Abstract
The pigment epithelium–derived factor (PEDF) belongs to the superfamily of serine protease inhibitors (serpin). There have been 2 distinct functions attributed to this factor, which can act either as a neurotrophic or as an antiangiogenic factor. Besides its localization in the eye, PEDF was recently reported to be present also in human plasma. We found that PEDF purified from plasma is a phosphoprotein, which is extracellularly phosphorylated by protein kinase CK2 (CK2) and to a lesser degree, intracellularly, by protein kinase A (PKA). CK2 phosphorylates PEDF on 2 main residues, Ser24 and Ser114, and PKA phosphorylates PEDF on one residue only, Ser227. The physiologic relevance of these phosphorylations was determined using phosphorylation site mutants. We found that both CK2 and PKA phosphorylations of PEDF markedly affect its physiologic function. The fully CK2 phosphorylation site mutant S24, 114E abolished PEDF neurotrophic activity but enhanced its antiangiogenic activity, while the PKA phosphorylation site mutant S227E reduced PEDF antiangiogenic activity. This is a novel role of extracellular phosphorylation that is shown here to completely change the nature of PEDF from a neutrophic to an antiangiogenic factor.
Introduction
The pigment epithelium–derived factor (PEDF) was originally identified in conditioned medium of fetal human retinal pigment epithelium cell cultures.1,2 It shares sequence and structure homology with members of the superfamily of serine protease inhibitors (serpin),3 however, it does not serve as an inhibitor of any protease activity.4 PEDF was first described as a neurotrophic factor that induces a specific neuronal phenotype in retinoblastoma cells.3 The neurotrophic activity of PEDF was also demonstrated by its ability to support neuronal survival,5 and its ability to protect neurons against neurotoxic effects.6 Structure-function studies have shown that this neurotrophic activity is exerted by the amino terminal edge (44-mer, amino acid residues 78-121) of the human PEDF, and that it is mediated through an approximately 80 kDa membranal receptor, which is abundant in retinoblastoma cells7 and in neural retinal cells.8
Beside its neurotrophic activity, PEDF was recently demonstrated as one of the most potent natural inhibitors of angiogenesis.9 Thus, it was found that PEDF inhibits not only basic fibroblast growth factor (bFGF)–induced migration of endothelial cells under in vitro conditions, but also bFGF-induced neovascularization in an avascular rat cornea. Furthermore, addition of anti-PEDF antibodies (Abs) to rat corneas was found to stimulate the invasion of new vessels into these corneas,9 suggesting that PEDF plays a physiologic regulatory role in retinal angiogenesis. Additionally, PEDF was shown to be a very potent inhibitor of neovascularization in a murine model of ischemia-induced retinopathy.10 The antiangiogenic activity of PEDF was associated with endothelial cell apoptosis,10 probably by increasing Fas ligand (FasL) mRNA and surface FasL in these cells.11
It was recently reported that besides its expression in multiple sites in the eye, PEDF is also present in human plasma at a physiologically relevant concentration.12 In the last decade, several reports have described the possibility that protein kinases might function as a regulatory device not only intracellularly but also in the cell exterior (for review, see Redegeld et al13 and Korc-Grodzicki et al14 ). These reports described the presence of membrane-bound ectoprotein kinases (on the outer cell surface) and soluble secreted exoprotein kinases (detached from the cell). Additionally, it was shown that these ectoprotein or exoprotein kinases do have several substrates in the circulating blood, including the coagulation cofactors Va and VIII15,16 as well as vitronectin.14 The main protein kinases that seem to exert exokinase activity are protein kinase A (PKA14,17 ) and protein kinase CK2 (CK213 ). For example, it was shown that vitronectin is phosphorylated by PKA,14 and this phosphorylation modulates its interaction with plasminogen activator inhibitor 1 (PAI-1).18 In addition, phosphorylation by CK2 changes intracellular signaling by vitronectin,19 indicating that both PKA and CK2 play an important regulatory role in the circulating blood.
Since PEDF was found to be present in human plasma, we aimed to determine whether PEDF in circulating blood serves as a substrate for exokinases. We report here that PEDF purified from human plasma is a phosphoprotein. It is phosphorylated in the serum mainly by CK2 on 2 main residues, Ser24 and Ser114, but also by PKA on Ser227. We found that PEDF is functionally modulated by extracellular phosphorylation. The CK2 phosphorylated PEDF had a reduced neurotrophic activity, while its antiangiogenic activity was significantly increased. On the other hand, PKA phosphorylation reduced the PEDF antiangiogenic activity but had only slight effect on its neurotrophic activity. This is a novel role of extracellular phosphorylation that is shown here to completely change the nature of the physiologic activity of a circulating protein.
Materials and methods
Reagents and antibodies
Recombinant human CK2 was from Calbiochem (Darmstadt, Germany); the catalytic subunit of PKA was purified as described.20 Active extracellular signal-regulated kinase (ERK) was purified as described.21 Full-length human PEDF cDNA was provided by Dr N. Bouck (Northwestern University, Chicago, IL). Phosphothreonine Ab was from Zymed Laboratories (San Francisco, CA). Phosphotyrosine Ab (PY99) was from Santa Cruz Biotechnology (Santa Cruz, CA). Phosphorylated ERK (pERK), general ERK (gERK) and phosphoserine Abs, bFGF, α-casein, and dephosphorylated casein were from Sigma (Rehovot, Israel). Polyclonal Ab against PEDF was developed by the Ab Unit of the Weizmann Institute of Science.
Cell cultures
Human Y-79 retinoblastoma cells (American Type Culture Collection [ATCC], Manassas, VA) were grown in modified essential medium (MEM) supplemented with 2 mM l-glutamine and 15% fetal calf serum (FCS). HEK-293T cells were cultured in Dulbecco modified Eagle medium (DMEM) F-12 supplemented with 10% FCS. Human umbilical vein endothelial cells (HUVECs) were grown in M-199 supplemented with 20% FCS, 25 μg/mL endothelial cell growth supplement (ECGS) mitogen (BT-203; Biomedical Technologies, Stoughton, MA), and 5 U/mL heparin.
Construction of recombinant PEDF (rPEDF) mutants
Full-length PEDF cDNA was used as a template for oligonucleotide site-directed mutagenesis kit (Clontech, Palo Alto, CA). Pure polymerase chain reaction (PCR) products digested by HindIII and EcoRI were ligated into the multicloning site of pcDNA3. DNA sequencing analysis confirmed the nucleotide sequence of the PEDF mutants.
Transient expression of mutants in HEK-293T cells
pcDNA3 carrying mutants were introduced into HEK-293T cells using the LipofectAMINE reagent (Life Technologies, Grand Island, NY) according to the manufacturer's instructions. The transfected cells were serum starved (3 days, serum-free), after which the PEDF mutants were purified on a Ni+2 column (Pharmacia Biotech, Uppsala, Sweden) according to the manufacturer's instructions.
Purification of PEDF from human plasma
plPEDF was purified from human citrated plasma (1 L) by a 9% to 20% polyethylene glycol (PEG) cut followed by diethylamino ethanol (DEAE)–Sephacel column (2.9 × 40 cm) and heparin agarose column that was developed stepwise. The fractions were pooled (∼ 20 mL), dialyzed against buffer D (20 mM Tris HCl, pH 7.4), and applied onto a Mono Q-FPLC column (1 mL; Pharmacia Biotech, Uppsala, Sweden), which was developed with a linear NaCl gradient in buffer D. PEDF was eluted at 0.2 M NaCl and usually yielded 1 mg pure PEDF (4°C all steps).
Alkaline phosphatase treatment of PEDF
Recombinant PEDF (50 μg/mL) or plPEDF (50 μg/mL) were incubated with alkaline phosphatase conjugated to acrylic beads (50 U/mL) or with sepharose CL-4B beads as control (45 minutes, 30°C). Beads were pre-equilibrated with bovine serum albumin (BSA, 1 mg/mL), Tris-HCL (50 mM, pH 8.0), and EDTA (ethylenediaminetetraacetic acid, 0.1 mM). Reaction was arrested by centrifugation. The supernatant was further subjected to in vitro phosphorylation.
In vitro phosphorylation of PEDF
The phosphorylation assay (40 μL) contained either rPEDF, plPEDF, or rPEDF mutants (50 μg/mL). For CK2, the constituents were CK2 (4 μg/mL), glycerol (2%), NaCl (20 mM), β-mercaptoethanol (0.1 mM), MgCl2 (20 mM), [γ32P]–adenosine triphosphate (ATP, 10 μM), poly-l-lysine (200 nM), and Tris-HCL (50 mM, pH 7.4). For PKA, the constituents were pure catalytic subunit of PKA (2.5 μg/mL), MgCl2 (10 mM), heparin (50 μg/mL), [γ32P]-ATP (10 μM), and Tris-HCL (50 mM, pH 6.5). For human plasma, the constituents were phosphatase-treated PEDF (30 μg/mL), fresh human plasma, MgCl2 (20 mM), [γ32P]-ATP (10 μM), and Tris (50 mM, pH 7.4), with or without PKA inhibitor (PKI, 1 μg/mL) or heparin (100 μg/mL). Reactions were for 45 minutes at 30°C. Then, boiled sample buffer was added, and the samples were subjected to 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE).
Determination of ERK phosphorylation
Serum-starved cells were treated with rPEDF, plPEDF, or the various rPEDF mutants (10 nM unless otherwise specified) for the indicated times. Following stimulation, pERK and gERK were detected using the appropriate Abs.
Neurite outgrowth assay
Human Y-79 retinoblastoma cells were assayed for neurite outgrowth as previously described.22 Briefly, 1 mL of a Y-79 cell suspension (2.5 × 105 cells/mL) was incubated with rPEDF, plPEDF, or the various rPEDF mutants (20 nM) in the cell's medium. After 7 days, the cells were transferred to poly-d-lysine–coated plates, and their neurite outgrowth was monitored by light microscopy at various periods of time.
Aortic ring assay
The aortic ring assay was modified from Nicosia and Ottinetti.23 Briefly, thoracic aortas were dissected from 10- to 12-week-old BALB/C mice and transferred to a Petri dish containing BIO-MPM-1. After removing excess perivascular tissue, transverse cuts of 1-mm long were made. The rings were embedded in collagen (prepared as in Strom and Michalopoulos24 ) mix (7 parts collagen, 1 part 10 × MEM, and 2 parts 0.15-M NaHCO3, 800 μL) in 24-well plates. Medium (500 μL BIO-MPM-1 containing penicillin-streptomycin and the examined reagent) was added to the embedded rings, and the plates were incubated at 37°C in a humidified incubator. Medium containing reagents was replaced 3 times a week. After 10 to 12 days, the rings were fixed with 4% formaldehyde and stained with crystal violet (0.02%). The effect of each factor was examined in 2 wells (4 rings) per assay, and was repeated at least 3 times.
Matrigel plug angiogenesis assay
Matrigel (0.5 mL/mouse; BD Biosciences, Bedford, MA) containing bFGF (300 ng/mL), with or without PEDF (20 nM), was injected subcutaneously into the flank of 8-week-old nude mice as described.25 On day 7, mice were killed, and plugs were removed, fixed (4% formaldehyde), paraffin embedded, and sectioned. Sections were stained using hematoxylin-eosin (H&E). Endothelial cells/microvessels infiltrating the Matrigel were confirmed by Masson trichrome staining.
Results
PEDF in plasma is a phosphoprotein
PEDF, which was identified as a neurotrophic and antiangiogenic factor in the eye, was recently found to be present also in circulating blood.12 Since it was demonstrated that exokinases are able to phosphorylate plasma proteins, we studied whether PEDF can be a target for phosphorylation by these kinases. The 2 forms of PEDF used in the study were PEDF purified from human plasma (plPEDF) and recombinant PEDF (rPEDF), which was expressed in HEK-293T cells and purified from the serum-free medium of these cells. To examine whether plPEDF is indeed a phosphoprotein, we first immunoblotted samples of plPEDF and rPEDF with various antiphospho amino acid Abs. Both proteins were specifically recognized by antiphospho-Ser Ab but not by antiphospho-Thr or by antiphospho-Tyr Abs (Figure 1A). As positive controls, we used active phosphorylated ERK (pERK), which was recognized both by antiphospho-Tyr and antiphospho-Thr, and α casein, which was recognized only by antiphospho-Ser Ab. We therefore concluded that plPEDF and rPEDF are phosphorylated on Ser residue(s).
PEDF in plasma is a phosphoprotein. (A) Aliquots of rPEDF, plPEDF active (phosphorylated) ERK, phosphorylated α-casein (phos cas), and dephosphorylated α-casein (dephos cas) were subjected to 10% SDS-PAGE (1 μg/lane) and immunoblotted with antiphospho-Ser, -Thr, or -Tyr Abs in the presence or absence of the appropriate phosphorylated amino acid (2.5 mM). As a control, the samples were blotted with anti-PEDF, anti–phosphorylated ERK (αpERK), and anti–general ERK (αgERK) Abs, or stained with gel code for the α-casein. (B) rPEDF (2 μg) or plPEDF (2 μg) were incubated with alkaline phosphatase (APase) conjugated to acrylic beads (2 U) or with protein A sepharose CL-4B (1.6 mg) for 45 minutes at 30°C. Following incubation, samples were centrifuged in order to remove the phosphatase, and the supernatants were subjected to in vitro CK2 or PKA phosphorylation as described in “Materials and methods.” Phosphorylated products were analyzed by 10% SDS-PAGE and blotting, followed by exposure to autoradiography (Auto, top panel) and immunoblot with anti-PEDF Ab (bottom panel). (C) Quantitative analysis of the experiment depicted in panel B is presented as a mean ± SD (n = 4). (D) PEDF purified from human plasma was subjected to alkaline phosphatase treatment as described in “Materials and methods”. Thereafter, aliquots (1.5 μg) were incubated with fresh human plasma (8 μL) and [γ32P]-ATP (10 μM, 6 Ci/mmol [222 GBq/mmol]) in the presence or absence of PKA inhibitor (PKI, 1 μg/mL) or heparin (100 μg/mL). Control samples were subjected to in vitro CK2 or PKA phosphorylation as described in panel B. Phosphorylated products were analyzed as described in panel B. Vn indicates plasma vitronectin.
PEDF in plasma is a phosphoprotein. (A) Aliquots of rPEDF, plPEDF active (phosphorylated) ERK, phosphorylated α-casein (phos cas), and dephosphorylated α-casein (dephos cas) were subjected to 10% SDS-PAGE (1 μg/lane) and immunoblotted with antiphospho-Ser, -Thr, or -Tyr Abs in the presence or absence of the appropriate phosphorylated amino acid (2.5 mM). As a control, the samples were blotted with anti-PEDF, anti–phosphorylated ERK (αpERK), and anti–general ERK (αgERK) Abs, or stained with gel code for the α-casein. (B) rPEDF (2 μg) or plPEDF (2 μg) were incubated with alkaline phosphatase (APase) conjugated to acrylic beads (2 U) or with protein A sepharose CL-4B (1.6 mg) for 45 minutes at 30°C. Following incubation, samples were centrifuged in order to remove the phosphatase, and the supernatants were subjected to in vitro CK2 or PKA phosphorylation as described in “Materials and methods.” Phosphorylated products were analyzed by 10% SDS-PAGE and blotting, followed by exposure to autoradiography (Auto, top panel) and immunoblot with anti-PEDF Ab (bottom panel). (C) Quantitative analysis of the experiment depicted in panel B is presented as a mean ± SD (n = 4). (D) PEDF purified from human plasma was subjected to alkaline phosphatase treatment as described in “Materials and methods”. Thereafter, aliquots (1.5 μg) were incubated with fresh human plasma (8 μL) and [γ32P]-ATP (10 μM, 6 Ci/mmol [222 GBq/mmol]) in the presence or absence of PKA inhibitor (PKI, 1 μg/mL) or heparin (100 μg/mL). Control samples were subjected to in vitro CK2 or PKA phosphorylation as described in panel B. Phosphorylated products were analyzed as described in panel B. Vn indicates plasma vitronectin.
The existence of an extracellular PKA as well as CK2 activities is well documented.14,16 Analysis of the primary amino acid sequence of PEDF revealed the existence of several putative phosphorylation sites for CK2, as well as for PKA. In order to examine whether PEDF can be phosphorylated by one of these protein kinases, we have pretreated rPEDF and plPEDF with immobilized alkaline phosphatase prior to in vitro phosphorylation reaction by CK2 and PKA. Phosphorylated products were subjected to SDS-PAGE followed by Western blot, and the membrane used was first exposed to autoradiography and then immunoblotted with anti-PEDF Ab. Pretreatment of plPEDF with alkaline phosphatase (Figure 1B) significantly increased CK2, and to a lesser extent PKA phosphorylation of the protein. The PKA and CK2 phosphorylation of rPEDF following phosphatase treatment was also increased, but not as significantly as plPEDF (Figure 1C).
To further verify that CK2 phosphorylation of PEDF can occur in plasma, we pretreated plPEDF with alkaline phosphatase following its phosphorylation by fresh human plasma. A phosphorylated product that corresponds exactly to PEDF was detected by the autoradiogram (Figure 1D, left panel). Heparin, which is an inhibitor of CK2,26 and PKI,27 which inhibits PKA, inhibited this reaction (Figure 1D, right panel). Taken together, our results indicate that PEDF is phosphorylated in the circulating blood on the CK2 sites. The small amount of phosphorylation in the secreted rPEDF may be a result of cellular phosphorylation.
CK2 and PKA phosphorylate PEDF in vitro
After showing that plPEDF is a phosphoprotein that can be phosphorylated by CK2 and PKA, we undertook to further analyze these phosphorylations. Thus, rPEDF and plPEDF were incubated with CK2 and [γ32P]-ATP, with an increasing concentration of poly-l-lysine, which activates CK2 in vitro.28 Both rPEDF and plPEDF were phosphorylated by CK2 (Figure 2A), and as reported for calmodulin,28 the phosphorylation of PEDF was dependent on the presence of poly-l-lysine. Additionally, CK2 phosphorylation of rPEDF was stronger than the phosphorylation of an identical amount of plPEDF (Figure 2A), indicating that some of the plPEDF sites are already phosphorylated. Heparin was found to inhibit CK2 phosphorylation of PEDF (Figure 2B).
CK2 and PKA phosphorylate PEDF in vitro. (A) rPEDF and plPEDF were incubated with CK2 holoenzyme, [γ32P]-ATP, and increasing concentrations of poly-L-lysine (PLL) as described in “Materials and methods.” As a control, rPEDF and plPEDF were incubated in the same mix in the absence of CK2. After 45 minutes at 30°C, the reaction was arrested by boiling for 5 minutes in sample buffer, and the samples were subjected to 10% SDS-PAGE. The gel was stained with Coomassie blue (Coom, bottom panel), dried, and subjected to autoradiography (Auto, top panel). (B) rPEDF and plPEDF (50 μg/mL) were incubated with CK2 holoenzyme (4 μg/mL), [γ32P]-ATP (10 μM, 6 Ci/mmol [222 GBq/mmol]), poly-l-lysine (200 nM), and increasing concentrations of heparin (Hep). Phosphorylation and analysis were performed as in panel A. (C) rPEDF and plPEDF (50 μg/mL) were incubated with the pure catalytic subunit of PKA (2.5 μg/mL), heparin (50 μg/mL), and [γ32P]-ATP (10 μM, 6 Ci/mmol [222 GBq/mmol]). Phosphorylation and analysis were conducted as in panel A. (D) rPEDF was digested with trypsin as described in “Materials and methods.” At the indicated times, aliquots were removed from the reaction mixture and centrifuged, and sample buffer was added to the supernatant. Samples were boiled and subjected to 12.5% SDS-PAGE followed by silver stain. Right panel: rPEDF was phosphorylated by CK2 and loaded on a G25 Sephadex column to remove the excess of [γ32P]-ATP. The eluted fraction was then subjected to trypsin digestion and subjected to 12.5% SDS-PAGE followed by autoradiography. (E) Alignment of the tryptic peptides revealed by mass spectrometry and N-terminus sequence analysis that were obtained from the trypsin-digested fragments of rPEDF within a schematic representation of PEDF.
CK2 and PKA phosphorylate PEDF in vitro. (A) rPEDF and plPEDF were incubated with CK2 holoenzyme, [γ32P]-ATP, and increasing concentrations of poly-L-lysine (PLL) as described in “Materials and methods.” As a control, rPEDF and plPEDF were incubated in the same mix in the absence of CK2. After 45 minutes at 30°C, the reaction was arrested by boiling for 5 minutes in sample buffer, and the samples were subjected to 10% SDS-PAGE. The gel was stained with Coomassie blue (Coom, bottom panel), dried, and subjected to autoradiography (Auto, top panel). (B) rPEDF and plPEDF (50 μg/mL) were incubated with CK2 holoenzyme (4 μg/mL), [γ32P]-ATP (10 μM, 6 Ci/mmol [222 GBq/mmol]), poly-l-lysine (200 nM), and increasing concentrations of heparin (Hep). Phosphorylation and analysis were performed as in panel A. (C) rPEDF and plPEDF (50 μg/mL) were incubated with the pure catalytic subunit of PKA (2.5 μg/mL), heparin (50 μg/mL), and [γ32P]-ATP (10 μM, 6 Ci/mmol [222 GBq/mmol]). Phosphorylation and analysis were conducted as in panel A. (D) rPEDF was digested with trypsin as described in “Materials and methods.” At the indicated times, aliquots were removed from the reaction mixture and centrifuged, and sample buffer was added to the supernatant. Samples were boiled and subjected to 12.5% SDS-PAGE followed by silver stain. Right panel: rPEDF was phosphorylated by CK2 and loaded on a G25 Sephadex column to remove the excess of [γ32P]-ATP. The eluted fraction was then subjected to trypsin digestion and subjected to 12.5% SDS-PAGE followed by autoradiography. (E) Alignment of the tryptic peptides revealed by mass spectrometry and N-terminus sequence analysis that were obtained from the trypsin-digested fragments of rPEDF within a schematic representation of PEDF.
We then analyzed whether PEDF is an in vitro substrate of PKA as well. Therefore, rPEDF and plPEDF were incubated with the pure catalytic subunit of PKA and [γ32P]-ATP in the presence of heparin, which stimulates PKA phosphorylation of several substrates.29 Both rPEDF and plPEDF were equally phosphorylated by PKA in the presence of heparin (Figure 2C) in a PKI-inhibited manner (not shown), indicating that both proteins contain only a small amount of phosphate incorporated to the PKA site.
Localization of the CK2 phosphorylation site(s) in PEDF
CK2 phosphorylates Ser or Thr immersed in acidic sequence within proteins and peptides.30 The minimum requirement for CK2 phosphorylation is depicted by the sequence S/T-X-X-D/E. The presence of additional Asp or Glu residues at positions -3, +1, +2, +4, +5, or +7 improves the phosphorylation efficacy. By examining the primary sequence of PEDF for potential phosphorylation sites, 11 putative sites that meet the minimal consensus requirements were found. These are S24, S114, T121, S195, T219, T226, S227, T287, S328, S336, and T354. Of these, S24, S114, S195, T226, S227, and T287 were considered as preferred targets since they contain additional acidic residues in the preferred positions.
In an attempt to identify the actual CK2 phosphorylation site(s) in PEDF, rPEDF was digested with trypsin. This partial digestion yielded 2 major fragments with an apparent molecular weight of 20 kDa and 30 kDa (Figure 2D). We then phosphorylated rPEDF by CK2 and digested the phosphorylated protein with trypsin. Only the 20-kDa fragment was phosphorylated by CK2 (Figure 2D), indicating that the CK2 phosphorylation site is located within the 20-kDa fragment. We found that the fragment could not be sequenced by Edman degradation since it was blocked, indicating that it is the N-terminal fragment of PEDF. The 30-kDa fragment was sequenced by Edman degradation and was found to start at the amino acid Glu198. Mass spectrometry revealed more peptides in the 30-kDa fragment (Figure 2E), confirming its C-terminal position. Since the CK2 phosphorylation sites are located within the 20-kDa fragment, we concluded that Ser24 and/or Ser114 are the sites of CK2 phosphorylation. However, because the combined mass of the fragments is smaller than that of full-length rPEDF, it is possible that an additional CK2 phosphorylated fragment, which runs out of the gel, was also formed.
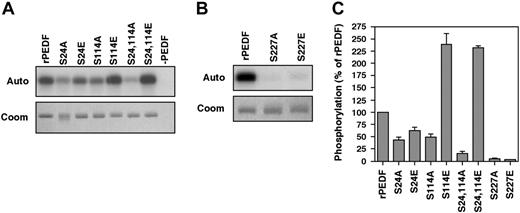
Identification of the CK2 phosphorylation sites by site-directed mutagenesis
To further study the CK2 phosphorylation sites in PEDF, we constructed single or double site mutants by replacing Ser at position 24, 114 with Ala (S24A, S114A, and S24, 114A) or with Glu (S24E, S114E and S24, 114E). rPEDF and its mutants were purified from the medium of the transfected HEK-293T cells and subjected to phosphorylation by CK2. Mutation of S24A significantly reduced CK2 phosphorylation (Figure 3), while the S24E mutation reduced phosphorylation only to a moderate extent. The S114A mutant significantly reduced CK2 phosphorylation, while the double mutant S24, 114A almost completely abolished this phosphorylation. We concluded that both Ser24 and Ser114 are the main sites for CK2 phosphorylation of PEDF. Surprisingly, both S114E and S24, 114E mutations significantly increased phosphorylation compared with CK2 phosphorylation of rPEDF (Figure 3). This unexpected result implies that mutation of this residue to Glu leads to the exposure of additional potential phosphorylation sites that were normally covered. Analysis of the 3-dimensional structure of PEDF31 revealed that Thr121 is spatially close to Ser114 and may serve as the additional site. However, since these sites may be covered upon phosphate incorporation to Ser24 and Ser114, it is possible that Thr354 is the other phosphorylated site. This site might have been phosphorylated by CK2 but was not detected in the tryptic digest because it was included in a small fragment that was not present on the gels. Nonetheless, our results indicate that PEDF is phosphorylated by CK2 mainly on residues Ser24 and Ser114.
Identification of CK2 and PKA phosphorylation sites of PEDF by site-directed mutagenesis. (A) rPEDF and rPEDF mutants (indicated) were phosphorylated by CK2 as described in “Materials and methods.” Reaction was arrested by sample buffer and samples were subjected to 10% SDS-PAGE. The gel was stained with Coomassie blue (Coom, bottom panel), dried, and subjected to autoradiography (Auto, top panel). (B) rPEDF and rPEDF mutants (indicated) were phosphorylated by PKA as described in “Materials and methods.” Samples were subjected to 10% SDS-PAGE, stained with Coomassie blue (Coom, bottom panel), dried, and subjected to autoradiography (Auto, top panel). (C) Quantitative analysis of the autoradiogram depicted in panels A-B is presented as a mean ± SD of 6 distinct experiments.
Identification of CK2 and PKA phosphorylation sites of PEDF by site-directed mutagenesis. (A) rPEDF and rPEDF mutants (indicated) were phosphorylated by CK2 as described in “Materials and methods.” Reaction was arrested by sample buffer and samples were subjected to 10% SDS-PAGE. The gel was stained with Coomassie blue (Coom, bottom panel), dried, and subjected to autoradiography (Auto, top panel). (B) rPEDF and rPEDF mutants (indicated) were phosphorylated by PKA as described in “Materials and methods.” Samples were subjected to 10% SDS-PAGE, stained with Coomassie blue (Coom, bottom panel), dried, and subjected to autoradiography (Auto, top panel). (C) Quantitative analysis of the autoradiogram depicted in panels A-B is presented as a mean ± SD of 6 distinct experiments.
Identification of the PKA phosphorylation site by site-directed mutagenesis
PKA phosphorylates Ser or Thr residues adjacent to at least 2 consecutive basic residues, depicted by the consensus sequence of R/K-R/K-X-S/T.32 By examining the primary sequence of PEDF for potential PKA phosphorylation sites, we found one such putative site at Ser227. In order to confirm this PKA phosphorylation site in PEDF, we constructed a single site mutant by replacing Ser227 either with Ala (S227A) or with Glu (S227E). The rPEDF and the mutants were purified and subjected to phosphorylation by PKA. Mutation of Ser227 to Ala or Glu completely abolished PKA phosphorylation of both rPEDF and plPEDF (Figure 3), indicating that this residue is indeed the PKA site in PEDF.
A 3-dimensional structure analysis31 of the CK2 and PKA phosphorylation sites in PEDF revealed that Ser114 and Ser227 residues are exposed and can be accessible to interact with potential kinases. Ser24 is not included in the crystal structure, however the location of the N-terminus is spatially converging to Ser114. Therefore, from the structural point of view, these residues may well serve as substrate candidates for phosphorylation.
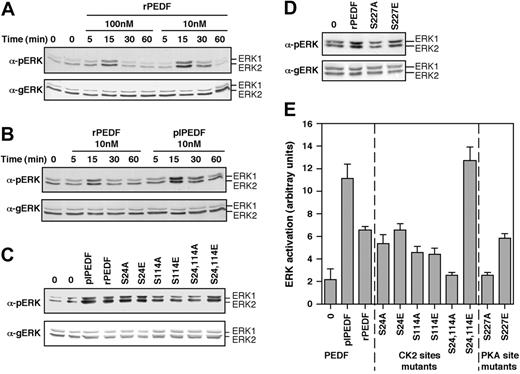
Activation of ERK by PEDF in endothelial cells
It was previously shown that CK2 phosphorylation of vitronectin significantly enhances endothelial cell adhesion.19 We undertook to study the effect of PEDF and its phosphorylated forms on the signaling and physiologic responses of endothelial cells. Therefore, serum-starved endothelial cells were incubated with rPEDF or with plPEDF, and cell lysates were analyzed for mitogen-activated protein kinases (MAPKs) and protein kinase B (PKB) activity using antiphospho Abs. PKB as well as JUN N-terminal kinase (JNK), p38MAPK, or ERK5 were not significantly affected in any of the conditions used (not shown). On the other hand, rPEDF caused a small (× 5) but reproducible activation of ERK phosphorylation in endothelial cells, whether obtained from a human source (HUVECs; Figure 4A) or from a bovine source (bovine aortic endothelial cells [BAECs]; not shown). The maximal activation of ERK1/2 was obtained after 15 minutes with 10 nM PEDF. Interestingly, the activation obtained with plPEDF was higher than that with rPEDF in HUVECs (Figure 4B) as well as in BAECs (not shown).
The effect of rPEDF, plPEDF, and the various rPEDF mutants on ERK/MAPK activation in HUVECs. (A) HUVECs were serum starved for 16 hours and then stimulated with different concentrations of rPEDF for the indicated times. Cytosolic extracts (30 μg) were subjected to immunoblotting with anti-pERK (αpERK, top panel) or anti-gERK (αgERK, bottom panel) Abs. The positions of ERK2 and ERK1 are indicated. (B) HUVECs were serum starved for 16 hours and then stimulated with rPEDF (10 nM) or with plPEDF (10 nM) for the indicated times. Cytosolic extracts (30 μg) were subjected to immunoblotting with anti–phospho ERK Ab (pERK, top panel) or with anti–general ERK Ab (gERK, bottom panel). (C-D) HUVECs were serum starved for 16 hours and then stimulated with rPEDF (10nM), plPEDF (10 nM), or with the various rPEDF mutants (10 nM) for 15 minutes. Cytosolic extracts (30 μg) were subjected to immunoblotting as described in panel A. (E) Quantitative analysis of immunoblots depicted in panels C-D is presented as a mean ± SD of 5 distinct experiments.
The effect of rPEDF, plPEDF, and the various rPEDF mutants on ERK/MAPK activation in HUVECs. (A) HUVECs were serum starved for 16 hours and then stimulated with different concentrations of rPEDF for the indicated times. Cytosolic extracts (30 μg) were subjected to immunoblotting with anti-pERK (αpERK, top panel) or anti-gERK (αgERK, bottom panel) Abs. The positions of ERK2 and ERK1 are indicated. (B) HUVECs were serum starved for 16 hours and then stimulated with rPEDF (10 nM) or with plPEDF (10 nM) for the indicated times. Cytosolic extracts (30 μg) were subjected to immunoblotting with anti–phospho ERK Ab (pERK, top panel) or with anti–general ERK Ab (gERK, bottom panel). (C-D) HUVECs were serum starved for 16 hours and then stimulated with rPEDF (10nM), plPEDF (10 nM), or with the various rPEDF mutants (10 nM) for 15 minutes. Cytosolic extracts (30 μg) were subjected to immunoblotting as described in panel A. (E) Quantitative analysis of immunoblots depicted in panels C-D is presented as a mean ± SD of 5 distinct experiments.
The effect of rPEDF mutants on ERK activation
Because of the differences in ERK activation between plPEDF and rPEDF, we used this system to examine whether the phosphorylation mutants indeed mimic the effect of phosphorylation on PEDF activity. When used to stimulate HUVECs, the CK2 phosphorylation site mutants S24A and S24E did not have a significant effect, while S114A and S114E mutants demonstrated slightly reduced ability to stimulate ERK phosphorylation (Figure 4C). However, significant effects were found with the double mutants, as S24, 114A had a reduced effect, while S24, 114E enhanced ERK phosphorylation. These effects were even stronger than the effects of rPEDF or plPEDF. The higher activity of S24, 114E suggests that the 2 Glu residues indeed mimic the activity of phosphorylated PEDF. However, plPEDF is incompletely phosphorylated in contrast to the existence of negatively charged residues in positions 24 and 114 of all molecules of S24, 114E. Similarly, the activity of S24, 114A was lower than that of rPEDF, suggesting that a small fraction of the rPEDF molecule is phosphorylated on Ser24 and Ser114. Thus, the mutants S24, 114E and S24, 114A further extend the phosphorylation-dependent differences between plPEDF and rPEDF.
Differences in ERK activation were observed also with the PKA mutants. Thus S227A completely inhibited the ability of rPEDF to induce ERK1/2 phosphorylation, whereas the S227E mutant had only a slight inhibitory effect (Figure 4D). Similar results were obtained with BAECs (not shown). These results further indicate that rPEDF is secreted as a phosphorylated protein on residue 227, in agreement with the phosphatase study described in Figure 1. Removal of the phosphate abolishes the PEDF-induced ERK phosphorylation, while Glu at this position elevated the PEDF effect. Taken together, our results indicate that the Glu or Ala mutants indeed mimic either the phosphorylated or nonphosphorylated forms of PEDF.
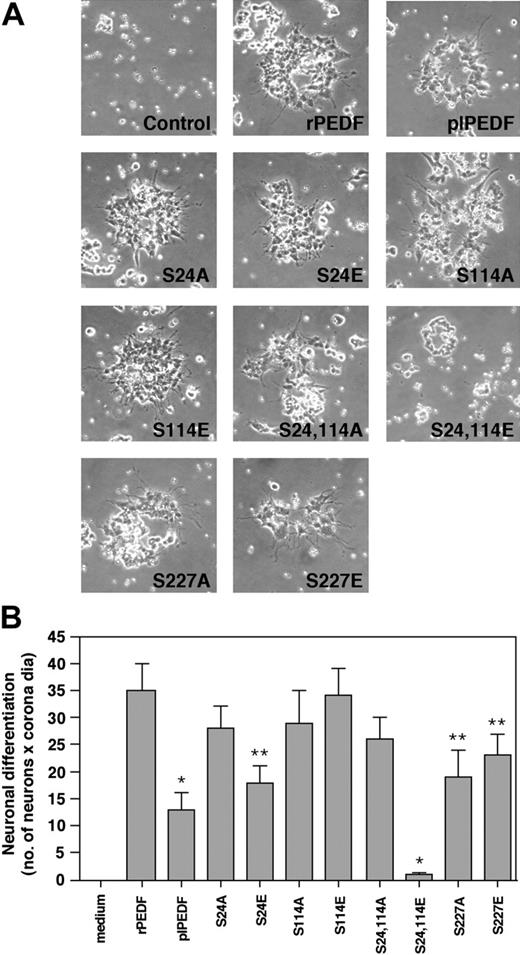
The effect of rPEDF mutants on PEDF's neurotrophic activity
We examined whether CK2 as well as PKA phosphorylation of PEDF can modulate its neurotrophic activity.3 Thus, we tested rPEDF, plPEDF, and the mutants for their ability to induce differentiation in human retinoblastoma Y-79 cells in culture. Indeed rPEDF and plPEDF induced neuronal differentiation (cell aggregation and neurite outgrowth) in Y-79 cells, where the effect of rPEDF was more pronounced compared with plPEDF (Figure 5). The CK2 phosphorylation site mutants S24E/S24A and S114E/S114A had only small effects, as they all induced neuronal differentiation of the Y-79 cells. However, much fewer neurite-like processes and cell aggregates were observed when cells were treated with the S24, 114E mutant. With this mutant, the cells formed small coronalike structures but were very compact without any sprouts projecting from the cells, and this inhibitory effect was stronger than that of plPEDF. On the other hand, cells treated with the S24, 114A mutant exhibit neurite outgrowth and big aggregates similar to rPEDF. Mutation of the PKA phosphorylation site S227E revealed a different phenotype, where colonies were smaller, fewer, and randomly spread, although their processes were clearly observed. Therefore, PKA phosphorylation has a limited influence on the neurotrophic effect of PEDF, while CK2 phosphorylation significantly reduces this neurotrophic effect.
The effect of rPEDF, plPEDF, and the various rPEDF mutants on PEDF neurotrophic activity. The cells (2.5 × 105 cells/mL) were incubated with rPEDF, plPEDF, or the various rPEDF mutants (all at 20 nM) in MEM supplemented with 2 mM l-glutamine, antibiotics, and 0.1% 5 mg/mL insulin, 5 mg/mL transferrin, 5 mg/mL selenium (ITS). After 7 days in culture, the cells were transferred onto poly-d-lysine–coated plates, and their morphology and differentiation state were monitored by inverted microscope (Nikon TE 2000U connected to DUC camera) at various periods of time. The Y-79 morphology at 10 days after attachment is shown. (B) Quantitative analysis of the results presented in panel A is presented as a mean ± SD of 6 distinct experiments. Student t test was used to analyze statistical significance of the differences between cells treated with rPEDF and cells treated with the various PEDF forms (*P < .01; **P < .05). dia indicates diameter.
The effect of rPEDF, plPEDF, and the various rPEDF mutants on PEDF neurotrophic activity. The cells (2.5 × 105 cells/mL) were incubated with rPEDF, plPEDF, or the various rPEDF mutants (all at 20 nM) in MEM supplemented with 2 mM l-glutamine, antibiotics, and 0.1% 5 mg/mL insulin, 5 mg/mL transferrin, 5 mg/mL selenium (ITS). After 7 days in culture, the cells were transferred onto poly-d-lysine–coated plates, and their morphology and differentiation state were monitored by inverted microscope (Nikon TE 2000U connected to DUC camera) at various periods of time. The Y-79 morphology at 10 days after attachment is shown. (B) Quantitative analysis of the results presented in panel A is presented as a mean ± SD of 6 distinct experiments. Student t test was used to analyze statistical significance of the differences between cells treated with rPEDF and cells treated with the various PEDF forms (*P < .01; **P < .05). dia indicates diameter.
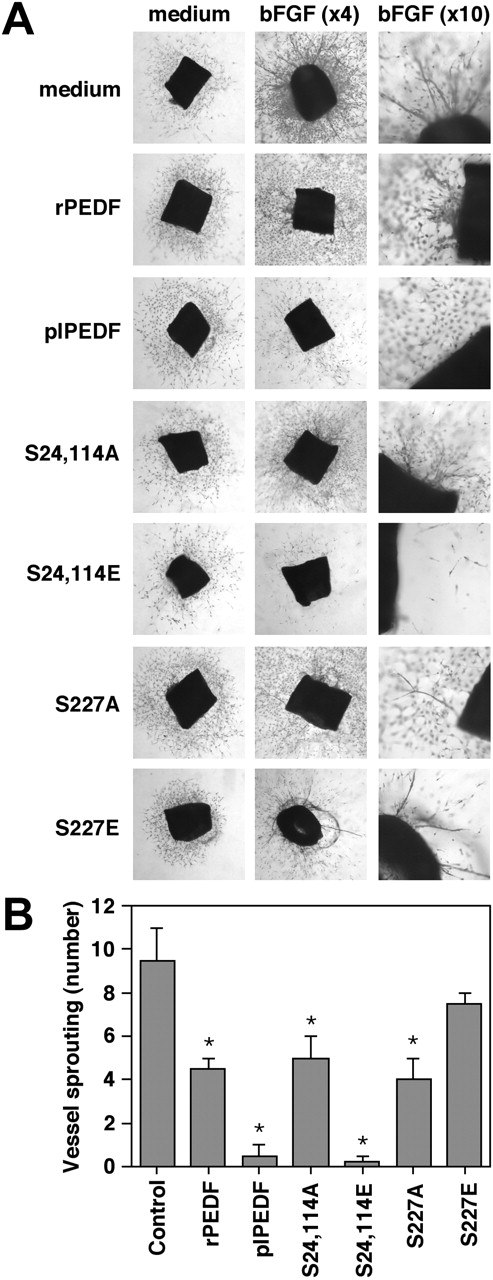
The effect of rPEDF mutants on PEDF's antiangiogenic activity ex vivo
To examine the effect of phosphorylation on the antiangiogenic activity of PEDF,9 we used the ex vivo aortic ring assay in the presence of bFGF as an angiogenic model.23 In the presence of bFGF (50 ng/mL), aortic rings from BALB/C mice developed numerous vessel-like sprouts compared with the rings that were treated with serum-free medium (Figure 6). As expected, plPEDF significantly inhibited the bFGF-induced vessel formation. However, the inhibitory effect of rPEDF was less pronounced than that of plPEDF, as rearrangement toward vessel formation and small numbers of vessel structure were observed when rPEDF and bFGF were added together.
The antiangiogenic activity of the various rPEDF forms on bFGF-induced vessel sprouting in the ex vivo aortic ring assay. (A) Aortic rings from BALB/C mice embedded in collagen matrix were exposed to rPEDF, plPEDF, S24, 114A mutant, S24, 114E mutant, S227A mutant, or S227E mutant (10 nM) in the presence or absence of bFGF (50 ng/mL) in the serum-free BIO-MPM-1 medium. Control rings were treated with serum-free BIO-MPM-1 medium or with bFGF (50 ng/mL). Following 10 days of incubation, rings were fixed and stained with crystal violet (0.02%) to illustrate sprouting and vessel formation. Representative micrographs of ring of each arm of the experiment are shown. Micrographs were taken using inverted microscope (Nikon TE 2000U) connected to DUC camera under × 4 and × 10 objective. (B) Quantitative analysis of the assay described in panel A is presented as a mean ± SD of 6 distinct experiments. Student t test was used to analyze statistical significance of the differences between rings treated with bFGF and rings treated with the combination of bFGF and the various PEDF forms (*P < .01).
The antiangiogenic activity of the various rPEDF forms on bFGF-induced vessel sprouting in the ex vivo aortic ring assay. (A) Aortic rings from BALB/C mice embedded in collagen matrix were exposed to rPEDF, plPEDF, S24, 114A mutant, S24, 114E mutant, S227A mutant, or S227E mutant (10 nM) in the presence or absence of bFGF (50 ng/mL) in the serum-free BIO-MPM-1 medium. Control rings were treated with serum-free BIO-MPM-1 medium or with bFGF (50 ng/mL). Following 10 days of incubation, rings were fixed and stained with crystal violet (0.02%) to illustrate sprouting and vessel formation. Representative micrographs of ring of each arm of the experiment are shown. Micrographs were taken using inverted microscope (Nikon TE 2000U) connected to DUC camera under × 4 and × 10 objective. (B) Quantitative analysis of the assay described in panel A is presented as a mean ± SD of 6 distinct experiments. Student t test was used to analyze statistical significance of the differences between rings treated with bFGF and rings treated with the combination of bFGF and the various PEDF forms (*P < .01).
We then examined the effects of the phosphorylation site mutants. When incubated together with bFGF, the CK2 nonphosphorylated double mutant, S24, 114A, exhibited an antiangiogenic activity that was similar to or slightly less than that of rPEDF, where rearrangement toward vessels could be seen, but clear vessels did not form. On the other hand, the CK2 phosphorylated mutant, S24, 114E, appeared to be a very significant antiangiogenic factor, even stronger than plPEDF, as it did not allow any vessel formation. The PKA nonphosphorylated mutant, S227A, inhibited the bFGF-induced vessel formation similarly to rPEDF, while the PKA phosphorylated mutant, S227E, had less antiangiogenic activity. S227E alone was not proangiogenic and its effect on the bFGF-induced angiogenesis was reduced compared with rPEDF. We therefore conclude that phosphorylation of PEDF on its CK2 sites significantly enhanced the antiangiogenic activity of PEDF, while the phosphorylation on its PKA site may slightly reduce its antiangiogenic activity.
The effect of rPEDF mutants on PEDF's antiangiogenic activity in vivo
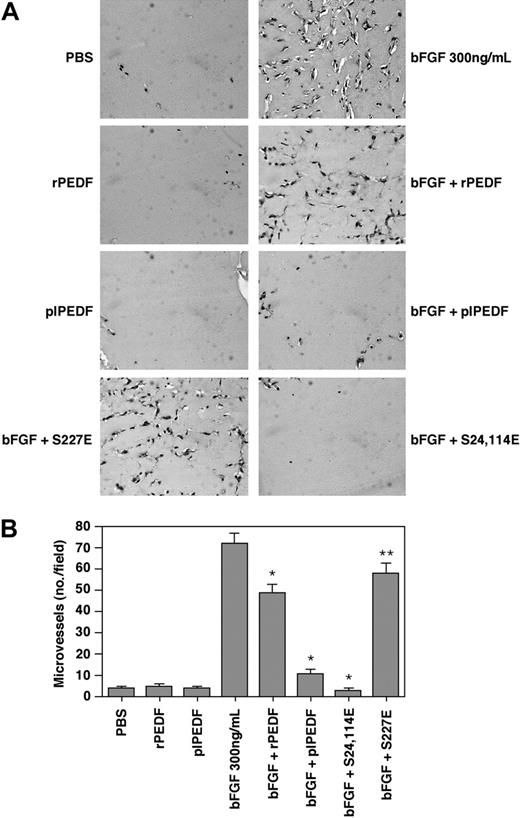
To further assess the effect of phosphorylation on PEDF antiangiogenic activity in vivo we have used the Matrigel plug assay25 in the presence of bFGF as an angiogenic model. Thus, liquid Matrigel supplemented with the various treatments was injected subcutaneously into CD-1 nude mice. The Matrigel polymerized to form a plug, which was removed after a week and analyzed for its angiogenic response. As expected, control plugs treated with phosphate-buffered saline (PBS) or PEDF alone showed very little angiogenic response (Figure 7). bFGF-impregnated plugs elicited a robust angiogenic activity, as judged by the large number of blood vessels infiltrating into the plug. plPEDF significantly inhibited the bFGF-induced vessel infiltration, while the inhibitory effect of rPEDF was significantly less pronounced. As shown in the aortic ring assay, the S24, 114E mutant had even stronger antiangiogenic activity relative to plPEDF, as plugs treated with this mutant had very little angiogenic response. In contrast, plugs treated with bFGF and S227E had much less antiangiogenic activity reflected in many infiltrating vessels. In addition plugs treated with bFGF and S24, 114A mutant or S227A mutant appeared similar to those treated with bFGF and rPEDF (not shown). These results further support that CK2-phosphorylated PEDF enhanced the antiangiogenic activity of PEDF, while the phosphorylation on its PKA site may reduce this activity.
The antiangiogenic activity of the various rPEDF forms on bFGF-induced neovascularization in the in vivo Matrigel plug assay. A) CD-1 nude mice were subcutaneously injected with 0.5 mL Matrigel containing rPEDF, plPEDF, S24, 114E mutant, and S227E mutant (all at 20 nM) in the presence or absence of bFGF (300 ng/mL). Control plugs were combined with PEDF (20 nM) or bFGF (300 ng/mL) only. After 7 days, mice were killed and Matrigel plugs were excised, fixed in 4% formaldehyde, embedded in paraffin, sectioned, and stained. Representative fields of H&E staining of thin sections from Matrigel plugs of each arm of the experiments were taken using light microscope (Nikon E600 connected to DxM 1200 F camera) (× 40 magnification). (B) Angiogenesis was measured by counting the number of blood vessels/field for 3 different cross-sectional areas of each Matrigel plug. Student t test was used to analyze statistical significance of the differences between plugs treated with bFGF and plugs treated with the combination of bFGF and the various PEDF forms (*P < .01; **P < .05; n = 3).
The antiangiogenic activity of the various rPEDF forms on bFGF-induced neovascularization in the in vivo Matrigel plug assay. A) CD-1 nude mice were subcutaneously injected with 0.5 mL Matrigel containing rPEDF, plPEDF, S24, 114E mutant, and S227E mutant (all at 20 nM) in the presence or absence of bFGF (300 ng/mL). Control plugs were combined with PEDF (20 nM) or bFGF (300 ng/mL) only. After 7 days, mice were killed and Matrigel plugs were excised, fixed in 4% formaldehyde, embedded in paraffin, sectioned, and stained. Representative fields of H&E staining of thin sections from Matrigel plugs of each arm of the experiments were taken using light microscope (Nikon E600 connected to DxM 1200 F camera) (× 40 magnification). (B) Angiogenesis was measured by counting the number of blood vessels/field for 3 different cross-sectional areas of each Matrigel plug. Student t test was used to analyze statistical significance of the differences between plugs treated with bFGF and plugs treated with the combination of bFGF and the various PEDF forms (*P < .01; **P < .05; n = 3).
Discussion
In this report, we demonstrated that PEDF is present in the human plasma as a phosphoprotein, with phosphates incorporated to 2 of its CK2 sites and to a lesser extent on a PKA site. rPEDF is also phosphorylated on the sites of these kinases but to a much lesser extent. Indeed, rPEDF can be phosphorylated by CK2 and PKA in vitro (Figure 1A-C), and these phosphorylations can be achieved also by the whole plasma (Figure 1D). These results indicate that the phosphorylation may occur, at least in part, in the circulating blood and may serve as a regulatory mechanism of PEDF activity.
The 2 different sources of PEDF used in our study, rPEDF and plPEDF exhibited different physiologic activities in the assays used. We examined whether the physiologic differences between the 2 proteins were induced by the differences in their phosphorylation. Thus, we constructed several phosphorylation site mutants that were supposed to mimic either the phospho (Glu) or nonphospho (Ala) forms of PEDF. First we determined whether the mutants indeed behave like their phosphorylated counterparts. Therefore, we compared the mutants with rPEDF and plPEDF for their ability to induce ERK phosphorylation. We found that these constructs even amplify the differences in phosphorylation between plPEDF and rPEDF (Figure 4). This could be due to the fact that while rPEDF is slightly phosphorylated by CK2, S24, 114A is not phosphorylated at all on these sites. On the other hand, some of the molecules of plPEDF seem to be phosphorylated on the CK2 site, while the S24, 114E represent a homogeneous PEDF population with a negative charge at positions 24 and 114. Unlike the significant differences in the CK2 sites, plPEDF and rPEDF were found to be phosphorylated to a small extent on Ser227. However, S227A represent molecules that are not phosphorylated at all, while S227E represents fully phosphorylated Ser227. We conclude that the amount of negative charges on residues 24 and 114 is in the following order S24, 114E > plPEDF > > rPEDF > S24, 114A, while the phosphorylation on Ser227 is in the order S227E > > plPEDF = rPEDF > S227A.
Previous reports demonstrated that the recombinant His-tagged PEDF is functionally identical to the PEDF isolated from bovine eyes.9,10,33 Here we show that although rPEDF may induce part of the activities of plPEDF, these 2 proteins still represent significant differences in their effects, which are dependent on their degree of phosphorylation. Thus, we observed a CK2-dependent difference in the ability of PEDF to induce neuronal differentiation in retinoblastoma cells where both rPEDF and S24, 114A mutants induced neuronal differentiation, while the S24, 114E had almost no neurotrophic effect. These results correlated with the extent of CK2 phosphorylation, and clearly indicate that lack of CK2 phosphorylation makes PEDF a superior neurotrophic factor. On the other hand, the results with the PKA mutants indicated that although this phosphorylation may play a role in the proliferation of the cells, it probably does not affect the neurotrophic activity of PEDF.
Dependence on the extent of phosphorylation was also observed in the 2 angiogenic assays used in this study, namely the aortic rings assay (Figure 6) and the Matrigel plug assay (Figure 7). In both assays, however, the phosphorylation increased, rather than decreased, the effect. Thus, S24, 114E appeared to be a very significant antiangiogenic factor, and its inhibitory effect was more pronounced when compared with plPEDF antiangiogenic activity. Treatment with S227E reduced the antiangiogenic effect of PEDF, and in both assays the effect of this mutant was even smaller than that of rPEDF. Therefore, it is possible that phosphorylation of weak Ser227 and to a lesser extent also Ser24 and Ser114 induces a general weak antiproliferative effect of PEDF.
The present data reveal different physiologic functions of PEDF that are dependent on the phosphorylation state of the protein. Indeed the various functions of PEDF that are well described in the literature reveal a protein that can maintain dual activity. PEDF is a very effective neuroprotective,5,6 as well as antiangiogenic,9,10 factor. The mechanism involved in PEDF's various functions has not yet been revealed. This is mainly due to lack of information on the nature of the receptor for PEDF. Although an approximately 80-kDa interacting protein has been reported for PEDF in retinoblastoma (Y-79) and neuronal cells,7,8 it is not clear whether this is indeed the receptor or another type of regulatory protein. Therefore, it is possible that PEDF is exerted by 2 distinct receptors or by 1 receptor that may be influenced differently by the phosphorylated or nonphosphorylated PEDF.
Additional support for a functional change of PEDF that is phosphorylation dependent was demonstrated by the degree of ERK activation. A significant effect was found with the double mutants, as S24, 114A reduced ERK activation, while S24, 114E enhanced ERK activation to a higher degree than the stimulation achieved by plPEDF. Apparently, none of the forms of PEDF that has been used was able to significantly inhibit the bFGF-induced ERK activation (data not shown). Therefore, there is no correlation between the activation of ERK and either the antiangiogenic or the neurotrophic activities of PEDF. The lack of correlation together with the low extent of the ERK activation indicate that PEDF signaling is not mediated by the ERK cascade.
Evidence for the existence of an extracellular PKA and CK2-like activity was documented in several laboratories. One of the substrates for these extracellular kinases is vitronectin that was found to be a major substrate for PKA in blood.14 This phosphorylation attenuates the vitronectin binding to PAI-1, and consequently modulates this important physiologic function of vitronectin.18 Additionally it was shown that urokinase plasminogen activator (u-PA) is phosphorylated on Tyr residue,34 and the phosphorylated u-PA exhibits a lower affinity for PAI-1 and PAI-2 than the nonphosphorylated form.35 These studies together with our current findings support the involvement of extracellular protein phosphorylation as a mechanism of regulatory cellular processes. Thus, our findings indicate that the extracellular phosphorylation of PEDF may well be a physiologic regulatory mechanism that controls PEDF's multifaceted functions, and determines its specific outcome.
In summary, we have shown that PEDF purified from human plasma (plPEDF) is a phosphoprotein, which is phosphorylated in the circulation by CK2 and to a lesser degree also by PKA. The physiologic relevance of these phosphorylations was determined by comparing rPEDF with the phosphorylation site mutants in regard to their neurotrophic and antiangiogenic activities. We found that both CK2 and PKA phosphorylations of PEDF markedly affect its physiologic function. The fully CK2 phosphorylation site mutant S24, 114E abolished PEDF neurotrophic activity and enhanced its antiangiogenic activity, while the PKA phosphorylation site mutant S227E reduced PEDF antiangiogenic activity. Therefore, phosphorylation may determine the specific physiologic outcome of PEDF, and as such, is the first demonstration that extracellular phosphorylation causes a complete change of function of a circulating protein.
Prepublished online as Blood First Edition Paper, September 16, 2004; DOI 10.1182/blood-2004-04-1569.
Supported by a grant from La Foundation Raphael et Regina Levy (R.S.) and a grant from the Israel Academy of Sciences and Humanities (S.S.).
Shmuel Shaltiel died on April 5, 2002.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank T. Kreizman, I. Schwartz, M. Eisenstein, H. Shao, S. Klachenko, R. Margalit, and T. Berkutzki for their contribution to this study.

![Figure 1. PEDF in plasma is a phosphoprotein. (A) Aliquots of rPEDF, plPEDF active (phosphorylated) ERK, phosphorylated α-casein (phos cas), and dephosphorylated α-casein (dephos cas) were subjected to 10% SDS-PAGE (1 μg/lane) and immunoblotted with antiphospho-Ser, -Thr, or -Tyr Abs in the presence or absence of the appropriate phosphorylated amino acid (2.5 mM). As a control, the samples were blotted with anti-PEDF, anti–phosphorylated ERK (αpERK), and anti–general ERK (αgERK) Abs, or stained with gel code for the α-casein. (B) rPEDF (2 μg) or plPEDF (2 μg) were incubated with alkaline phosphatase (APase) conjugated to acrylic beads (2 U) or with protein A sepharose CL-4B (1.6 mg) for 45 minutes at 30°C. Following incubation, samples were centrifuged in order to remove the phosphatase, and the supernatants were subjected to in vitro CK2 or PKA phosphorylation as described in “Materials and methods.” Phosphorylated products were analyzed by 10% SDS-PAGE and blotting, followed by exposure to autoradiography (Auto, top panel) and immunoblot with anti-PEDF Ab (bottom panel). (C) Quantitative analysis of the experiment depicted in panel B is presented as a mean ± SD (n = 4). (D) PEDF purified from human plasma was subjected to alkaline phosphatase treatment as described in “Materials and methods”. Thereafter, aliquots (1.5 μg) were incubated with fresh human plasma (8 μL) and [γ32P]-ATP (10 μM, 6 Ci/mmol [222 GBq/mmol]) in the presence or absence of PKA inhibitor (PKI, 1 μg/mL) or heparin (100 μg/mL). Control samples were subjected to in vitro CK2 or PKA phosphorylation as described in panel B. Phosphorylated products were analyzed as described in panel B. Vn indicates plasma vitronectin.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/105/2/10.1182_blood-2004-04-1569/6/m_zh80020572460001.jpeg?Expires=1769111849&Signature=qlMgjVsQHUeekh5RlHzgHbujGe4sUPNVMuVgovmkz5HaTDEdvykXH67HYELynGevvMIKfvWKobE2CUoPFyeyS3V9cd-OwbcoouRjzLmhyxYXV91tY3WkFsOEJ~l2JKXqzVBAIh-XxMPpGJnGVrv9KgdcU7tav4YMZ6OAqwNxMPpa73fiVZCmfFftcxWC93Tp0JpPy7LqR2SAMyldc~zBGzVaSWKHNwz-Vx7gFuGP8isQtys7y4xmSXjeHeSJUeqHMIWtcYgfBklLqLqeCh65lixyC5~0KkrX547AvYJQWWicqlZmXart6Fn18S8Zw~DBmiRajNjav4Pp6x~Kz0QKEQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 2. CK2 and PKA phosphorylate PEDF in vitro. (A) rPEDF and plPEDF were incubated with CK2 holoenzyme, [γ32P]-ATP, and increasing concentrations of poly-L-lysine (PLL) as described in “Materials and methods.” As a control, rPEDF and plPEDF were incubated in the same mix in the absence of CK2. After 45 minutes at 30°C, the reaction was arrested by boiling for 5 minutes in sample buffer, and the samples were subjected to 10% SDS-PAGE. The gel was stained with Coomassie blue (Coom, bottom panel), dried, and subjected to autoradiography (Auto, top panel). (B) rPEDF and plPEDF (50 μg/mL) were incubated with CK2 holoenzyme (4 μg/mL), [γ32P]-ATP (10 μM, 6 Ci/mmol [222 GBq/mmol]), poly-l-lysine (200 nM), and increasing concentrations of heparin (Hep). Phosphorylation and analysis were performed as in panel A. (C) rPEDF and plPEDF (50 μg/mL) were incubated with the pure catalytic subunit of PKA (2.5 μg/mL), heparin (50 μg/mL), and [γ32P]-ATP (10 μM, 6 Ci/mmol [222 GBq/mmol]). Phosphorylation and analysis were conducted as in panel A. (D) rPEDF was digested with trypsin as described in “Materials and methods.” At the indicated times, aliquots were removed from the reaction mixture and centrifuged, and sample buffer was added to the supernatant. Samples were boiled and subjected to 12.5% SDS-PAGE followed by silver stain. Right panel: rPEDF was phosphorylated by CK2 and loaded on a G25 Sephadex column to remove the excess of [γ32P]-ATP. The eluted fraction was then subjected to trypsin digestion and subjected to 12.5% SDS-PAGE followed by autoradiography. (E) Alignment of the tryptic peptides revealed by mass spectrometry and N-terminus sequence analysis that were obtained from the trypsin-digested fragments of rPEDF within a schematic representation of PEDF.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/105/2/10.1182_blood-2004-04-1569/6/m_zh80020572460002.jpeg?Expires=1769111849&Signature=0ySx7G3GPQPEBiepnUk1mcOALuGTwUsX0DNOiF1bhfXkkrm3f96SEnjjTMGKnN0bc4CHYsOjtn0yxzJKYHEti78GiLG2sLnztL3nGaC6Iv4AScn1Numv5MTsmpJNmgEIR2d5noTU7PnfIRh4vyKiznqVPOrAxHJaRhHJVHqWYSICncis~FijsyQVsZ6G0~8K0V39o7mDzwPliWHof12B7cSrg0kkT6qa80nkdjwAZcMTk-n5Bk6zYAiqSIrK4EwMd8oz8jUVe-GmS6-h7R1gTLtcHYspprLBuudgPSFPv~rzlmkXTIMZhFT1za3iRR8xGDWuGIqmOfbl5y5FKeJpCQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal