrounded by perivascular cells). However, proliferation or apoptosis of HUVECs in the Matrigel was not influenced by endostatin. Apossible explanation might be that the local concentration of endostatin in our Matrigel plugs was insufficient, as those in vitro studies which reported an effect on apoptosis and mitosis exposed their endothelial cell cultures to substantially higher doses of endostatin than we observed in the serum of our mice.10,26,37,38 This interpretation would also fit in vitro studies which used endostatin in the nanogram range, and in which case only migration was affected.19,36
The effect of endostatin on endothelial cell migration deserves further discussion, in particular because lack of vascular assembly does not exclude the possibility that single endothelial cells were still capable of random, nondirected migration. However, the strong reduction of cells with a migratory endothelial cell phenotype (F-actin positive, pseudopod-extending) in endostatin-treated animals is good evidence that endostatin indeed prevented random cell migration. Moreover, our observation is well in line with several in vitro studies that have shown effects on actin cytoskeletal organization in response to endostatin. For example, Wickstrom et al33,56 have shown that endostatin leads to disassembly of actin stress fibers, whereas Dixelius et al30 reported an initial increase in actin polymerization followed by a decrease to initial levels. Moreover, Keezer et al32 showed that endostatin reduced the levels of the actin-depolymerizating protein cofilin and also induced decreased activity of the actin-capping protein hsp27 through phosphorylation, leading to a weakening of actin stress fibers. Although these reports used different sets of experimental in vitro conditions, they nevertheless fit nicely with our data showing that endostatin in a 3D gel in vivo situation will maintain cells in a state with low numbers of stress fibers and apparently reduce their ability to extend migratory cellular protrusions.
Our study provides new insight to how endostatin affects in vivo angiogenesis because previous detailed analyses in growing tumors or healing wounds have either failed to detect an effect of endostatin,41 observed subtle signs of vascular dysfunction42 or a reduction of global parameters such as vascular density, perfusion, and vessel diameter.57 Our finding that endostatin also affects mural cell recruitment and maturation into circulated vascular constructs adds substantially to the current understanding of how endostatin works in vivo. Moreover, by showing that endothelial cells in endostatin-treated mice are strongly restricted in their ability to synthesize PDGFB, we provide a molecular hypothesis in line with the well-documented role of PDGFB on mural cell recruitment.53,54 However, we cannot rule out that endostatin also has a direct effect on perivascular precursor recruitment. Moreover, our data are at variance with those of 2 recent studies in which cultured endothelial cells were exposed to endostatin but did not alter their expression level of PDGFB27,34 (A. Abdollahi, personal e-mail communication, September 2004). Thus, extensive comparison of in vivo and in vitro expression profiles associated with endostatin treatment deserves further attention.
The vascular morphogenesis that became evident after 40 days can be explained by reduced levels of endostatin due to the exhaustion of the osmotic pumps that were designed for delivery up to 28 days. However, the endostatin levels measured in serum on day 40 were only reduced about 40% from those found on day 20. Given that the half-life of human recombinant endostatin in humans has been found to be 10.7 hours,58 and that a similar half-life might be expected in mice, one may assume that the delivery of endostatin may indeed have exceeded 28 days, perhaps with decreasing amounts. We, therefore, cannot exclude that the remodeling observed might reflect an override of the mechanisms inhibited by endostatin by exploiting redundant, endostatin-insensitive molecular pathways for vascular assembly.
While this study is focused on observing human endothelial cell behavior in the presence of endostatin, it is perfectly possible that human endostatin also affects the behavior of other murine cells, including endothelial cells and macrophages. To this end, the density of murine vessels does not appear to change substantially at the edge of the Matrigel in the presence of endostatin (D.K. S. and G.H., unpublished observations, 2002). However, endostatin does affect ingrowth of murine vessels in a matrigel assay that lacks human endothelial cells,59 and it is, therefore, tempting to speculate that the complete absence of human vessels that were filled with mouse erythrocytes may in part be a direct effect of endostatin on murine endothelial cells.
While our model appears to reflect several aspects of angiogenesis well and may open new venues to observing this process in vivo, we do realize that the initial distance between single endothelial cells may not accurately reflect the in vivo conditions. Thus, it remains unknown whether, for example, the lack of agreement with other reports on endothelial cell proliferation may also have a basis in this difference. Nevertheless, our surrogate model of human angiogenesis does provide opportunities in terms of reproducibility and controlled experimental conditions that clinical or experimental tumor samples cannot afford.
In conclusion, this study strongly indicates that the in vivo effect of endostatin on human endothelial cell behavior is several fold as we found migration, vascular morphogenesis, and perivascular cell recruitment to be strongly affected. However, it remains to be seen whether endostatin administered past 30 to 40 days, for example by exchanging the osmotic pump, maintains its angiostatic effect. Furthermore, it will be interesting to examine the effect of other dose levels and regimens in our model, including for example the effect on immature vessels that have assembled in the absence of endostatin. Finally, the apparent ability of endostatin to affect vascular maturation deserves increased focus.
Prepublished online as Blood First Edition Paper, October 5, 2004; DOI 10.1182/blood-2004-03-1164.
Supported by the Norwegian Cancer Society (B02 085), the Research Council of Norway (13932/300) Anders Jahre's Fund, and the Independent Order of Odd Fellows. D.K.S. and M.J.T.V. are research Fellows of the Norwegian Cancer Society, and G.H. is a Career Investigator of the Research Council of Norway.
M.J.T.V. and D.R.S. contributed equally to this paper.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We would like to thank the technical staff at the Institute of Pathology for expert technical assistance and to Kari Alitalo, Per Brandtzaeg, Jim Campbell, Ole Petter Clausen, Finn Eirik Johansen, and Axel Kuchler for helpful discussions and critical review of the manuscript. We are also grateful to Karl Schenck for providing the antibody to TGFβ1, to EntreMed for supporting us with endostatin and the endostatin ELISA kit, to Christer Betsholtz and Alexandra Abramsson for PDGFB probes, to Espen Baekkevold for assistance with riboprobe constructs, to the staff at the Department of Gynecology and Obstetrics, Rikshospitalet, for providing umbilical cords, and the staff at the Section of Medical Statistics, University of Oslo, for the statistical calculations and advice.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal